Abstract
Chronic monocular deprivation induces severe amblyopia that is resistant to spontaneous reversal in adulthood. However, dark exposure initiated in adulthood reactivates synaptic plasticity in the visual cortex and promotes recovery from chronic monocular deprivation. Here we show that chronic monocular deprivation significantly decreases the strength of feedforward excitation and significantly decreases the density of dendritic spines throughout the deprived binocular visual cortex. Dark exposure followed by reverse deprivation significantly enhances the strength of thalamocortical synaptic transmission and the density of dendritic spines on principle neurons throughout the depth of the visual cortex. Thus dark exposure reactivates widespread synaptic plasticity in the adult visual cortex, including at thalamocortical synapses, during the recovery from chronic monocular deprivation.
Introduction
The ocular dominance of binocular neurons in the primary visual cortex is established prenatally and maintained postnatally by a competition between the synapses serving the two eyes. Brief monocular deprivation in juveniles induces a rapid shift in ocular dominance due to a rapid decrease in the strength of synapses serving the deprived input. The deprived eye depression is similar to the homosynaptic long-term depression (LTD) of excitatory synapses induced by low frequency stimulation1-3. Accordingly, both LTD and the deprived eye depression are induced by de-correlation of pre- and postsynaptic activity4-5. LTD and the deprived eye depression are also both mediated by endocytosis of synaptic AMPARs and both decline over the course of postnatal development3, 6-7. Importantly, deprived eye depression occludes subsequent LTD induced by low frequency stimulation to layer IV1.
The majority of excitatory synaptic transmission in the mammalian cortex occurs on the heads of dendritic spines that harbor postsynaptic densities. In the visual cortex, approximately 95% of all dendritic spines form synapses, and spine head volume is strongly correlated with the area of the postsynaptic density8. Morphological features of dendritic spines, such as spine head diameter or neck length, as well as overall spine density are regulated by sensory input9-10 and state of arousal11. In addition, prolonged low-frequency stimulation of excitatory synapses in the hippocampus induces an NMDAR-dependent shrinkage of spine heads12 and depolymerization of actin13-14. Similarly, LTD is accompanied by a retraction in dendritic spines15 and a decrease in the number of contacts between presynaptic boutons and dendritic spines16-17.
In addition to the deprived eye depression induced by brief monocular deprivation in juveniles, prolonged monocular deprivation induces a potentiation of the response to stimulation of the non-deprived eye18-19. Accordingly, monocular deprivation induces a transient decrease in the density of dendritic spines on the apical dendrites of supragranular neurons, which recovers following prolongation of the monocular deprivation20. Monocular deprivation in juveniles also increases dendritic spine turnover along the length of layer 5 apical dendrites in layer II/III and V, but not layer IV21. In contrast, anatomical reorganization following monocular or binocular deprivation in adults is limited20-22, but can be encouraged with repeated episodes of monocular deprivation23 or enzymatic degradation of the extracellular matrix24.
The developmental decline in ocular dominance plasticity also constrains the recovery of the binocular visual cortex from monocular deprivation. For example, in juveniles, the ocular dominance shift induced by brief monocular deprivation is rapidly reversed if the occlusion is removed early in postnatal development25-27. In contrast, chronic monocular deprivation, from eye opening to adulthood, induces a significant shift in ocular dominance that does not reverse spontaneously following removal of the occlusion. Chronic monocular deprivation is also predicted to induce considerable degradation of feedforward excitation, which contributes to the constraint of recovery in adults. Indeed, a significant retraction of thalamocortical afferents is observed following prolonged monocular deprivation28-31. However, we have recently reported that binocular visual deprivation, through dark exposure, reactivates robust ocular dominance plasticity in adults and promotes the recovery of spatial acuity in the chronically-deprived eye32-33. This complements a growing list of experimental interventions developed to promote the recovery of vision following chronic monocular deprivation24, 34-35. The ability to recover spatial acuity suggests that some feedforward excitation remain intact following chronic monocular deprivation, however this prediction is at odds with the assumption that chronic monocular deprivation induces significant degradation of the pathway serving the chronically-deprived eye. Alternatively, interventions that promote the recovery from chronic monocular deprivation by may enhance synaptic plasticity in horizontal cortico-cortical connectivity to compensate for the absence of plasticity in a degraded, feedforward circuit.
Therefore we examined the anatomical integrity of excitatory synapses before and after the recovery from chronic monocular deprivation, using an unbiased labeling method (Diolistic staining36) to track the density and morphology of spines along pyramidal neuron basolateral dendrites. Following chronic monocular deprivation, from eye opening to adulthood, we observed a significant, parallel decrease in dendritic spine density throughout the depth of the visual cortex, including the thalamo-recipient zone. Nonetheless, stimulation of the chronically-deprived eye continued to evoke residual neuronal spiking and thalamocortical synaptic potentials. Recovery from chronic monocular deprivation induced by dark exposure followed by reverse deprivation, resulted in a parallel enhancement in dendritic spine densities throughout the depth of the visual cortex and a recovery of visually evoked activity including thalamocortical synaptic potentials.
Results
Decrease in dendritic spine density induced by chronic monocular deprivation and recovery following dark exposure and reverse deprivation
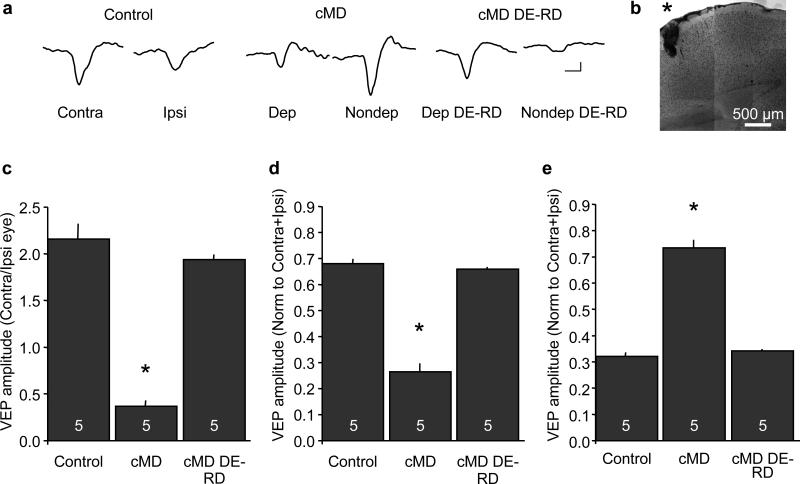
We used visually evoked potentials (VEPs) recorded in layer IV of the binocular visual cortex in response to high contrast gratings (0.04 cycles/degree reversing at 1 Hz) to assess the physiological consequences of chronic monocular deprivation in Long Evans rats. The contralateral bias of the rodent visual system is reflected as a two-fold larger VEP in response to stimulation of the contralateral eye relative to the ipsilateral eye (Figure 1). Chronic monocular deprivation of the dominant contralateral eye, from eye opening to adulthood (postnatal day P14 to postnatal day 148±11.65), induced a significant decrease in the VEP contralateral bias. The effects of chronic monocular deprivation could be reversed by binocular visual deprivation, through dark exposure, followed by reverse deprivation (open the chronically-deprived eye and close the chronically non-deprived eye; VEP amplitude in response to stimulation of contralateral/ipsilateral eye average ± SEM: control = 2.16±0.16, n=5; chronic monocular deprivation (cMD) = 0.37±0.06, n=5; chronic monocular deprivation + dark exposure + reverse deprivation (cMD DE-RD) = 1.93±0.05, n=5; one-way ANOVA, F(2,14)=94.5117, p<0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc; Figure 1C). Chronic monocular deprivation and subsequent reversal by dark exposure and reverse deprivation, induced bidirectional regulation of the VEP evoked by stimulation of the deprived (contralateral) eye and non-deprived (ipsilateral) eye (normalized deprived eye VEP average ± SEM: control = 0.68 ±0.02, cMD = 0.27 ±0.03, cMD DE-RD = 0.66 ±0.01; one-way ANOVA, F(2,14)=155.2638, p<0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc, Figure 1D; non-deprived eye VEP normalized to C+I average ± SEM: control = 0.32 ±0.02, cMD = 0.73 ±0.03, cMD DE-RD = 0.34 ±0.01; one-way ANOVA, F(2,14)=114.2875, p<0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc, Figure 1E).
Figure 1.
Regulation of layer IV VEP contralateral bias by chronic monocular deprivation. A. Representative VEP waveforms recorded from layer IV evoked by horizontal gratings (average of 100 presentations of 0.04 cycles/degree, 96% contrast, reversing at 1 Hz; scale bars=50 ms, 50 μvolts). B. Photomontage of nissl stain confirms electrode placement (*) in layer IV of visual cortex. C. Reduction of VEP contralateral bias following chronic monocular deprivation and recovery following dark exposure and reverse deprivation (DE-RD; one-way ANOVA, F(2,14)=94.5117, p<0.0001, *p<0.05 versus control Tukey-Kramer HSD post-hoc). D. Bidirectional regulation of the deprived eye VEP (one-way ANOVA F(2,14)=155.2638, p<0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc). E. Bidirectional regulation of the ipsilateral eye VEP (one-way ANOVA F(2,14)=114.2875, p<0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc).
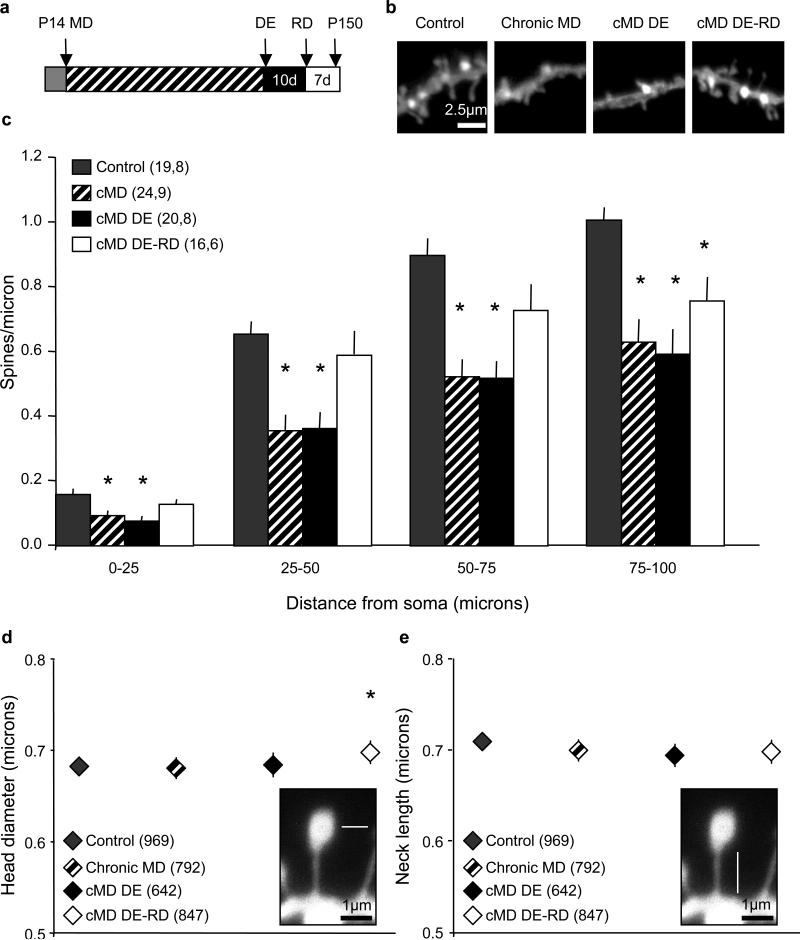
To probe for changes in the anatomy of excitatory circuitry that may underlie the bidirectional regulation of visual responses following chronic monocular deprivation and subsequent recovery, we tracked the density and morphology of dendritic spines. We focused on basolateral dendrites of principle neurons, which contain the majority of feedforward excitatory input37 and limited our analysis to dendrites that could be traced back to pyramidal neuron cell bodies in the binocular primary visual cortex contralateral to the occluded eye. Dendritic spine densities were binned in 25 micron segments starting from the soma, and analysis was performed for approximately 100 microns of the basolateral dendrite. Chronic monocular deprivation significantly decreased the density of dendritic spines in the deprived visual cortex. Surprisingly, dendritic spine densities were unchanged following dark exposure, however, dark exposure and reverse deprivation in adulthood significantly enhanced dendritic spine density (spines/micron binned by 25 micron segment average ± SEM: 0-25 μm control=0.16±0.02, chronic MD=0.09±0.01, cMD DE=0.08±0.01, cMD DE-RD=0.13±0.02, one-way ANOVA, F(3,78)=5.7840, p=0.0013, *p<0.05 versus control, Tukey-Kramer HSD post-hoc; 25-50 μm control=0.65±0.04, chronic MD=0.36±0.05, cMD DE=0.36±0.05, cMD DE-RD=0.59±0.07, one-way ANOVA, F(3,78)=7.1713, p<0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc; 50-75 μm control=0.90±0.05, chronic MD=0.52±0.05, cMD DE=0.52±0.05, cMD DE-RD=0.73±0.08, one-way ANOVA, F(3,75)=10.0410, p<0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc; 75-100 μm: control=1.01±0.04, chronic MD=0.63±0.07, cMD DE=0.59±0.08, cMD DE-RD=0.76±0.07, one-way ANOVA, F(3,63)=8.2569, p=0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc, Figure 2C). Complementary changes in dendritic spine density were not observed in the non-deprived visual cortex (ipsilateral to the occluded eye). However, a significant decrease in dendritic spine density was observed in the non-deprived visual cortex following dark exposure and reverse deprivation, consistent with the activation of robust anatomical plasticity by dark exposure in adulthood (Supplemental figure 1).
Figure 2.
Bidirectional regulation of dendritic spine density in deprived visual cortex by chronic monocular deprivation. A. Experimental timeline. B. Representative DiI labeled basolateral dendrites from pyramidal neurons from binocular visual cortex in a control, chronically monocularly-deprived (cMD), chronically monocularly-deprived plus dark exposure (cMD DE) and chronically monocularly-deprived plus dark exposure and reverse deprivation (cMD DE-RD) subject. In each case the dendritic segment shown is 75–100 μm from the cell body. C. A significant reduction in spine density is observed in all dendritic segments following chronic monocular deprivation, but is unchanged following 10 days of dark exposure. However, dark exposure plus reverse deprivation induces a significant increase in dendritic spine density in 0-75 μm segments (one-way ANOVA for each segment p<0.0001; *p<0.05 versus within-segment control, Tukey-Kramer HSD post-hoc; n=neurons, subjects). D. No change in dendritic spine head diameter following chronic monocular deprivation or dark exposure. However an increase in dendritic spine head diameter is observed when reverse deprivation follows chronic monocular deprivation and dark exposure (one-way ANOVA, F(3,3249) p<0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc; n=spine number). Inset: Schematic depicting the measurement of spine head diameter (white line). E. No change of dendritic spine lengths following manipulations of monocular visual experience. Inset: Schematic depicting the measurement of spine neck length (white line).
Although chronic monocular deprivation induced a significant reduction in dendritic spine density in the deprived visual cortex, a substantial number of dendritic spines persist. To ask if the spared dendritic spines showed evidence for compensation for the spine loss, we measured the head diameter and neck length of residual spines across all experimental conditions. Surprisingly, we observed no change in the average diameter of spine heads following chronic monocular deprivation or dark exposure. However, dark exposure followed by reverse deprivation significantly increased the diameter of spine heads along the basolateral dendrite (spine head diameter average ± SEM: control=0.68±0.01, chronic MD=0.68±0.01, cMD DE=0.68±0.01, cMD DE-RD=0.70±0.01; oneway ANOVA, F(3,3249)=17.609, p<0.0001, *p<0.05 versus control, Tukey-Kramer HSD post-hoc, Figure 2D). No change in dendritic spine neck lengths were observed prolonged manipulations of visual experience (average ± SEM control= 0.71±0.01, chronic MD=0.70±0.01, cMD DE=0.69±0.01, cMD DE-RD=0.70±0.01, Figure 2E).
Parallel, bidirectional regulation of dendritic spine density in all cortical laminae
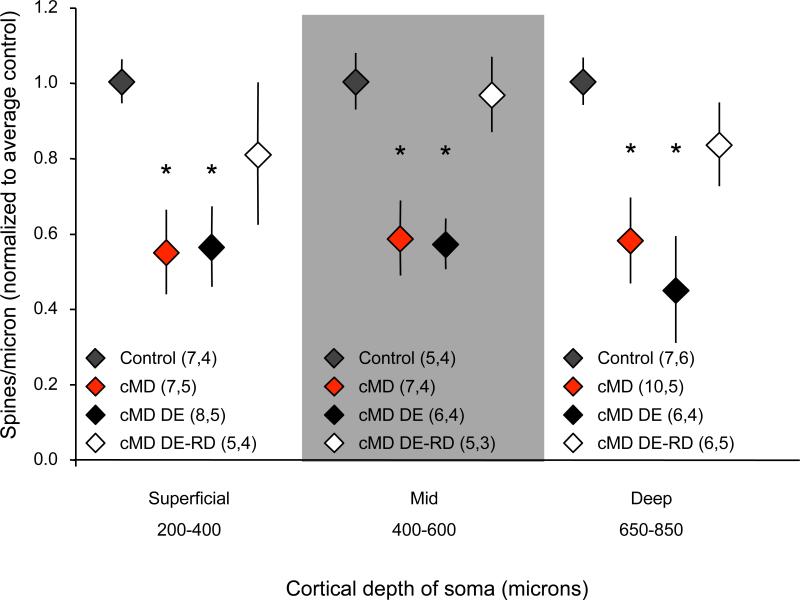
The Diolistic method allows for tracking of dendritic spine density from neurons at all depths from the dura surface. Therefore we used Diolistic labeling to ask if monocular visual experience differentially affects spine density of neurons at different depths of the visual cortex. Dendrites were sorted into three groups based on the distance of the parent somata from the dura: 200 – 400 microns = superficial, 400 – 600 microns = middle and 650-850 microns = deep. Sorting the dendritic spine density data by lamina revealed a parallel loss of spine density in all groups following chronic monocular deprivation. Dark exposure alone did not regulate the density of dendritic spines at any cortical depth. Remarkably, however, dark exposure followed by reverse deprivation significantly increased the dendritic spine density at all cortical depths, including the middle zone which is enriched for thalamocortical synapses (normalized spine density average ± SEM: superficial control=1.00±0.06, chronic MD=0.55±0.11, cMD DE=0.56±0.11, cMD DE-RD=0.81±0.19, one-way ANOVA, F(3,26)=3.7793, p=0.0243, *p<0.05 versus control, Tukey-Kramer HSD post-hoc; mid control=1.00±0.08, chronic MD=0.59±0.10, cMD DE=0.57±0.07, cMD DE-RD=0.97±0.10, one-way ANOVA, F(3,22)=6.7756, p=0.0027, *p<0.05 versus control, Tukey-Kramer HSD post-hoc; deep control=1.00±0.06, chronic MD=0.58±0.11, cMD DE=0.45±0.14, cMD DE-RD=0.83±0.11, one-way ANOVA, F(3,28)=4.5740, p=0.0110, *p<0.05 versus control, Tukey-Kramer HSD post-hoc, Figure 3). This suggests that the recovery from chronic monocular deprivation is accompanied by significant spinogenesis throughout the visual cortex, including the thalamo-recipient zone.
Figure 3.
Parallel regulation of dendritic spine density in all laminae of the binocular region of the deprived visual cortex. A reduction in spine density (normalized to within in lamina average control) is observed in all cortical laminae following chronic monocular deprivation (cMD; superficial, mid and deep determined by distance between soma and dura: 200-400 μm, 400-600 μm and 650-850 μm respectively). Dendritic spine density is unchanged in all cortical laminae following dark exposure (DE), but is increased following dark exposure plus reverse deprivation (DE-RD; average ± SEM; one-way ANOVA p<0.01 for each segment, *p<0.05 versus within lamina control, Tukey-Kramer HSD post-hoc; n=neurons, subjects).
Dark exposure reactivates experience-driven thalamocortical plasticity in adulthood
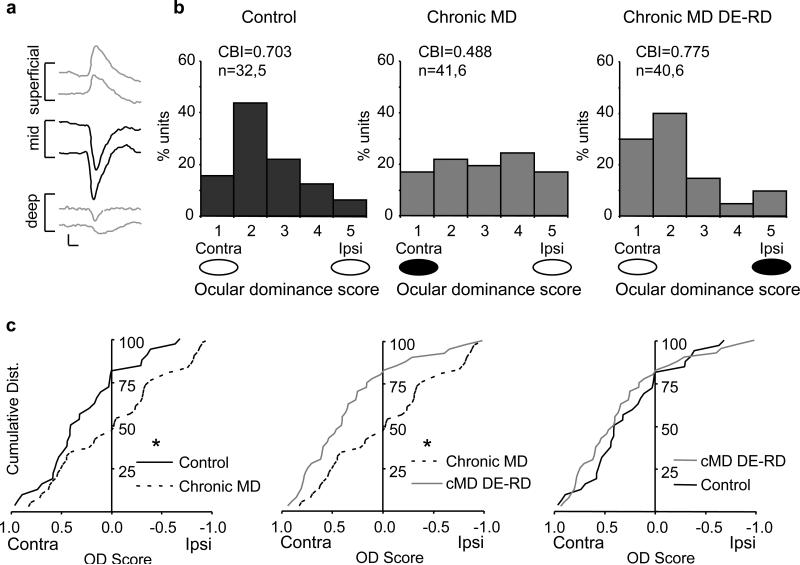
The parallel spinogenesis at all cortical depths following dark exposure and reverse deprivation in adulthood is surprising given the evidence that plasticity at thalamocortical synapses in brain slices is constrained early in the postnatal period 6, 38-40, 56-58, although direct electrical stimulation of the thalamus continues to drive plasticity at adult thalamocortical synapses42. Therefore, we used two complementary strategies to further examine the contribution of plasticity at thalamocortical synapses to the recovery from chronic monocular deprivation in adulthood. First we examined the regulation of ocular dominance of single units isolated from layer IV of the primary visual cortex by chronic monocular deprivation and reversal following dark exposure and reverse deprivation. We used the shape of the VEP waveform to guide electrode placement in the thalamo-recipient zone and simultaneously recorded VEPs (50 Hz low pass filter) and neuronal spiking (300 Hz high pass and a 3 kHz low pass filters41-42, Figure 4A). We observed the expected contralateral bias in the ocular dominance of single units acquired from the thalamo-recipient zone of control subjects7, 43 reflecting the complement of thalamocortical projections44. Chronic monocular deprivation significantly shifted the ocular dominance of single units away from the deprived (contralateral) eye, which was reversed by dark exposure and reverse deprivation in adulthood (Figure 4B). The bidirectional changes in the response properties of single units were reflected in the changes in the contralateral bias index ([(N1-N5)+(N2-N4)+(Ntot/2Ntot)] where N(tot) is the total number of cells, and N1-N5 represent the number of neurons in classes 1-5 using a modified version of the scale introduced by Hubel and Wiesel (see methods). The cumulative distribution of ocular dominance scores (OD scores=spike rate in response to stimulation of contra – ipsi eye/contra +ipsi eye) revealed a significant shift in ocular dominance away from the contralateral eye following chronic monocular deprivation (Figure 4C, left, KS test, *p=0.018). Surprisingly, despite chronic monocular deprivation, visual stimulation of the chronically-deprived eye continued to evoke single unit activity (~34% of neurons retained ocular dominance scores > 0.20 following chronic monocular deprivation). Dark exposure and reverse deprivation significantly shifted the distribution of single unit ocular dominance to the previously deprived, contralateral eye (Figure 4C, middle; *p=0.007, KS test), which was indistinguishable from non-deprived controls (Figure 4C, right).
Figure 4.
Regulation of ocular dominance in the thalamo-recipient zone by chronic monocular deprivation. A. Low pass filter (50 Hz) allows acquisition of a VEP. The shape of the VEP waveform changes as the recording electrode moves vertically through the cortex. A VEP with a large negative component confirms placement of the recording electrode in the thalamo-recipient zone (deep layer III and layer IV; black waveforms; average of 100 presentations of 0.02 cycles/degree, 96% contrast gratings reversing at 1 Hz, scale bars = 50 ms, 100 μvolts). B. A band pass filter (300 Hz high pass and a 3 kHz low pass) allows acquisition of unit activity. Chronic monocular deprivation induces a shift in the responses of single units recorded from the thalamo-recipient zone away from the deprived eye (black oval) and towards the non-deprived eye (white oval). Dark exposure and reverse deprivation restore the normal contralateral bias. The increase in the contralateral bias index (CBI) also reflects the recovery of the normal distribution of single units following dark exposure and reverse deprivation. C. Cumulative distribution of the OD scores of single units obtained from thalamo-recipient zone reveals a significant shift away from contralateral eye preference following chronic monocular deprivation. Ocular dominance scores shift back to control levels after chronic monocular deprivation is reversed by dark exposure and reverse deprivation, *p<0.020, KS test.
The recovery of normal ocular dominance of single units from the thalamo-recipient zone suggests regulation of thalamocortical synapses may contribute to the recovery from chronic monocular deprivation in adulthood. However, changes in the spiking output of thalamo-recipient neurons could also reflect changes in the strength of cortico-cortical synaptic connections. To distinguish between these possibilities, we used a strategy to pharmacologically isolate activity at thalamocortical synapses. A combination of the GABAA receptor agonist muscimol (4mM, to silence cortical spiking) and the GABAB receptor antagonist SCH50911 (6mM, to block activation of presynaptic GABAB receptors by muscimol) delivered intracranially has recently been used to isolate the thalamocortical component of the auditory and visually evoked potential45, 4. Silencing cortico-cortical spiking with musc+SCH (500 nL delivered intracranially) inhibited the cortico-cortical contribution to the VEP. Cortical spiking and the cortico-cortical contribution of the VEP recovered following washout of the drug (Figure 5A). Interestingly, visual stimulation of the chronically-deprived eye continues to evoke a residual thalamocortical VEP (chronically deprived eye VEP amplitude μvolts; average ± SEM, pre drug application: 129.8±3.6, chronically deprived eye post drug application: 39.8±11.8, n=4 Figure 5B, paired t-test, *p=0.004). The thalamocortical contribution of the VEP was also be isolated in subjects that received dark exposure and reverse deprivation to reverse the effects of chronic monocular deprivation (pre drug application 323.3±48.5, post drug application: 172.5±22.4, n=4, Figure 5C, paired t-test, *p=0.005). Comparison of the normalized thalamocortical VEPs reveals a significant increase in the amplitude following dark exposure and reverse deprivation (average ± SEM cMD: 0.31±0.10, n=4; cMD DE-RD: 0.54±0.01, n=4; Figure 5D, unpaired t-test, *p=0.027). No non-specific regulation in VEP amplitude or contralateral bias was observed following intracranial delivery of vehicle alone (VEP amplitude normalized to pre-vehicle amplitude average ± SEM pre: 1.0±0.0; post veh: 0.93±0.05, n=2, paired t-test, p=0.13, data not shown). Importantly, silencing cortical spiking did not affect the VEP contralateral bias (C/I average ± SEM pre drug application cMD: 0.38±0.070; post drug application cMD: 0.38±0.12, n=4; pre drug application cMD DE-RD: 1.97±0.05; post drug application cMD DE-RD: 2.07±0.12, n=4, Figure 5E).
Figure 5.
Enhancement of the thalamocortical VEP in the deprived visual cortex during the recovery from chronic monocular deprivation. A. Top: Post stimulus time histograms plot action potentials (in 50 ms bins) versus time using a visual stimulus reversing every 500 ms (arrows). Intracortical infusion of muscimol+SCH50911 induces a reversible silencing of cortical spiking and reduction in VEP amplitude. Cortical spiking and VEP amplitude recover following washout (~ 10 hours), scale bars = 50 ms, 50 μvolts. B and C. Pharmacological isolation of the thalamocortical VEP in subjects that received chronic monocular deprivation and chronic monocular deprivation followed by dark exposure and reverse deprivation (*p<0.005 paired t-test). D. Normalization of the thalamocortical VEP to the total cortical VEP reveals the recovery from chronic monocular deprivation is accompanied by an enhancement of thalamocortical VEP amplitude (*p=0.027 unpaired t-test). E. Inhibition of cortical spiking does not modify the VEP contralateral bias in subjects that received chronic monocular deprivation, or after chronic monocular deprivation is reversed by dark exposure and reverse deprivation.
Discussion
Chronic MD induces a significant decrease in dendritic spine density
Chronic monocular deprivation induces severe amblyopia that is resistant to spontaneous reversal46. The obstacles to recovery include the developmental reduction in physiological and anatomical plasticity in the visual cortex, and the considerable degradation of feedforward excitation predicted to result from chronic monocular deprivation. Indeed, we show that chronic monocular deprivation induced a significant reduction in the density of basolateral dendritic spines of principle neurons throughout the deprived visual cortex. However, the reversal of chronic monocular deprivation by dark exposure and reverse deprivation in adulthood recovered dendritic spine density recovered throughout the depth of the visual cortex. Importantly, increases in the amplitude of the total and thalamo-cortical VEP were observed during the recovery from chronic monocular deprivation following dark exposure and reverse deprivation, indicating a strengthening of thalamocortical synapses. Thus dark exposure in adulthood promotes widespread synaptic plasticity throughout the visual cortex, including thalamocortical synapses, which precedes the recovery from chronic monocular deprivation.
Interestingly, the anatomical response to recovery from chronic monocular deprivation differed along the length of the basolateral dendrite. The recovery of spine density following dark exposure and reverse deprivation was limited to the proximal s (0-75 μm from the soma) segment of the basolateral dendrite. This suggests that synapses in the proximal portion of the dendrite may have a lower threshold for experience-induced spinogenesis following dark exposure and reverse deprivation. Indeed, a higher concentration of voltage gated sodium channels was recently described on proximal dendritic segments47, which may serve to stabilize synaptic structures by enhancing the correlations between pre- and postsynaptic activity. Proximal synapses are also more likely to potentiate than distal synapses in response to the same stimulus parameters48.
Anatomical plasticity is robust in juveniles, evident by the rapid decrease in the density of spines on the apical dendrites of layer II/III pyramidal neurons following brief monocular deprivation. However, extending the duration of the monocular deprivation in juveniles reveals that the decrease in dendritic spine density is transient, likely a reflection of the generation of new excitatory synapses during the strengthening of non-deprived eye responses19-20. Brief monocular deprivation in juveniles also induces a decrease in the length of thalamocortical afferents serving the deprived eye31, which are supplemented by afferents serving the non-deprived eye49. In contrast, we show that prolonged monocular deprivation resulted in a net reduction in dendritic spine density, suggesting the deprived eye depression dominates the response to chronic monocular deprivation initiated at a very young age. Alternatively, the first 100 microns of basolateral dendrites may be dominated by inputs from the contralateral eye.
Nonetheless, the reduction in the dendritic spine density following chronic monocular deprivation was observed throughout the depth of the visual cortex. The parallel anatomical response to monocular deprivation across laminae is consistent with a final common pathway for deprived eye depression, despite laminar differences in the mechanism for induction of long-term synaptic depression and AMPAR endocytosis3,43,50. However, it is estimated that < 10 % of excitatory input onto principle neurons in thalamo-recipient zone originate from the thalamus in the cat51, and a similarly small complement of excitatory inputs onto thalamo-recipient neurons is predicted to be mediated by thalamic afferents in the rodent. Therefore, the ~40% decrease in dendritic spine density we observed in response to chronic monocular deprivation suggests that long-term depression and subsequent elimination at cortico-cortical synapses also contributes to the deprived eye depression.
Dark exposure in adulthood does not regulate dendritic spine density
Surprisingly, we did not observe an increase in dendritic spine density in the adult visual cortex following dark exposure. There were also no compensatory changes in spine head diameter or neck length of residual spines following chronic monocular deprivation or dark exposure. Compensatory scaling of spine numbers or morphologies requires a significant reduction in neuronal activity levels52. The persistence of spontaneous feedforward excitation, as is seen following lid suture in juvenile mice5, may suppress homeostatic scaling of residual synapses in adults. Alternatively, dark exposure in adulthood may reactivate synaptic plasticity by enhancing the motility of residual spines. Indeed dark-rearing mice until the peak of the critical period induces a persistent enhancement of the dynamics of dendritic spines on layer V pyramidal neurons53.
Reactivation of juvenile-like anatomical plasticity by dark exposure in adulthood
In the absence of dark exposure, reverse deprivation does not promote the recovery of dendritic spine density or spatial acuity24, 32 consistent with the absence of rapid ocular dominance plasticity and the stability of dendritic spines in the adult visual cortex54. Nonetheless, we see a significant increase in dendritic spine density during the recovery from chronic monocular deprivation by dark exposure and reverse deprivation in adulthood. The new spines that emerge following reverse deprivation in dark-exposed adults are likely to form functional synapses and contribute to the increase in the amplitude of the deprived eye VEP observed during the recovery from chronic monocular deprivation. The observation that reverse deprivation significantly increases spine density suggests that spinogenesis may be triggered specifically by competitive processes, as observed in multi-whisker septal neurons in the barrel cortex10 and binocular neurons in the visual cortex20, 21, 23, 55.
Previous examinations of dendritic spine density by manipulation of visual experience have been limited to tracking anatomical changes in single cortical lamina9, 20, 24 or dendritic compartments that receive substantial cortico-cortical excitatory input21. By tracking dendritic spines on pyramidal neurons throughout the depth of the visual cortex, we demonstrated a significant loss and recovery of dendritic spine density in all cortical laminae, including thalamo-recipient zone. Surprisingly, visual stimuli presented to the chronically-deprived eye continues to evoke residual single unit activity in the thalamo-recipient zone and a residual thalamocortical VEP, demonstrating the persistence of thalamocortical synaptic transmission following chronic monocular deprivation. The residual feedforward excitation likely provides the anatomical scaffold on which anatomical plasticity, once reactivated in the adult visual cortex by dark exposure, can promote the recovery from chronic monocular deprivation.
Materials and Methods
Subjects
Long Evans rats received chronic monocular deprivation via lid suture, from eye opening (~ postnatal day 14), into adulthood (average ± SEM age at experiment postnatal day 148±11.65). In some cases chronic monocular deprivation was followed by 10 days of dark exposure +/− 7 days of reverse deprivation (opening chronically-deprived eye and suturing non-deprived eye). Controls subjects were raised in a normal visual environment (12 hours light:12 hours dark per day), and all subjects had food and water available ad libitum. All procedures conformed to the guidelines of the U.S. Department of Health and Human Services and the University of Maryland Institutional Animal Care and Use Committee.
Anatomy
A modification of the diolistic staining method of Gan et al., 200036, was used. Briefly, transcardial perfusion (100 mls, 2% paraformaldehyde in 0.1M phosphate buffer, pH 7.3) was followed by post fixation of the brain (1 hr in 2% paraformaldehyde at RT). Coronal sections of visual cortex (200 μm thickness) were prepared in ice-cold phosphate buffered saline (PBS, pH 7.3) on a vibratome. The lipophilic dye, DiI (1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate), was coated onto tungsten particles (1.3 μm) and delivered into slices with a Helios gene gun (Bio-Rad; 60-80 psi helium pressure). Cortical slices were incubated in PBS (2.5 hrs at RT) to allow the dye to diffuse followed by a second post-fix (4% paraformaldehyde for 30 min at RT). Slices were mounted in ProLong gold antifade reagent (Invitrogen). Neuronal selection within the binocular region of the visual cortex was based on characteristic pyramidal soma, distinct basolateral and apical dendrites at low magnification (10×), since dendritic spines are not detectable at this magnification. Neurons were sorted into categories (superficial, mid and deep) based on depth of the parent cell body from the dura and other identifying landmarks. Confocal images were acquired using a Zeiss 710 confocal microscope. A typical z-stack consisted of ~30 optical image planes each of 436×436 pixels. We used a 63×/1.4 Oil DIC Plan Apo objective with 3× zoom with 0.42 μm z-steps. Maximum intensity projections were exported and photomontages were constructed in Photoshop (Adobe). Dendrite segments, spine morphology (head diameter and neck length) and spine density were manually marked and quantified in an image processing toolkit (Reindeer Graphics).
Physiology
Visually evoked potentials (VEPs) were recorded from the thalamo-recipient laminae of the binocular visual cortex (7 mm posterior of bregma, 4 mm lateral of midline) with tungsten microelectrodes (1 MΩ) following urethane anesthesia (1.4 mg/kg, i.p.). Visual stimuli were 1 Hz full screen square wave gratings with a spatial frequency of 0.02-0.04 cycles per degree, 96.28% maximal contrast and 25 cd/m2 luminance. The amplitude of the primary negative (thalamo-recipient) component of the evoked potential was quantified to assess the cortical response to visual stimulation. VEP waveforms were averaged from 100 visual stimuli presentations. Subdural electrode placement allowed for the simultaneous collection of thalamo-recipient VEPs (50 Hz low pass filter) and neuronal spiking (300 Hz high pass and a 3 kHz low pass) in response to visual stimulation. Multi-unit activity was sorted into single-units based on waveform shape and principal component analysis using OpenEx software (TDT). Ocular dominance scores were calculated based on individual eye peak activity responses in PSTHs (contra eye - ipsi eye)/(contra eye + ipsi eye) and were binned in a modified version of the classic scale introduced by Hubel and Wiesel: 1= 1.00 – 0.60, 2= 0.59 – 0.20, 3= 0.19 – −0.19, 4= −0.20 – −0.59, 5= −0.60 – −1.00; where 1 represents units primarily driven by the contralateral eye and 5 represents units primarily driven by the ipsilateral eye. Contralateral bias index was calculated by: [(N1-N5)+(N2-N4)+(Ntot/2Ntot)] where N(tot) is the total number of cells and N1 – 5 is the number of neurons in bins 1 – 5 respectively. The thalamocortical location of the electrode was confirmed anatomically by a post hoc reconstruction of the electrode track. Prior to transcardial perfusion (4% paraformaldehyde) the electrode was cemented to the skull with dental acrylic to maintain electrode placement during fixation. Following overnight post-fix (4% paraformaldehyde), coronal sections (50 microns) were collected on a vibratome in PBS. A cresyl violet nissl stain was used to reveal the laminar organization of the visual cortex, and the placement of the recording electrode in layer IV. Brightfield images (Figure 1B) were collected on a Zeiss 710 confocal microscope in transmitted light mode using a 10× air objective. A photomontage was constructed in Photoshop to reveal the laminar location of the electrode track. Only contrast or brightness was adjusted as needed for the photomontage.
Pharmacology
The thalamocortical component of the VEP was isolated using a combination of the GABAA receptor agonist muscimol (4mM, to silence cortical spiking) and the GABAB receptor antagonist SCH50911 (6mM to prevent activation of presynaptic GABAB receptors) as described4,45. A small craniotomy was made ~500 microns posterior to the recording craniotomy (7 mm posterior of bregma, 4 mm lateral of midline). The drug or vehicle (ACSF) was delivered intracranially (~500nL) via a guide cannula (26 gauge, Plastics One; attached to surrounding skull with dental acrylic) allowed to infuse for ~60 minutes.
Statistics
Distributions of two independent experimental groups were assessed with the Kolmogorov-Smirnov Test to determine statistical significance (p<0.05). When appropriate, an unpaired, one-tail t-test was used to determine statistical significance for two independent experimental groups or a paired, one-tail t-test was used to determine statistical significance for two dependent experimental groups (p<0.05; Excel). One-way ANOVAs were used to determine statistical significance between three or more independent experimental groups (p<0.05). A subsequent Tukey-Kramer HSD post-hoc analysis was used to make pair-wise comparisons (JMP).
Supplementary Material
References
- 1.Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat. Neurosci. 2003;6:854–62. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- 2.Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces long-term depression in visual cortex. Nature. 1999;397:347–50. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- 3.Yoon BJ, Smith GB, Heynen AJ, Neve RL, Bear MF. Essential role for a long-term depression mechanism in ocular dominance plasticity. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9860–5. doi: 10.1073/pnas.0901305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khibnik LA, Cho KK, Bear MF. Relative contribution of feedforward excitatory connections to expression of ocular dominance plasticity in layer 4 of visual cortex. Neuron. 2010;66:493–500. doi: 10.1016/j.neuron.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linden ML, Heynen AJ, Haslinger RH, Bear MF. Thalamic activity that drives cortical plasticity. Nat. Neurosci. 2009;12:390–2. doi: 10.1038/nn.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang B, Treviño M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J. Neurosci. 2007;27:9648–52. doi: 10.1523/JNEUROSCI.2655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci. 1996;16:3274–86. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007;1:131–43. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace W, Bear MF. A morphological correlate of synaptic scaling in visual cortex. J. Neurosci. 2004;24:6928–38. doi: 10.1523/JNEUROSCI.1110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilbrecht L, Holtmaat A, Wright N, Fox K, Svoboda K. Structural plasticity underlies experience-dependent functional plasticity of cortical circuits. J. Neurosci. 2010;30:4927–32. doi: 10.1523/JNEUROSCI.6403-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popov VI, Medvedev NI, Patrushev IV, Ignat'ev DA, Morenkov ED, Stewart MG. Reversible reduction in dendritic spines of CA1 of rat and ground squirrel subjected to hypothermia-normothermia in vivo: A three-dimensional electron microscope study. Neuroscience. 2007;149:549–60. doi: 10.1016/j.neuroscience.2007.07.059. [DOI] [PubMed] [Google Scholar]
- 12.Medvedev NI, et al. The N-methyl-D-aspartate receptor antagonist CPP alters synapse and spine structure and impairs long-term potentiation and long-term depression induced morphological plasticity in dentate gyrus of the awake rat. Neuroscience. 2010;165:1170–81. doi: 10.1016/j.neuroscience.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–57. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat. Neurosci. 2004;7:1104–12. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 15.Nägerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–67. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3123–7. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nägerl UV. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron. 2008;60:590–97. doi: 10.1016/j.neuron.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Sawtell, et al. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 19.Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–23. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–41. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron. 2004;44:1021–30. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Majewska A, Sur M. Motility of dendritic spines in the visual cortex in vivo: changes during the critical period and effects of visual deprivation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:16024–9. doi: 10.1073/pnas.2636949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–7. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8517–22. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giffin F, Mitchell DE. The rate of recovery of vision after early monocular deprivation in kittens. J. Physiol. 1978;274:511–37. doi: 10.1113/jphysiol.1978.sp012164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell DE, Gringas G. Visual recovery after monocular deprivation is driven by absolute, rather than relative, visually evoked activity levels. Curr. Biol. 1998;21:1179–1182. doi: 10.1016/s0960-9822(07)00489-7. [DOI] [PubMed] [Google Scholar]
- 27.Liao DS, Mower AF, Neve RL, Sato-Bigbee C, Ramoa AS. Different mechanisms for loss and recovery of binocularity in the visual cortex. J. Neurosci. 2002;22:9015–9023. doi: 10.1523/JNEUROSCI.22-20-09015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J. Comp. Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 29.Tieman SM. Effects of monocular deprivation on geniculocortical synapses in the cat. J. Comp. Neurol. 1984;222:166–76. doi: 10.1002/cne.902220203. [DOI] [PubMed] [Google Scholar]
- 30.Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J. Physiol. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–21. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- 32.He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat. Neurosci. 2007;10:1134–6. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan HM, Eaton NC, Quinlan EM. Dark exposure promotes visual perceptual learning and the recovery from chronic monocular deprivation. J. Neurosci. 2011 Submitted. [Google Scholar]
- 34.Sale A, et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat. Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- 35.Maya Vetencourt JF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 36.Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–25. doi: 10.1016/s0896-6273(00)00031-3. [DOI] [PubMed] [Google Scholar]
- 37.Cajal SRY. In: Histology of the Nervous System. Neely, Swanson Larry., editors. I. Oxford University Press, Oxford; New York, NY: 1995. [Google Scholar]
- 38.Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–8. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 39.Dudek SM, Friedlander MJ. Developmental down-regulation of LTD in cortical layer IV and its independence of modulation by inhibition. Neuron. 1996;16:1097–106. doi: 10.1016/s0896-6273(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 40.Wang XF, Daw NW. Long term potentiation varies with layer in rat visual cortex. Brain Res. 2003;989:26–34. doi: 10.1016/s0006-8993(03)03321-3. [DOI] [PubMed] [Google Scholar]
- 41.Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- 42.Heynen AJ, Bear MF. Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. J. Neurosci. 2001;21:9801–13. doi: 10.1523/JNEUROSCI.21-24-09801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CH, Heynen AJ, Shuler MG, Bear MF. Cannabinoid receptor blockade reveals parallel plasticity mechanisms in different layers of mouse visual cortex. Neuron. 2008;58:340–5. doi: 10.1016/j.neuron.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Coleman JE, Law K, Bear MF. Anatomical origins of ocular dominance in mouse primary visual cortex. Neuroscience. 2009;161:561–71. doi: 10.1016/j.neuroscience.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat. Neurosci. 2007;10:1594–600. doi: 10.1038/nn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell DE, MacKinnon S. The present and potential impact of research on animal models for clinical treatment of stimulus deprivation amblyopia. Clin. Exp. Optom. 2002;85:5–18. doi: 10.1111/j.1444-0938.2002.tb03067.x. [DOI] [PubMed] [Google Scholar]
- 47.Lorincz A, Nusser Z. Molecular identity of dendritic voltage-gates sodium channels. Science. 2010;328:906–9. doi: 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon U, Polsky A, Schiller J. Plasticity compartments in basal dendrites of neocortical pyramidal neurons. J. Neurosci. 2006;26:12717–12726. doi: 10.1523/JNEUROSCI.3502-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman JE, Nahmani M, Gavornik JP, Haslinger R, Heynen AJ, Erisir A, Bear MF. Rapid structural remodeling of thalamocortical synapses parallels experience-dependent functional plasticity in mouse primary visual cortex. J. Neurosci. 2010;30:9670–9682. doi: 10.1523/JNEUROSCI.1248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1383–8. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Binzegger T, Douglas RJ, Martin K. A quantitative map of the circuit of cat primary visual cortex. J. Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrak LJ, Harris KM, Kirov SA. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J. Comp. Neurol. 2005;484:183–90. doi: 10.1002/cne.20468. [DOI] [PubMed] [Google Scholar]
- 53.Tropea D, Majewska AK, Garcia R, Sur M. Structural dynamics of synapses in vivo correlate with functional changes during experience-dependent plasticity in visual cortex. J. Neurosci. 2010;30:11086–11095. doi: 10.1523/JNEUROSCI.1661-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific growth in the neocortex. Nature. 2006;441:979–83. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 55.Keck T, Mrsic-Flogel TD, Vaz Afonso M, Eysel UT, Bonhoeffer T, Hübener M. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat. Neurosci. 2008;11:1162–7. doi: 10.1038/nn.2181. [DOI] [PubMed] [Google Scholar]
- 56.Beaver CJ, Ji Q, Daw NW. Layer differences in the effect of monocular vision in light- and dark-reared kittens. Vis. Neurosci. 2001;18:811–20. doi: 10.1017/s0952523801185147. [DOI] [PubMed] [Google Scholar]
- 57.Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J. Neurophysiol. 1992;67:197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- 58.Glazewski S, Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J. Neurophysiol. 1996;75:1714–29. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.