Abstract
Purpose
This study aimed at evaluating the bacterial colonization in dental implants inserted in the crestal or supracrestal position and correlated it to radiographic bone measurements.
Methods
Thirty-five implants with regular platform in nine patients (mean age 62.4±11.2 years) were inserted either at the bone crest level (control group) or at a suprecrestal level (test group). Radiographic examination was performed at baseline (implant installation) and after 6 months. Clinical and microbiological data were collected after 6 months. Digital radiography was used to assess bone remodeling (marginal bone loss and optical alveolar density). Bacterial profile was analyzed by checkerboard DNA–DNA hybridization, including a panel of 40 bacterial species.
Results
After 6 months, there were significantly higher counts of Actinomyces gerencseriae (p=0.009) and Streptococcus constellatus (p=0.05) in the test group. No significant differences between test and control groups were observed for marginal bone loss (p=0.725) and optical alveolar density (p=0.975). Probing depth was similar in both groups.
Conclusion
Significantly higher counts of A. gerencseriae and S. constellatus were found in implants placed at the supracrestal level compared to the ones placed at the bone level. No relation was found between the installation level of dental implants and peri-implant bone remodeling.
Keywords: dental implants, bacteria, Actinomyces, Streptococcus, bone remodeling
Replacing missing teeth by dental implants represents a breakthrough in dentistry, being a well-accepted treatment modality (1) and one of the first treatment options for rehabilitation of edentulous patients (2). Despite high successful rates achieved by implant-supported restorations and osseointegration of different implant designs (3–5), some factors are still not elucidated, especially concerning the bacterial colonization that might influence bone remodeling around dental implants.
At the moment of installation, pristine implant surfaces are devoid of an indigenous microbiota. The colonization of the implant-supported restoration leads to an increase in peri-implant inflammation, altering the local habitat. In turn, the composition of the microbiota adjacent to implants is influenced by the local environment established at the interface between peri-implant mucosa and implant surface (6). Peri-implant microbiota composition has been evaluated along with clinical parameters. Some studies have shown that an increase in proportions of spirochetes and motile organisms was associated with an increase in probing depth around implants (7, 8). Other studies have found greater levels of periodontal pathogens around implants with marginal bone loss (9, 10).
A supracrestal position of the implant platform may favor the establishment of a biological width, and also push aside the microgap and its bacterial contamination from peri-implant bone crest, besides reducing the inflammatory infiltrate. Studies have shown that the absence of a microgap at the bone crest level, obtained with a supracrestal installation, was associated with reduced inflammatory peri-implant cells and with minimal bone loss (11, 12). Authors have shown that minor bone loss is observed when the microgap is coronally moved away from the bone crest. In turn, the contrary may be observed as far as the microgap is moved apically (13).
Peri-implant bone remodeling is one of the healing phases, which involves hemostasis, an inflammatory phase, a proliferative phase, and finally, a remodeling phase (14), and occurs since the implant is exposed to the oral environment, in a second surgical procedure or when the prosthetic component is immediately connected after implant installation (immediate loading protocol) (15). The remodeling process implicates marginal bone resorption both horizontally and vertically that may be affected by several biological and mechanical factors, for instance, a traumatic surgical technique, excessive loading conditions, peri-implant and microgap bacterial colonization, and implant-neck geometry (6, 15, 16). Thus, the aim of this study was to evaluate the bacterial colonization in dental implants inserted in crestal or supracrestal position and correlated it to radiographic measurements of bone remodeling.
Methods
A total of 10 patients were included in this study. They were selected from the Prosthodontics Clinic at the Rio de Janeiro State University. All patients signed a written informed consent. The study protocol was approved by the Ethics Committee in Research of the Pedro Ernesto University Hospital – Rio de Janeiro State University. Patients were totally edentulous; had absence of medical conditions that compromise the immunological status; wore complete superior and inferior dentures, which were aesthetically and functionally adequate; and had enough bone to support four dental implants with 4.1 mm in diameter in the anterior portion of the mandible between the mental foramens. Exclusion criteria were use of antibiotics (minimum of 6 months), corticosteroids, non-steroidal anti-inflammatory drugs (minimum of 3 months), use of oral antiseptics, and smoking.
The included patients were randomly divided into two groups of five patients each, according to the implants insertion protocol: Group 1 (test group) – implants placed at the supracrestal level; and Group 2 (control group) – implants placed at the crestal level. All implants were external hexagon implants with NeoPoros surface, prepared by sandblasting and acid etching (Titamax Ti Cortical, Neodent, Curitiba, Brazil). Patients were evaluated at baseline (T1) and after 6 months (T2). In T1, radiographic examination was performed. In T2, clinical, radiographic, and microbiologic data were gathered.
The surgical protocol for implant installation consisted of a full thickness flap for mandibular bone exposure and mental foramina localization. The two distal implants were inserted 5 mm far from each mental foramen. The two other implants were inserted in an equidistant manner, counting four implants for each patient. The drill sequence was performed according to implant system recommendations. According to the bone availability determined by the computed tomography, the implants’ length was selected (11 or 13 mm). In the test group, implants were inserted with smooth neck surface of the implant localized supra-crestally. In the control group, implants were inserted with the implant platform located at the bone level. The minimal insertion torque considered for immediate loading was 35 N/cm.
After implant installation, mini abutments with height varying from 3.0 to 4.0 mm were connected to implant platforms so that all components presented the same final height. Protectors were inserted over mini abutments and the suture was performed. Patients had taken antibiotics (amoxicillin 500 mg for 7 days), a non-steroidal anti-inflammatory drug (potassium diclofenac 50 mg for 3 days), and an oral antiseptic (chlorhexidine digluconate 0.2% for 10 days). Ten days after the surgical procedures (T1), standardized radiographies were taken for each implant. A polyvinyl siloxane (Express XT – 3M, Sumaré, Brazil) mold was used to standardize the radiography sensor in the second assessment. The total fixed prosthesis was made based on a complete removable denture using the NeodentTM system.
Clinical examination
The same examiner assessed probing depth using a calibrated periodontal probe (Colorvue®, Hu-Friedy, Chicago, IL) at the mesial and distal sites of each implant.
Microbiological analysis
Two sites for each implant were selected. After removing the supragingival plaque, the most apical subgingival biofilm was collected using sterile Teflon curettes (Implacare™ IC4R/4L – Hu-Friedy Co., Chicago, IL). Samples were placed in separate microtubes containing 0.15 mL TE (10 mM Tris-HCl and 1 mM EDTA, pH 7.6). Freshly prepared 0.5 M NaOH was added to each tube so that the bacterial DNA remained viable. Samples were kept under −20°C until analysis.
Counts of 40 bacterial species were determined in each sample using checkerboard DNA–DNA hybridization. The analyses were performed at the Laboratory of Microbiology of Guarulhos University as previously described (17). A single blinded examiner performed radiography films readings twice in two different days. Each signal produced by a probe in the sample was compared, in its intensity, to the signal produced by the same probe in two control lines that contained 105 and 106 bacteria. Thus, score 0 was attributed to sample when no signal was detected; score 1 corresponded to a signal with intensity lower than 105 control; score 2 corresponded to 105 cells; score 3 corresponded between 105 and 106 cells; score 4 corresponded to approximately 106 cells; and score 5 was attributed to more than 106 cells.
Radiographic bone assessment
Radiographic assessment was performed in the mesial and distal sites of each implant. Periapical radiographies were obtained at baseline and after 6 months, by paralleling technique, using the Kodak® 2200 Intraoral X-ray system (Trophy, France) and the intraoral positioner Super-Bite® sensor anterior/posterior (WA). The X-ray device was calibrated with 70 kV and 7 mA. The exposure time was 0.077 for all implants. Seventy sites (32 sites for the test group and 38 sites for the control group) were radiographed and followed. Two radiographic parameters were assessed: linear bone loss and optical alveolar density. All assessments were performed by the same examiner. Examiner calibration was performed by assessing 20 radiographies twice. Intra-examiner concordances were 93.7% for linear measurements within ±0.1 mm and 99% for optical density assessments within ±3 pixels.
Linear measurements corresponded to the distance from the prosthetic component/implant abutment interface to the most coronal point of the bone/implant contact and were obtained in millimeters. The bone regions of interest (ROIs), approaching 1 mm2, were positioned laterally to each implant, at the mesial and distal most coronal point of the implant/bone contact, in the alveolar bone crest (without touching the implant). For the ROIs confection, a radiodense net with 1 mm2 was positioned above the digital sensor and was used as a calibrator to the X-ray system. Optical alveolar density was determined by the average intensity of the grayscale in a diagonal line from the left inferior vertex to the right superior vertex of the ROI. The grayscale varied from 0 to 256 pixels, where 0 corresponded to black and 256 to white.
Statistical analysis
Since many implants were analyzed in each patient, imposing an internal dependence of observed data, generalized linear models with generalized estimation equation were used to analyze marginal bone loss and optical alveolar density variations. In these models, the statistical significance was obtained by Wald statistics. Pearson and Spearman's rank coefficients, whenever appropriate, were used to assess linear correlations between variables. Statistical significance was set at 0.05.
Based on the difference observed for the counts of Actinomyces gerencseriae, this study presented power >80% with 16 implants per group and α=0.05.
Results
From the 10 patients who were included, 9 remained in the T2 analysis. One patient from test group dropped out of the study. One implant was lost in a patient from the control group. Mean age (±standard deviation) of the nine patients (three males and six females) was 62.4±11.2 years.
Checkerboard DNA–DNA hybridization
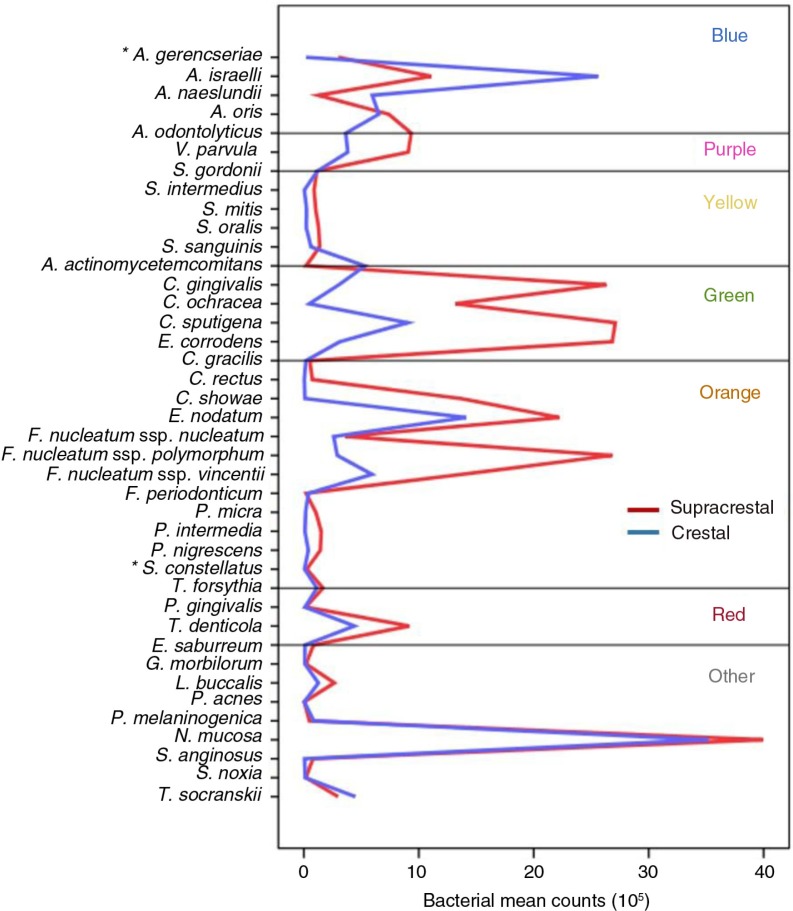
Regarding the microbial profile, the test group showed higher counts of A. gerencseriae (p=0.009) and Streptococcus constellatus (p=0.05). There was also a trend to higher counts of S. oralis (p=0.06) and Gemella morbillorum (p=0.08) in the test group. No bacterial count was significantly higher than in the control group. Microbiological data are depicted in Fig. 1.
Fig. 1.
Bacterial profiles in test and control groups (*p<0.05).
The median total bacterial load was 59.5×105 (32.5–93.0×105) in the test group and 57.0×105 (36.0–65.0×105) in the control group, without significant difference between the groups.
Radiographic bone analysis
Bone loss and optical alveolar density in T1 and T2 are presented in Table 1. Both groups showed a statistically significant increase in radiographic bone loss from T1 to T2 (p<0.001). There was no significant difference in optical alveolar density from T1 to T2 in the test and control groups (p>0.05). There were no significant differences between groups in both parameters at T2 (p>0.05).
Table 1.
Bone remodeling in baseline (T1) and after 6 months (T2) in test and control groups
| Test group (n=16) | Control group (n=19) | ||
|---|---|---|---|
| Linear measurement | T1 | 4.00 (±0.33) | 4.31 (±0.31)** |
| T2 | 4.68 (±0.48) | 4.93 (±0.33) | |
| Bone loss (Δ T2 – T1) | 0.68 (±0.36)* | 0.62 (±0.54)* | |
| Optical alveolar density | T1 | 53.4 (±18.5) | 61.3 (±16.0) |
| T2 | 57.0 (±28.1) | 64.6 (±30.4) |
Data are presented as mean (standard deviation). p-Value calculated using Wald test applying generalized estimating equations.
p<0.001 for intragroup difference.
p<0.001 for the difference between test and control group.
In T2, the probing depth was 2.32 mm (±0.37) and 2.38 mm (±0.71) for the test and the control groups, respectively. There was no significant difference in probing depth between groups (p>0.05).
Correlational analysis
No significant correlation was established between bone loss (ΔT2 – T1) and variation in optical alveolar density (ΔT2 – T1) in both groups. Also, there was no significant correlation between radiographic measures and probing depth. There was no significant correlation between bacterial profile and radiographic measures and between bacterial profile and probing depth.
Discussion
There is a discordance regarding insertion level of dental implants related to bone crest (and consequently the localization of the microgap) and its influence on peri-implant bone remodeling. Thus, this study was performed to evaluate the effect of the implant insertion level on bacterial profile and peri-implant bone remodeling. We found higher counts of A. gerencseriae and S. constellatus in the supracrestal group. There were also trends for higher counts of S. oralis and G. morbillorum in the supracrestal group. The total bacterial count was not significantly different between the groups. Canullo et al. (6) have found no significant differences in bacterial colonization between implants restored with a traditional approach and those restored with a platform-switching approach.
Our patients were fully edentulous, while Canullo's patients were partially edentulous. This may account for the conflicting results. A systematic review has shown a difference in submucosal peri-implant microbiota between fully edentulous and partially edentulous patients (18). Our study and that from Canullo et al. (6) used sandblasted and acid-etched implant surfaces. Bacterial adhesion is dependent on the surface roughness (19, 20).
A. gerencseriae and S. constellatus are early colonizers from the blue and orange complexes, respectively. Peri-implant disease was associated with higher levels of Actinomyces and non-mutans Streptococcus than healthy implants (21). On the other side, higher proportions of Actinomyces were shown in healthy implants (22). However, we found no significant correlation between bacterial profiles and bone remodeling and between bacterial profiles and probing depth. In agreement with our finding, Canullo et al. (6) also found no significant correlation between mean bone loss and levels and proportions of subgingival species in the peri-implant plaque.
Some studies have shown that the absence of microgap at the bone level with non-submerged implant systems would result in minimal peri-implant bone loss (4, 11, 23). Meanwhile, other studies have found advantages with subcrestal placement of dental implants (24, 25). Our study found no statistically significant difference when bone remodeling was evaluated in two-piece implants inserted 1 mm supracrestally and at the bone level. This result is in accordance with other studies, which found no effect of the microgap location in bone remodeling (26–28).
Several factors may account for the conflicting results, as interface implant/abutment and healing time. In our study, the interface implant/abutment in the supracrestal group was located 1 mm above the bone crest. Boynueğri et al. (1) have located the microgap 2.8 mm above the bone crest. Piattelli et al. (13) located the interface implant/abutment 1–2 mm above the bone crest and found that this position was favorable to a minor bone loss in this group. In addition, our study used an immediate loading protocol. On the other hand, healing time before loading varied from 3 to 6 months in other studies (1, 11, 12), which may account for the different results. However, Guruprasada et al. (29) and Piattelli et al. (13) found no significant differences in peri-implant bone remodeling comparing conventional loading and immediate loading protocols.
We also found no significant difference in probing depth between the groups. This result is in agreement with that showed by Boynueğri et al. (1).
A reduced sample and analysis of multiple implants in the same patient are limitations of our study, which imposed an internal dependence in the observed data. Nevertheless, we used generalized linear models with a generalized estimation equation to take into account this dependence. Also, we assessed a limited number of bacterial species, and modern sequencing techniques have been shedding light on the peri-implant microbiome complexity (21, 30). Studies with greater sample sizes and longer follow-ups are needed to investigate if these results and their clinical implications will be maintained. In conclusion, significantly higher counts of A. gerencseriae and S. constellatus were found in implants placed at the supracrestal level compared to the ones placed at the bone level. No relation was found between the installation level of dental implants and peri-implant bone remodeling.
Conflict of interest and funding
The authors declare no conflict of interest.
References
- 1.Boynueğri AD, Yalim M, Nemli SK, Erguder BI, Gokalp P. Effect of different localizations of microgap on clinical parameters and inflammatory cytokines in peri-implant crevicular fluid: a prospective comparative study. Clin Oral Investig. 2012;16:353–61. doi: 10.1007/s00784-010-0497-4. [DOI] [PubMed] [Google Scholar]
- 2.Rasperini G, Siciliano VI, Cafiero C, Salvi GE, Blasi A, Aglietta M. Crestal bone changes at teeth and implants in periodontally healthy and periodontally compromised patients. A 10-year comparative case-series study. J Periodontol. 2014;85:e152–9. doi: 10.1902/jop.2013.130415. [DOI] [PubMed] [Google Scholar]
- 3.Buser D, Mericske-Stern R, Bernard JP, Behneke A, Behneke N, Hirt HP, et al. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997;8:161–72. doi: 10.1034/j.1600-0501.1997.080302.x. [DOI] [PubMed] [Google Scholar]
- 4.Buser D, Mericske-Stern R, Dula K, Lang NP. Clinical experience with one-stage, non-submerged dental implants. Adv Dent Res. 1999;13:153–61. doi: 10.1177/08959374990130010501. [DOI] [PubMed] [Google Scholar]
- 5.Lekholm U, Gunne J, Henry P, Higuchi K, Linden U, Bergstrom C, et al. Survival of the Branemark implant in partially edentulous jaws: a 10-year prospective multicenter study. Int J Oral Maxillofac Implants. 1999;14:639–45. [PubMed] [Google Scholar]
- 6.Canullo L, Quaranta A, Teles RP. The microbiota associated with implants restored with platform switching: a preliminary report. J Periodontol. 2010;81:403–11. doi: 10.1902/jop.2009.090498. [DOI] [PubMed] [Google Scholar]
- 7.Papaioannou W, Quirynen M, Nys M, van Steenberghe D. The effect of periodontal parameters on the subgingival microbiota around implants. Clin Oral Implants Res. 1995;6:197–204. doi: 10.1034/j.1600-0501.1995.060401.x. [DOI] [PubMed] [Google Scholar]
- 8.Renvert S, Roos-Jansaker AM, Lindahl C, Renvert H, Rutger Persson G. Infection at titanium implants with or without a clinical diagnosis of inflammation. Clin Oral Implants Res. 2007;18:509–16. doi: 10.1111/j.1600-0501.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 9.Hultin M, Gustafsson A, Klinge B. Long-term evaluation of osseointegrated dental implants in the treatment of partly edentulous patients. J Clin Periodontol. 2000;27:128–33. doi: 10.1034/j.1600-051x.2000.027002128.x. [DOI] [PubMed] [Google Scholar]
- 10.Quirynen M, Alsaadi G, Pauwels M, Haffajee A, van Steenberghe D, Naert I. Microbiological and clinical outcomes and patient satisfaction for two treatment options in the edentulous lower jaw after 10 years of function. Clin Oral Implants Res. 2005;16:277–87. doi: 10.1111/j.1600-0501.2005.01127.x. [DOI] [PubMed] [Google Scholar]
- 11.Broggini N, McManus LM, Hermann JS, Medina RU, Oates TW, Schenk RK, et al. Persistent acute inflammation at the implant–abutment interface. J Dent Res. 2003;82:232–7. doi: 10.1177/154405910308200316. [DOI] [PubMed] [Google Scholar]
- 12.Broggini N, McManus LM, Hermann JS, Medina R, Schenk RK, Buser D, et al. Peri-implant inflammation defined by the implant–abutment interface. J Dent Res. 2006;85:473–8. doi: 10.1177/154405910608500515. [DOI] [PubMed] [Google Scholar]
- 13.Piattelli A, Vrespa G, Petrone G, Iezzi G, Annibali S, Scarano A. Role of the microgap between implant and abutment: a retrospective histologic evaluation in monkeys. J Periodontol. 2003;74:346–52. doi: 10.1902/jop.2003.74.3.346. [DOI] [PubMed] [Google Scholar]
- 14.Terheyden H, Lang NP, Bierbaum S, Stadlinger B. Osseointegration – communication of cells. Clin Oral Implants Res. 2012;23:1127–35. doi: 10.1111/j.1600-0501.2011.02327.x. [DOI] [PubMed] [Google Scholar]
- 15.Atieh MA, Ibrahim HM, Atieh AH. Platform switching for marginal bone preservation around dental implants: a systematic review and meta-analysis. J Periodontol. 2010;81:1350–66. doi: 10.1902/jop.2010.100232. [DOI] [PubMed] [Google Scholar]
- 16.Gultekin BA, Gultekin P, Leblebicioglu B, Basegmez C, Yalcin S. Clinical evaluation of marginal bone loss and stability in two types of submerged dental implants. Int J Oral Maxillofac Implants. 2013;28:815–23. doi: 10.11607/jomi.3087. [DOI] [PubMed] [Google Scholar]
- 17.Matarazzo F, Figueiredo LC, Cruz SE, Faveri M, Feres M. Clinical and microbiological benefits of systemic metronidazole and amoxicillin in the treatment of smokers with chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol. 2008;35:885–96. doi: 10.1111/j.1600-051X.2008.01304.x. [DOI] [PubMed] [Google Scholar]
- 18.de Waal YC, Winkel EG, Meijer HJ, Raghoebar GM, van Winkelhoff AJ. Differences in peri-implant microflora between fully and partially edentulous patients: a systematic review. J Periodontol. 2014;85:68–82. doi: 10.1902/jop.2013.130088. [DOI] [PubMed] [Google Scholar]
- 19.Badihi Hauslich L, Sela MN, Steinberg D, Rosen G, Kohavi D. The adhesion of oral bacteria to modified titanium surfaces: role of plasma proteins and electrostatic forces. Clin Oral Implants Res. 2013;24(Suppl A100):49–56. doi: 10.1111/j.1600-0501.2011.02364.x. [DOI] [PubMed] [Google Scholar]
- 20.Bürgers R, Gerlach T, Hahnel S, Schwarz F, Handel G, Gosau M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implants Res. 2010;21:156–64. doi: 10.1111/j.1600-0501.2009.01815.x. [DOI] [PubMed] [Google Scholar]
- 21.Kumar PS, Mason MR, Brooker MR, O'Brien K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol. 2012;39:425–33. doi: 10.1111/j.1600-051X.2012.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva ES, Feres M, Figueiredo LC, Shibli JA, Ramiro FS, Faveri M. Microbiological diversity of peri-implantitis biofilm by Sanger sequencing. Clin Oral Implants Res. 2014;25:1192–9. doi: 10.1111/clr.12231. [DOI] [PubMed] [Google Scholar]
- 23.Hermann JS, Cochran DL, Nummikoski PV, Buser D. Crestal bone changes around titanium implants. A radiographic evaluation of unloaded nonsubmerged and submerged implants in the canine mandible. J Periodontol. 1997;68:1117–30. doi: 10.1902/jop.1997.68.11.1117. [DOI] [PubMed] [Google Scholar]
- 24.Buser D, von Arx T. Surgical procedures in partially edentulous patients with ITI implants. Clin Oral Implants Res. 2000;11(Suppl 1):83–100. doi: 10.1034/j.1600-0501.2000.011s1083.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang B, Meng H, Piao M, Xu L, Zhang L, Zhu W. Influence of placement depth on bone remodeling around tapered internal connection implant: a clinical and radiographic study in dogs. J Periodontol. 2012;83:1164–71. doi: 10.1902/jop.2012.110617. [DOI] [PubMed] [Google Scholar]
- 26.Heydenrijk K, Raghoebar GM, Meijer HJ, van der Reijden WA, van Winkelhoff AJ, Stegenga B. Two-stage IMZ implants and ITI implants inserted in a single-stage procedure. A prospective comparative study. Clin Oral Implants Res. 2002;13:371–80. doi: 10.1034/j.1600-0501.2002.130405.x. [DOI] [PubMed] [Google Scholar]
- 27.Heijdenrijk K, Raghoebar GM, Meijer HJ, Stegenga B, van der Reijden WA. Feasibility and influence of the microgap of two implants placed in a non-submerged procedure: a five-year follow-up clinical trial. J Periodontol. 2006;77:1051–60. doi: 10.1902/jop.2006.050342. [DOI] [PubMed] [Google Scholar]
- 28.Todescan FF, Pustiglioni FE, Imbronito AV, Albrektsson T, Gioso M. Influence of the microgap in the peri-implant hard and soft tissues: a histomorphometric study in dogs. Int J Oral Maxillofac Implants. 2002;17:467–72. [PubMed] [Google Scholar]
- 29.Guruprasada Thapliyal GK, Pawar VR. A comparative analysis of periimplant bone levels of immediate and conventionally loaded implants. Med J Armed Forces India. 2013;69:41–7. doi: 10.1016/j.mjafi.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charalampakis G, Belibasakis GN. Microbiome of peri-implant infections: lessons from conventional, molecular and metagenomic analyses. Virulence. 2015;6:183–7. doi: 10.4161/21505594.2014.980661. [DOI] [PMC free article] [PubMed] [Google Scholar]