Abstract
Background and aim.
Patients with disabilities have a higher prevalence of caries and dental erosions than general population. This particularity may be assessed by the study of microcrystallization of saliva. We investigated the oral liquid microcrystallization in children with gastroesophageal reflux disease (GERD), a condition associated with dental erosions.
Material and methods.
54 children have been clinically examined: 27 children suffering from GERD with ages between 13 and 15, were included in the study group, and 27 healthy children - the control group. The study of crystallographic changes of the oral liquid was performed using the method developed by Shatohina, Razumov SN, Shabalin VN (2006) with the scanning electron microscope VEGA TESCAN TS 5130 MM.
Results
The degree of microcrystalization of the oral liquid in children with GERD was considerably reduced, (1.73±0.11 points) and was lower than in children in the control group (3.22±0.16 points) (p<0.01, RR=2). The degree of microcrystallization of oral liquid in children with GERD was 1.86 times lower than in healthy children. This was correlated with the duration of gastroesophageal reflux.
Conclusion
The study of structural particularities of dehydrated droplet of oral liquid in children with GERD has elucidated a number of markers of the changes produced in the oral cavity. These can be used in the screening research in prevention of caries and dental erosions.
Keywords: oral fluid, microcrystallization, GERD
Introduction
Body fluids are widely used in laboratory diagnosis. However, the process of obtaining them for research is accompanied by certain difficulties (the introduction in the internal environment of the organism, tissue damage, compliance with special temporary algorithms which are not always physiological) [1,2]. In recent years, the first steps were taken in the study of manifestations of various diseases on the structural organization of biological fluids [3–7]. The structures of the investigated fluids are obtained via the phasic transfer from liquid to solid state through dehydration. The results of experimental studies demonstrated that the information contained in the liquid phase at the molecular level in the dehydrating process is transferred at macroscopic level having different structures that become visible to the researcher [8].
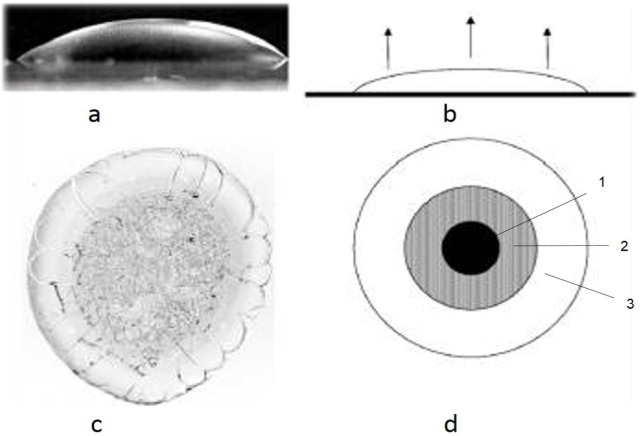
The research of the self-organization processes of various physiological and pathological liquid media elucidated the primary importance of the organic components present in biological fluids even in a small amount (from 0.01 microns to 100 g/l and above) in the structures formation. The structures separation into organic and inorganic compounds is carried out by the dehydration method, by applying the biological liquid onto a transparent surface in the form of drops, and then dehydrated under specific conditions. The volume of the droplet is determined by the ratio of the specific gravity of the liquid and its surface tension forces. According to theoretical concepts in biological fluid dehydration, specific mechanisms interact, that ensure the formation of structures of solid-phase systems and subsystems. The term “facies” refers to what is left from a drop of saliva, after drying out. A graphical representation of the action of these mechanisms provides the scheme of the biological liquid droplet (located on a horizontal plane) in sagittal section, developed by Tarasevici and Aiupova (2003) [9] (Fig. 1).
Figure 1.
- hemisphere shaped body liquid droplet;
- the beginning of the dehydration process;
- biological liquid microcrystalogram;
- schematic presentation of the dehydrated droplet of biological liquid with formed crystallization areas: 1 - the central area; 2 -the transient area and 3 - the peripheral area.
The authors noted that the evaporation of the liquid does not occur uniformly over the entire open area of the droplet. Due to the fact that the hemisphere has a different thickness in the central area as opposed to the peripheral area, the evaporation of the analyzed water droplet, the concentration of the dissolved substance changes non-uniformly, thus particularly in the peripheral area (which is of reduced thickness) is increased more rapidly than in the central zone (maximum thickness) of the droplet. In such processes osmotic and oncotic forces interact. Because the power of osmotic forces considerably exceeds the oncotic ones, the salts migrate to the centre of the droplet, to the low concentration of dissolved substances. In the central area, the proteins and other dissolved substances with high molecular weight yield water and are shifted to the periphery of the droplet. As a result, the marginal amorphous zone of the dehydrated droplet, is presented by structures of organic origin, while the central crystallized one – by salts. Thus, a shift of unstable structures is produced, rather dynamically at the molecular level in stable macrostructures of solid phase. According to the research of Annarelli et al. (2001), the central zone is called the crystallized structures zone, and the peripheral one-amorphous area [10]. The transitional zone can be seen only in the blood serum studies. Peripheral width area ratio of the diameter of the droplet is used for the determination of protein contents in biological fluids [11].
Therefore, taking into account the concept of classical crystallography, the early diagnosis of dental and systemic diseases, the method of crystallographic research of one of the most accessible biological liquid, i.e. oral liquid, was proposed [12]. Oral liquid is a very well organized specific biological environment, with unique, universal properties is a dynamic environment, reflecting any changes in the body, including pathological processes. Over the past decades, the first steps in studying the manifestations of various diseases in the structural organization of the oral liquid have been made [13–16].
The first investigations of crystalograms have revealed that after oral liquid droplets dehydration on the glass slide a sediment remains, its microcrystalline structure is dependent on the state of health of the oral cavity and the entire body. The results of the study of outlined microcrystalization types characterize the ability of the saliva remineralization. The optical properties of the crystals vary significantly depending on exo- and endogenous factors, this phenomenon being applied in the research, for diagnostic purposes [13]. The morphological image blurring or changing oral liquid is an indicator of qualitative and quantitative disorders of salivary proteins, mucins, being one of the earliest markers of pathological processes that occur in the body and can be used to detect diseases at the premorbid stage and to apply efficiently the preventive measures and minimally invasive treatment. The structural features of microcrystalograms (MCG) are determined by the balance of organic mineral components and physicochemical properties of oral liquid [6].
The use of oral liquid in the health study can be justified by many arguments:
Physicochemical properties of the oral liquid can be used as a marker for pathological changes not only in the salivary glands and oral cavity, but also in other organs and systems of human body [ 4,5,8,11,17–21]. Oral liquid composition reflects the psycho-emotional state [19], the intensity of metabolism [13], severity of inflammatory processes, structural and functional properties of enamel and its resistance to caries attack [21]. A disorder in the contents of saliva and salivary secretion rate was noted in case of cardiovascular diseases. Changing the shape of the crystals is a consequence of the modification of the physicochemical properties of saliva. Changes in the composition of the saliva can be related not only to the presence of inflammatory processes and diseases (acute and chronic), but also to the administration of drugs [22].
Structural peculiarities studies of oral liquid in the health study is promising due to the invasive method for obtaining biological material and simple application.
The research method of oral fluid crytalogenesis by dehydration obtaining microcrystalograms (MCG) does not disturb the contents of the test sample and provides an overview of the state of the whole body.
Thus, the main advantages of oral fluid microcrystalograms oral liquid MCG are non-invasiveness, simplicity of implementation and availability of substrate sampling study providing new insights into clinical trials [5]. Therefore, the study of crytalogenesis oral fluid dynamics is up to date to produce precise quantitative assessment parameters of fractal structures and further standardization of analysis process of oral liquid MCG.
The purpose of the paper consisted in the study of the oral liquid microcrystalization peculiarities in children with GERD.
Material and methods
To achieve the objective 54 children were clinically examined: 27 (50%) children with GERD were the research group and 27 (50%) healthy children – control group. Selecting children’s groups was randomly, the groups had the same age and sex. The two groups were matched regarding gender, age, residence environment and social-economic conditions.
We performed the clinical examination of the patients, including the assessment of the dental caries prevalence by estimating the DMFT index. The assessment of the erosive wear was performed on the palatine/lingual sides, on the vestibular and incisal/occlusal sides, noting down a single value for each sextant, corresponding to the dental side having the greatest erosion degree. The final score of the BEWE index was obtained by adding up the values of all sextants based on which the erosive wear was calculated. In addition, we assessed the enamel resistance to acids, using the enamel resistance test following the method proposed by Okushko, 2011, the pH of saliva, salivary flow rate, the buffering capacity of saliva and the remineralization potential of saliva, assessed by estimating the remineralization speed of saliva using the method elaborated by Leontiev et al. 2007 [23,24].
In this study, we performed a comparative analysis of the crystallographic modifications of the OF in children with and without decay according to the method developed by Shatohina et al, 2006 [25].
Oral liquid was collected with a sterile pipette in the amount of 0.2 to 0.3 ml of the oral cavity floor. Then, on a glass slide, pre-treated with alcohol and ether, three drops of oral liquid were applied. The dehydration of the OF product drops was produced into the unit at t=37°C, protected from dust. Micropreparations were examined under scanning electron microscope VEGA TS 5130 MM TESCAN. Using MCG and oral liquid research methods the central and peripheral areas of oral liquid facies were investigated. Fractal structures, separate crystals and amorphous substance were analyzed. Interpretation of crystaloscopic components was achieved by applying special tables with notification of characteristics of the studied structures [5].
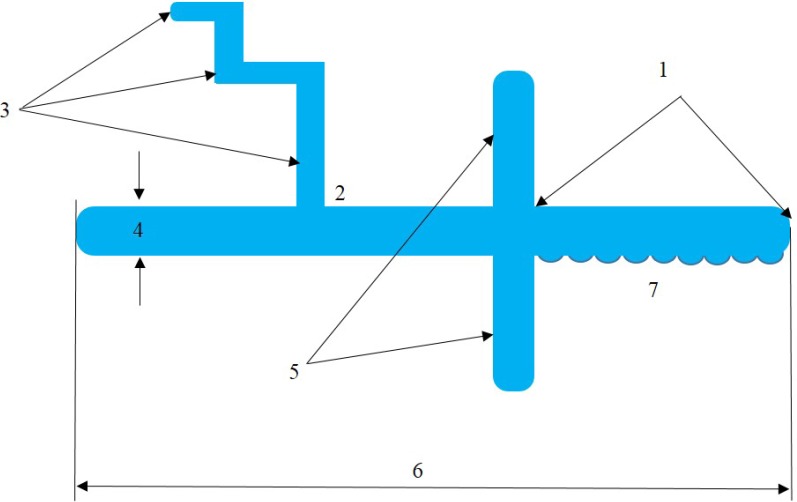
To quantify microcrystals, an algorithm was developed for the analysis of GCM, according to which the micropreparate is examined to highlight the facies layers and determine the base. For a description of the crystal the following parameters are estimated: length, width and degree of curvature of the main branch, main branch ratio of the width at the base and tip microcrystal report microcrystal surface perimeter and the frequency and angle of deviation degree dendrites, symmetrical ramifications of the dendrites degree from the mainline (Fig. 2).
Figure 2.
- the length of the crystal to the branch;
- angle of deviation of dendrites of the first degree;
- degree of dendrites (I, II, III, etc.);
- width;
- the symmetrical ramifications character of the first degree dendrites from the main line;
- crystal’s length;
- the number of microdendrites along the crystal.
Further on, the integrity mainline trunk and connection area with the first degree dendrites, dendritic tip shape (conical, oval or divided) was investigated. The organic inclusions were also assessed: the proportion of surface organic inclusions and field of view; the organic inclusions location (on the periphery in the central area or the entire area of the field of view); crystal report (membership or isolation).
The evaluation of MCG was performed after dried droplets examination by oral liquid, the results being expressed as the mean points depending on the types identified by the formation of crystals [4]: 0.0–1.0 - at a very low extent, 1.1–2.0 - low, from 2.1 to 3.0 - satisfactory 3.1–4.0 - high, 4.1 to 5.0 - very high degree of microcrystallization. The risk of deviations of microcrystallization (RDM) was calculated according to the formula RDM = (RR−1)/RR × 100, RR − relative risk.
Ethical issues
The study was approved by the local Ethics Committee and was conducted in accordance with ethical requirements, and with the written consent of the children’s parents or their legal representatives.
Statisitics
Data analysis was performed using Microsoft® Excel® 2013 şi IBM® SPSS Statistics 22.0 software using the functions and modules of these programs.
Results
At the initial clinical examination, performed in 27 children with GERD, dental decay was detected in 77.78% of them, the incidence of the DMFT index being 2.74±0.33. Dental erosion was detected in 59.26% of the total number of children, and the incidence of erosive wear was 3.96±0.7.
The average risk of erosive wear was detected in 6 (22.22%) children, and the reduced risk, respectively - at 7 (25.93%) children, depending on the duration of GERD. Affection by dental decay and the presence of dental erosion were found in 37.04% of the children suffering from GERD. The frequency of the dental decay in healthy children represented 55.56%, the incidence of the DMFT index is 1.26±0.14. Further on, in the study, we examined the central and peripheral areas of the facies obtained by dehydration of the OF droplets.
The outcome of the investigation specified the main types of oral liquid microcrystallization (MC). For the type of MC presence of large prismatic crystal structures form merger with “fern” is characteristic (Fig. 3). For quantitative data objectification, MC type I was assessed by 5 points. Fused random fractal structures are characteristic of type II MC, gaining 4 points (Fig. 4). In type III MC, in the central part star-shaped separated crystals were present, and remained at the periphery of larger fractal crystals (3 points) (Fig. 5). MC Type IV was characterized by the presence of the separated crystals in the form of a branch or stem, placed relatively evenly over the whole surface of the dried droplet (2 points) (Fig. 6). A large amount of separated stellate crystals of an oval and irregular shape located isometrically was characteristic of type V MC (1 point) (Fig. 7). In case of a complete lack of crystals, the type VI MC (0 points) was found.
Figure 3.
SEM. Facies of oral fluid, the type I of microcrystallization.
Figure 4.
SEM. Facies of oral fluid, the type II of microcrystallization.
Figure 5.
SEM. Facies of oral fluid, the type III of microcrystallization.
Figure 6.
SEM. Facies of oral fluid, the type IV of microcrystallization.
Figure 7.
SEM. Facies of oral fluid, the type V of microcrystallization.
We found MC type I and II in children with high resistance to caries, and in those with increased intensity of dental caries - types IV – VI MC prevailed. For children with GERD and dental erosions, the types IV–V MC were specific, while for children without any erosions and low risk of erosive wear we found types II – III MC.
Using the MCG research method of the oral liquid, we examined not only the fractal structures, separate crystals, but also the amorphous substance.
The analysis of MCG established that in the peripheral area of the oral liquid droplet pyramidal cruciform structures predominated, in the central part linear structures were present, with a dendritic image in the form of “fern”, amorphous bodies were of a reduced size and crystals of large size accumulation on the surface. In the peripheral area of the oral liquid in healthy children “crusade” and “prismatic” structure prevailed.
In patients with GERD affected by dental caries and those with low risk of erosive wear the crystals structure was disturbed considerably over the entire surface of the facia where crusader crystals of different sizes were present. In children suffering from GERD and carious lesions and those with low risk of erosive wear, the structure of the crystals was considerably disturbed, with different sizes of crusade crystals present on the entire surface of the facia. The peripheral area of the oral liquid facies was expanded and prominent, evidencing an abundance of elements that deteriorated the integrity of the structure (Fig. 8), which might have been caused by the high concentration of mucin in the saliva.
Figure 8.
SEM. Facies of oral fluid, prominent peripheral area of the facia.
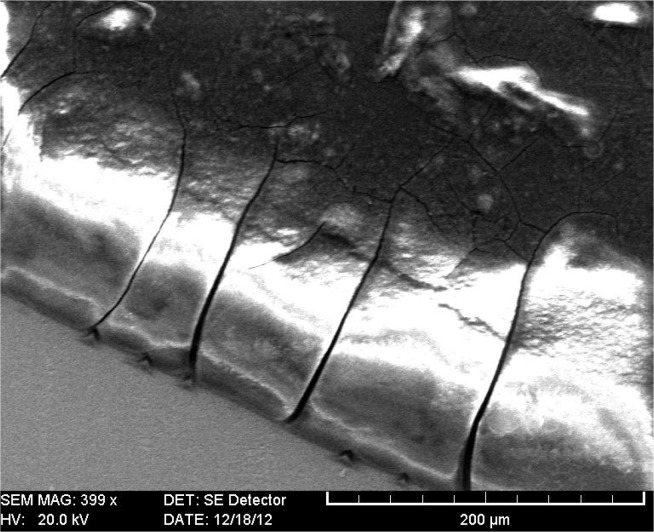
Oral liquid MCG in children with complicated caries, as well as in children with dental erosions, are marked by the highlighted, expanded and cracked character of the peripheral zone (Fig. 9) as well as the presence of crystalline structures of “fern” located both in the peripheral and central zones. Highlighted, expanded and cracked character of the peripheral area reflects increased protein contents and conduct oral fluid pathological processes caused by free radicals. The degree of facia destruction varies due to structural integrity disruption.
Figure 9.
SEM. Facies of oral fluid, radial cracks in the peripheral area.
It was found that in 96.29% of children from the control group and 7.41% of children with GERD the central area of the oral liquid MCG consisted mainly of dendritic crystals, but in the peripheral area additional inclusions were not found. In 3.7% of children in the control group and 92.59% of children in the study group a number of morphological features in the peripheral facies of oral fluid were established:
pathological mineral compounds crystallization in the protein surroundings, manifested by the presence of crystalline inclusions, which were detected in the peripheral zone of the oral fluid facia;
presence of the radial cracks network, which in clinical crystallography is considered a marker of tissue destruction processes (Fig. 10), detected in children with untreated dental caries or tooth decay complications;
presence of pigmentation portions (marker of intoxication), which may be associated with the phenomenon of pathological crystallization of salts in the protein surroundings, were detected in children with carious activity, intensely untreated caries or tooth decay complications.
Figure 10.
SEM. Facies of oral fluid, pathological crystallization of mineral compounds in the protein environment.
Next, we compared the specificities of MCG oral fluid in healthy children and those with GERD. Thus, in the majority of children with GERD a pathological crystallization of mineral compounds in the protein surroundings was found. It was assumed that this marker characterizes the crystals’ ability to “connect” microorganisms and products of their activity in crystalline aggregates with organic inclusions. In children with carious intense activity, untreated caries lesions and / or dental caries complications, and in chronic persistent infection odontogenic outbreaks, the marker intoxication and / or tissue damage was found.
It was found that the degree of MC of the oral liquid in children with GERD was considerably reduced, constituting 1.73±0.11 points, and was 1.86 times lower compared to the indicator considered in control children (3.22±0.16 points) (p<0.01, RR=2). Among children in the study group there was a tendency to reduce the degree of MC damage correlated to the duration of GERD. Thus, in children with GERD duration up to 1 year, the level of the MC of oral liquid was 0.9 times lower (p<0.05, RR = 1.9), duration of illness in children between 1 to 2 years - 1.36 times (p<0.01, RR = 2.4), and duration of illness in children between 2–3 years - 2.1 times lower as compared with the estimated values in children from the control group (p<0.05, RR = 3.8).
The analysis of the picture of dehydrated oral fluid droplet elucidated the relationship between the degree of the oral liquid and work MC caries process. Children with carious activity had MC moderate degree of the oral liquid 1.9 times (p<0.05, RR=2.1) and in children with intense carious activity - 2.3 times lower compared with controls (p<0.01, RR=2.6). The research results allow to conclude that the degree of oral liquid microcrystallization in children is directly related to the duration of impaired dental caries (r=0.647) and degree of carious activity (r=0.248).
Discussion
It is well known that saliva is a supersaturated liquid with calcium and phosphate ions and possesses the ability of enamel mineralization. The saturated liquid state is provided by its structure, its entire volume being distributed among the micelles. Under physiological conditions, saliva crystals have a branched form due to the presence in the oral liquid of micelles Ca3(PO4)2, protected by the aggregation of mucin, which has a branched form. Mucins are involved in the transport of transepithelial ions (Na+, K+, Cl−), and in the process of biocrystalization involving ions Ca2+, constituting the organic matrix which regulates the volume and configuration of the crystal structure and the formation of crystalline structures in dendritic drying oral liquid [25,26].
The optical properties of the crystals vary significantly depending on exo- and endogenous factors, this phenomenon being applied in research, for diagnostic purposes [4]. Under unfavourable changes, in the oral cavity the structure of saliva modifies, thereby its mineralization function is disturbed followed by changes in the shape and properties of the crystal structure [26–28].
Thus, the morphological image changes of the oral liquid due to its microcrystallization properties is an index of qualitative or quantitative disorders of salivary proteins, in particular the mucins, and is one of the earliest markers of pathological processes occurring in the organism and may be used to detect diseases in the premorbid phase, to efficiently implement the preventive and minimally invasive treatment. Therefore, the oral liquid microcrystallization disorder reflects the quantitative and qualitative disorders of mucins composition, which represents an increased risk of carious lesions [13,28].
Setting the hallmarks of crystallographic changes in saliva which are influenced by functional and metabolic changes increases accuracy of diagnosis and efficiency of caries risk prediction. Dehydration method (crystallization of bioliquids on the glass slide) can provide an overview of the health of the entire body. Changing the shape of the crystals is a consequence of changes of the physicochemical properties of saliva [21,27,28].
The demineralization of dental enamel in carious process reflects the properties of the saliva, and is manifested by changing crystal structures of the oral liquid MCG. The microcrystallization degree of the oral liquid in children is directly correlated with the GERD duration and activity of carious process.
Thus, the study of morphological features of the oral liquid may serve as a predictive test that identifies impaired oral cavity protection mechanisms. The knowledge of the mechanisms of these changes is required for the cariopreventive measures targeted on individual risk factors, while the availability of the oral liquid research, the research methodology simplicity, and the minimum costs allow its application in the dental practice.
The research method of oral liquid crystalogenesis may be applied not only in the diagnosis and prediction of diseases of the oral cavity, but it can also be used as a sensitive indicator of the operational status of the entire body. The development of a highly informative and noninvasive methodology of premorbid conditions diagnosis and the pre-research of methods for the appropriate diagnosis of the changes occurred in the health of the population represent the most important fields of research in medicine.
Conclusions
The research of the oral liquid microcrystallization is a simple, non-invasive and informative method of investigation that can be applied to assess the functioning not only of the dental apparatus, but also as an indicator of the functioning of the whole organism.
The degree of the oral liquid microcrystallization in children with GERD is 1.86 times lower than this indicator assessed in healthy children.
A low level of oral liquid microcrystallization involvement correlates with the duration of GERD and carious activity process.
The study of structural features of oral liquid dehydrated droplet in children with dental caries has elucidated a number of markers of the changes produced in the oral cavity, which can be later applied in screening studies in dental practice.
The applied method of oral liquid microcrystallization offers the opportunity to study the most important pathogenic mechanisms involved in the initiation and development of dental caries and their complications, to perform dental caries prediction in order to elaborate cariopreventive measures and evaluation of their effectiveness.
References
- 1.Haeckel R, Hanecke P. The application of saliva, sweat and tear fluid for diagnostic purposes. Ann Biol Clin (Paris) 1993;51:903–910. [PubMed] [Google Scholar]
- 2.Aps JK, Martens LC. Review: The physiology of saliva and transfer of drugs into saliva. Forensic Sci Int. 2005;150(2–3):119–131. doi: 10.1016/j.forsciint.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Veerman ECI, van den Keijbus PAM, Vissink A, van Nieuw Amerongen A. Human glandular salivas: their separate collection and analysis. Eur J Oral Sci. 1996;104:346–352. doi: 10.1111/j.1600-0722.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 4.Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50:140–161. doi: 10.1111/j.1875-595x.2000.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 5.Martusevici AK, Kamakin NF. Unified algorithm research and initiated free crystallogenesis biological fluids. Klinicheskaia laboratornaia diagnostika. 2007;6:21–24. [Russian] [PubMed] [Google Scholar]
- 6.Spielmann N, Wong DT. Saliva: diagnostics and therapeutic perspectives. Oral Dis. 2011;17(4):345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volojin AI, Vershinina GB. Peculiarities of local immunity of the oral cavity in children with anemia. Actual problems of dentistry. 2004:76–78. [Russian] [Google Scholar]
- 8.Miller SM. Saliva testing - a non-traditional diagnostic tool. Clin Lab Sci. 1994;7:39–44. [PubMed] [Google Scholar]
- 9.Tarasevici II, Aiupova AK. The effect of diffusion on the separation of the components of biological fluid with wedge dehydration. Journal of Technical Physics. 2003;5(73):13–18. [Russian] [Google Scholar]
- 10.Annarelli C, Fornazero J, Bert J, Colombania J. Crack patterns in drying protein solution drops. Eur Phys J E. 2001;5:599–603. [Google Scholar]
- 11.Mandel ID. A contemporary view of salivary research. Crit Rev Oral Biol Med. 1993;4:599–604. doi: 10.1177/10454411930040034701. [DOI] [PubMed] [Google Scholar]
- 12.Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, et al. Current developments in salivary diagnostics. Biomarkers Med. 2010;4(1):171–189. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamakin NF, Martusevici AK. Crystalloscopic pattern of biological fluids of a human organism. Klinicheskaia laboratornaia diagnostika. 2002;10:3–9. [Russian] [Google Scholar]
- 14.Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383(1–2):30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Cemikosova TS, Bakirov AB, Guleaeva OA. Occupational risk factors of formation of periodontal disease in workers of the organochlorine synthesis. Siberian medical journal. 2004;3:62–64. [Russian] [Google Scholar]
- 16.Biesbrock AR, Reddy MS, Levine MJ. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991;59:3492–3497. doi: 10.1128/iai.59.10.3492-3497.1991. [Russian] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komarova LG. Diagnosis of gastroduodenal diseases in children. Pediatrics. 1986;11:24–27. [Russian] [PubMed] [Google Scholar]
- 18.Perminov SV, Nichitina LP, Ivanova EN, Perminova TP. Biochemical parameters of human saliva. Clinical laboratory diagnostics. 1995;2:46–47. [Russian] [Google Scholar]
- 19.Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19:119–125. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 20.Petricek V, Dusek M, Jana Palatinus L. The crystallographic computing system. Institute of Physics; Praha: 2000. p. 2000. [Google Scholar]
- 21.Solomatina NN. Evaluation of crystallogram facies of oral fluid in chronic apical periodontitis. Journal of Volgograd State Medical University. 2011;4(40):46–49. [Russian] [Google Scholar]
- 22.Kamakin NF, Martusevich AK. Cristalloscopic study of the biological substrates. Guidelines Kirov. 2005:34. [Russian] [Google Scholar]
- 23.Leontiev VK. Caries and mineralization processes. Moskva: MMSI; 2007. p. 541. [Russian] [Google Scholar]
- 24.Okushko VR, Kozadaev SI, Potolya AV. Steps in computerizing the enamel resistance test. Saratov Journal of Medical Scientific Research. 2011;7(1):266–268. [Russian] [Google Scholar]
- 25.Shatohina SN, Razumov SN, Shabalin VN. Morphological pattern of oral fluid: diagnostic capabilities. Dentistry. 2006;4:14–17. [Russian] [Google Scholar]
- 26.Denisov AB. Role of salivary mucins in the protection of the oral cavity. Dentistry. 2006;7:15–20. [Russian] [Google Scholar]
- 27.Cemicosova TS, Guleaeva OA. Estimation of level of free radical oxidation by studying the composition of the oral fluid. Issues of dentistry. 2007;2:9–10. [Russian] [Google Scholar]
- 28.Martusevich AK, Kamakin NF. Crystallography of biological fluid. Bulletin of Experimental Biology and Medicine. 2007;3(143):358–360. doi: 10.1007/s10517-007-0118-7. [Russian] [DOI] [PubMed] [Google Scholar]