Summary
Nasopharyngeal (NP) pneumococcal carriage predisposes children to pneumococcal infections. Defining the proportion of pneumococcal isolates that are antibiotic-resistant enables the appropriate choice of empiric therapies. We have defined the antibiogram of NP carriage isolates derived from a pediatric population following introduction of the 13-valent pneumococcal conjugate vaccine.
Introduction
Nasopharyngeal (NP) colonization by Streptococcus pneumoniae predisposes young children to acute otitis media (AOM) and invasive pneumococcal disease 1, 2. Introduction of the seven-valent pneumococcal conjugate vaccine (PCV7) in 2000 significantly increased the proportion of penicillin-susceptible pneumococcal isolates (defined as penicillin MIC ≤ 0.06 μg/ml) isolated from NP carriers in the pediatric population of St. Louis, MO, USA 3. Definition of a high proportion of penicillin-susceptible isolates confirmed the appropriate use of low-dose amoxicillin (40-45 mg/kg per day) as first-line therapy for AOM in 2006 3, 4. Recently the American Academy of Pediatrics (AAP) recommended “high-dose” amoxicillin (90 mg/kg per day) for first-line therapy of AOM because of increasing penicillin resistance 4. The new guidelines posit that thirteen-valent PCV (PCV13) may result in reduced prevalence of antibiotic resistance among disease-causing pneumococcal isolates 4. Here, we performed a pneumococcal colonization prevalence study in the same geographic region as the 2006 study to evaluate trends in penicillin resistance following the introduction of PCV13 in 2010. Our objectives were to evaluate the prevalence of NP pneumococcal colonization and to assess the antimicrobial susceptibility profiles of pneumococcal isolates recovered.
Methods
Following approval by the Washington University Human Research Protection Office, informed consent for participation was obtained from the parents/legal guardians of otherwise healthy children aged 0 – 17 years requiring anesthesia for minor procedures (e.g. herniorrhaphies) in the St. Louis Children's Hospital Same Day Surgery center 5. Children with chronic illness, such as primary immunodeficiency or cystic fibrosis, were excluded. Children were also excluded if they were receiving an antibiotic, had an upper respiratory infection, a fever or hospital admission within the preceding 24 hours, or been previously enrolled. Vaccination coverage was determined by obtaining records from primary care pediatricians.
A single NP swab (E-swab Minitip, Copan) was obtained and eluate (100 μl) directly inoculated to 5% sheep's blood agar plates (BAP; BD Biosciences). To enrich for pneumococcus, 100 μl of eluate was also inoculated into 3 ml Todd Hewitt broth (BD Biosciences) containing 0.5% yeast (THY) extract and 0.5 ml rabbit serum. After incubation, 100 μl of inoculated broth was directly streaked onto BAP. Following overnight incubation at 35°C in 5% CO2, up to 12 α-hemolytic colonies were selected for analysis. Gram positive cocci in pairs and chains were determined to be pneumococci if they were catalase negative, optochin susceptible, and bile soluble 3.
Antibiotic susceptibility testing was performed in the Microbiology Laboratory at St. Louis Children's Hospital 6. The minimum inhibitory concentrations (MICs) of penicillin and cefotaxime were determined by gradient diffusion (Etest®, bioMérieux). Susceptibilities to erythromycin, clindamycin, sulfamethoxazole/trimethoprim, vancomycin, levofloxacin, and linezolid were determined by Kirby-Bauer disk diffusion. Chi-squared analyses were performed in GraphPad Prism. Confidence intervals (CI) were determined using the Wilson procedure for calculating the 95% CI for a binomial proportion.
Results
In a one-year period (July 2013 - June 2014), we enrolled 218 children, of whom 40 (18.3%; 95% CI 14-24%) were colonized with pneumococcus (Table). The prevalence of pneumococcal colonization of children < 2 years of age was 37% (95% CI 24-53%), of children aged 2- 6 years, 32% (95% CI 22-44%), and of children ≥ 7 years, 2.7% (95% CI 1-8%; p < 0.0001 by chi-squared test). The overall prevalence of colonization in children aged 6 years or younger was 34% (37 children colonized of 109 enrolled; 95% CI 26-43%). There was no significant correlation between receipt of ≥ 3 PCV vaccines and colonization (data not shown).
Table
| Age | Enrolled | Colonized (%; 95% CI) | Penicillin MIC ≤ 0.06 μg/ml (%; 95% CI) |
|---|---|---|---|
| < 2 years | 41 | 15 (37%; 24-52%) | 8 (53%; 30-75%) |
| 2 - 6 years | 68 | 22 (32%; 22-44%) | 10 (45%; 27-65%) |
| ≥ 7 years | 109 | 3 (2.7%; 1-8%)*** | 2 (67%; 21-94%) |
| Total | 218 | 40 (18.3%; 14-24%) | 20 (50%; 35-65%) |
Prevalence of NP pneumococcal colonization in children in the greater St. Louis area. Number of children enrolled and colonized are shown, grouped by age. Half of NP isolates demonstrated penicillin MICs of ≤ 0.06 μg/ml. Categorization of current isolates using the susceptibility criteria in use before 2006 allows direct comparison of our findings to those previously reported in our region 3. Prevalence is significantly lower in children ≥ 7 years than in children < 7 years old
p < 0.0001.
We determined the antimicrobial susceptibility profile (antibiogram) of the 40 carriage isolates recovered (Table, Figure; 6, 7). A statistically valid antibiogram requires a minimum of 30 isolates, as per guidelines established by the Clinical and Laboratory Standards Institute 7. To enable direct comparison to the earlier St. Louis study, we show the percentage of isolates with penicillin MIC ≤ 0.06 μg/ml (Table) 8. There was no significant difference in the penicillin susceptibility in different age groups, and we found that 20 (50%; 95% CI 35-65%) of current isolates had a penicillin MIC of ≤ 0.06 μg/ml (Table).
Figure.
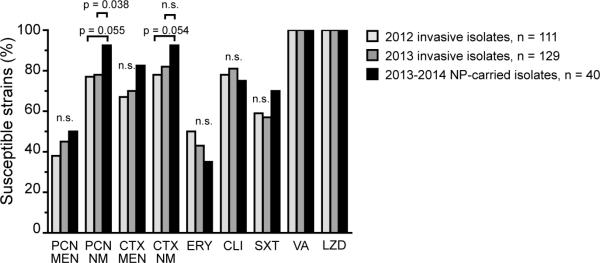
Percent of NP colonizing and invasive isolates susceptible to the indicated antibiotics, defined by 2014 CLSI guidelines 6, 7. PCN = penicillin, CTX = cefotaxime, ERY = erythromycin, CLI = clindamycin, SXT = sulfamethoxaxole/trimethroprim, VA = vancomycin, LZD = linezolid, MEN = meningitic breakpoint (PCN MIC ≤ 0.06 μg/ml, CTX MIC ≤ 0.5 μg/ml), NM = non-meningitic breakpoint (PCN MIC ≤ 2 μg/ml, CTX MIC ≤ 1 μg/ml).
Finally, we compared the antibiotic susceptibility profiles of the colonizing isolates to those of invasive pneumococcal isolates recovered from clinical specimens (blood, CSF, tracheal aspirates and bronchoalveolar lavage specimens) at St. Louis Children's Hospital 6 (Figure). The antibiotic susceptibility profile of colonizing isolates was similar to the 2012 and 2013 profiles of invasive isolates (Figure). The only significant difference between colonizing and invasive isolates was that the percentage of colonizing isolates that were penicillin susceptible under nonmeningitic conditions using intravenous administration (PCN-S-NM; p = 0.038).
Discussion
Our study is consistent with other studies indicating that PCV does not reduce overall pneumococcal carriage prevalence 1, 2, 9. We find that the prevalence of pneumococcal colonization in children under 7 years old has not been reduced from the prevalence of 39% (129 children colonized of 327 enrolled; 95% CI 26-43%) identified in the prior study 3. We also found no increase in penicillin susceptibility following the introduction of PCV13, as the proportion of isolates with penicillin MIC ≤ 0.06 μg/ml (50%) was lower than the proportion of 67% previously found 3. The stable prevalence of pneumococcal colonization and unchanged proportion of isolates with penicillin MIC ≤ 0.06 μg/ml are observed even though the study populations were slightly different; we excluded children with symptoms of an upper respiratory infection (URI) while they were included in the prior study 3. As URI symptoms are a risk factor for pneumococcal colonization 2, a lower prevalence in our study might have been expected based on this population difference. Our results contrast with recent studies performed in Israel and Atlanta, GA, USA, in which receipt of PCV13 increased the proportion of penicillin-susceptible isolates among colonizing isolates 2, 10. Colonizing isolates found in St. Louis were also more resistant to penicillin than those in a recent cross-sectional study performed in Milan 1. Higher penicillin MICs of NP pneumococci in St. Louis compared to those in other areas may be due to greater antibiotic use in the U.S., though confirmation of a causal relationship requires further study.
With the exception of slightly higher prevalence of beta-lactam-susceptible colonizing isolates, the antibiotic susceptibility profiles of colonizing isolates closely approximate those of invasive isolates, demonstrating that population surveys of colonizing isolates can be used to determine local choice of empiric antibiotic therapy. In summary, we have surveyed the prevalence of pneumococcal colonization in the pediatric population of the St. Louis area, finding no change in prevalence and, more importantly, no evidence of an increasing prevalence of penicillin-susceptible isolates following introduction of PCV13.
Highlights.
Prevalence of pneumococcal colonization in a U.S. pediatric population is 34%.
The 13-valent pneumococcal conjugate vaccine (PCV13) did not alter prevalence
PCV13 did not increase the prevalence of penicillin-susceptible isolates
Acknowledgements
The authors thank Phillip Tarr, Stephanie Fritz and Steen Hoffmann for critical review of the manuscript, and Patricia Sellenriek of the SLCH Microbiology Laboratory for advice and assistance. The authors also thank the faculty of the Washington University School of Medicine Department of Surgery and the Same Day Surgery nursing staff of St. Louis Children's Hospital for facilitating the enrollment of patients. Funding for the study was provided by the Children's Discovery Institute in St. Louis (grant number PD-II-2013-295 and PD-II-2014-366). S.C.M. is also a Scholar of the Child Health Research Center of Excellence in Developmental Biology at Washington University School of Medicine (grant number K12-HD01487).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zuccotti G, Mameli C, Daprai L, Garlaschi ML, Dilillo D, Bedogni G, Faccini M, Gramegna M, Torresani E, PneuMi Study G, Ballerini E, Benincaso A, Bonvissuto M, Bricalli D, Brioschi M, Calloni CS, Camiletti MI, Colella G, De Angelis L, Decarlis S, Di Nello F, Dozzi M, Galli E, Gandini V, Giuliani MG, Laviola F, Loda B, Macedoni M, Mazzucchi E, Metta MG, Moscatiello A, Nannini P, Petruzzi M, Picicco D, Picciotti M, Pisanelli S, Porta N, Ramponi G, Redaelli F, Rubini R, Sala N, Saitta V, Scelza G, Tiso RM, Tomasetto M, Torcoletti M, Travaini M, Valentini M, Vessia C. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine. 2014;32:527–34. doi: 10.1016/j.vaccine.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Desai AP, Sharma D, Crispell EK, Baughman W, Thomas S, Tunali A, Sherwood L, Zmitrovich A, Jerris R, Satola S, Beall B, Moore MR, Jain S, Farley MM. Decline in Pneumococcal Nasopharyngeal Carriage of Vaccine Serotypes After the Introduction of the 13-Valent Pneumococcal Conjugate Vaccine in Children in Atlanta, Georgia. Pediatr Infect Dis J. 2015 doi: 10.1097/INF.0000000000000849. in press. [DOI] [PubMed] [Google Scholar]
- 3.Garbutt J, Rosenbloom I, Wu J, Storch GA. Empiric first-line antibiotic treatment of acute otitis in the era of the heptavalent pneumococcal conjugate vaccine. Pediatrics. 2006;117:e1087–94. doi: 10.1542/peds.2005-2651. [DOI] [PubMed] [Google Scholar]
- 4.Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, Joffe MD, Miller DT, Rosenfeld RM, Sevilla XD, Schwartz RH, Thomas PA, Tunkel DE. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964–99. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 5.Colvin JM, Muenzer JT, Jaffe DM, Smason A, Deych E, Shannon WD, Arens MQ, Buller RS, Lee WM, Weinstock EJ, Weinstock GM, Storch GA. Detection of viruses in young children with fever without an apparent source. Pediatrics. 2012;130:e1455–62. doi: 10.1542/peds.2012-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute; Wayne, PA: 2014. [Google Scholar]
- 7.Clinical and Laboratory Standards Institute . Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data; Approved Guideline -- Fourth Edition. CLSI document M39-A4. Clinical and Laboratory Standards Institute; Wayne, PA: 2014. [Google Scholar]
- 8.Weinstein MP, Klugman KP, Jones RN. Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with antimicrobial susceptibility in an era of resistance. Clin Infect Dis. 2009;48:1596–600. doi: 10.1086/598975. [DOI] [PubMed] [Google Scholar]
- 9.van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MP, Harrison TG, Ladhani SN, Miller E. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine. 2014;32:4349–55. doi: 10.1016/j.vaccine.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Dagan R, Juergens C, Trammel J, Patterson S, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible Streptococcus pneumoniae. J Infect Dis. 2015;211:1144–53. doi: 10.1093/infdis/jiu576. [DOI] [PubMed] [Google Scholar]


