Abstract
Background
Idiopathic pulmonary fibrosis is a progressive interstitial lung disease with no current effective therapies. Treatment has focused on antifibrotic agents to stop proliferation of fibroblasts and collagen deposition in the lung. We present the first clinical trial data on the use of losartan, an antifibrotic agent, to treat idiopathic pulmonary fibrosis. The primary objective was to evaluate the effect of losartan on progression of idiopathic pulmonary fibrosis measured by the change in percentage of predicted forced vital capacity (%FVC) after 12 months. Secondary outcomes included the change in forced expiratory volume at 1 second, diffusing capacity of carbon monoxide, 6-minute walk test distance, and baseline/transition dyspnea index.
Methods
Patients with idiopathic pulmonary fibrosis and a baseline %FVC of ≥50 % were treated with losartan 50 mg by mouth daily for 12 months. Pulmonary function testing, 6-minute walk, and breathlessness indices were measured every 3 months.
Results
Twenty participants with idiopathic pulmonary fibrosis were enrolled and 17 patients were evaluable for response. Twelve patients had a stable or improved %FVC at study month 12. Similar findings were observed in secondary end-point measures, including 58, 71, and 65 % of patients with stable or improved forced expiratory volume at 1 second, diffusing capacity for carbon monoxide, and 6-minute walk test distance, respectively. No treatment-related adverse events that resulted in early study discontinuation were reported.
Conclusion
Losartan stabilized lung function in patients with idiopathic pulmonary fibrosis over 12 months. Losartan is a promising agent for the treatment of idiopathic pulmonary fibrosis and has a low toxicity profile.
Keywords: Pulmonary fibrosis, Angiotensin receptor blocker, Forced vital capacity, Dyspnea, Six-minute walk test
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disorder with no identifiable cause or proven effective treatment [1]. Even though IPF is considered rare, it is the most common idiopathic interstitial lung disease and has both high morbidity and mortality. The median survival of patients with IPF is 2–4 years, which has not changed over the past decade [2, 3]. There is considerable evidence that angiotensin II (AII) is involved in multiple models of fibrosis. Angiotensin II is known to activate the angiotensin II type 1 receptor, inducing transforming growth factor expression [4, 5], which stimulates lung fibroblast proliferation and lung procollagen production. Losartan’s ability to alleviate fibrosis by reducing the expression of transforming growth factor β (TGF-β) has been demonstrated in multiple animal and human studies [6–22]. This study’s main hypothesis was that losartan would attenuate progression of pulmonary fibrosis. We conducted a prospective 12 month uncontrolled pilot project, evaluating the potential of losartan to improve or stabilize lung function in patients with IPF. This study design was chosen to provide preliminary data to evaluate the efficacy and feasibility of using losartan in this rare patient population. We report our promising data to support conducting a larger placebo-controlled phase II study in IPF.
Methods
Patients with a diagnosis of IPF, based on the ATS/ERS international consensus statement [1], participated in this pilot project at the University of Cincinnati, Cincinnati, OH. Ethical/IRB approval was obtained prior to enrollment of patients. Twenty patients with IPF were followed for 12 months while taking losartan daily. Patients ≥21 years old with IPF, a baseline forced vital capacity (FVC) greater than or equal to 50 %, and a baseline 6-minute walk test (6MWT) distance greater than or equal to 200 m were included in the study. Patients who needed more than 6 L/min of oxygen to maintain SpO2 greater than 88 % during the 6MWT, were currently smoking or had quit within 6 months of study entry, had a lung transplant, or were taking any angiotensin II receptor blocker were excluded. Clinical use of non-angiotensin II receptor blocker antihypertensive agents was permitted during the study. Losartan 50 mg was administered orally once daily for 12 months. It was supplied at no cost to the patient or the enrolling center. Patients completed a daily log documenting when the study drug was taken and any potential side effects. Daily logs were reviewed at each study visit to identify adverse events or drug compliance issues. To specifically monitor for losartan-induced hypotension, patients also completed daily blood pressure diaries. Patients measured their blood pressure twice weekly while taking losartan and recorded the values in a blood pressure diary. The diaries were reviewed by research staff at study week 1, study week 4, and at each follow-up visit thereafter. Study visits occurred every 3 months for 12 months. These outpatient visits included a history and physical exam, urine pregnancy test if applicable, spirometry, lung volumes, diffusing capacity for carbon monoxide (DLCO), 6-minute walk test (6MWT), and assessment of dyspnea using the Baseline Dyspnea Index (BDI)/Transition Dyspnea Index (TDI). The BDI grades the severity of dyspnea at baseline. The TDI documents changes in dyspnea from a baseline assessment.
The primary end point of this study was change in %FVC from baseline to month 12. Secondary end points included other markers of lung function, including percent of predicted forced expiratory volume at 1 second (%FEV1), percent of predicted DLCO (%DLCO), and 6MWT distance. We also followed dyspnea scores using the BDI/TDI. The primary response assessment was stable or improved %FVC. We defined stable FVC as a −5 % change in %FVC from baseline up to a +5 % change from baseline due to the known variability of the test [23]. An improved FVC was defined as a greater than 5 % increase in the %FVC after 12 months of treatment. Improvement in secondary measures of pulmonary function was defined as a 10 % or greater increase in predicted values of %FEV1 and %DLCO, and a minimal importance difference (MID) of distance walked in the 6MWT of 28 m [24]. Patients who continued on the losartan for at least 6 months were evaluable for response. The change in %FVC was the primary study end point and determined the planned sample size. In addition, responders were compared to nonresponders in terms of %FEV, %DLCO, 6-minute walk test distance, and degree of dyspnea on the baseline and transition dyspnea index. These supplementary analyses were descriptive and secondary to the primary study question, which was to assess efficacy. Thus, neither power considerations nor adjustments for multiple comparisons were planned. Response rates and confidence intervals were constructed using exact methods. All the tests were two-sided with a significant level of 0.05. Probability (p) value <0.05 was considered to be statistically significant. Statistical analyses were performed using SAS ver. 9.2 (SAS Institute Inc., Cary, NC).
Results
Study Subjects
Twenty patients were accrued between April 2009 and October of 2010; 17 patients were evaluable for response. Of the three patients not evaluable, one had undergone a lung transplant; one had enrolled in the study but did not start losartan, and one had died due to complications associated with disease after 6 months in the study. Patient demographics and baseline characteristics are summarized in Table 1. The study population was predominantly male with a mean age of 67 years. Most patients had moderate lung disease at baseline, with a mean %FVC of 73.96 %, mean %DLCO of 43.65 %, and an average 6MWT distance of 464.89 m. Nine of the 20 patients enrolled required chronic O2 supplementation at baseline.
Table 1.
Baseline characteristics of the study population (N = 17)
| Age [mean (range)] (years) | 67 (58–81) |
| M:F | 13:4 |
| FVC% predicted (mean ± SD) | 73.96 ± 3.76 |
| FEV1% predicted (mean ± SD) | 85.44 ± 3.79 |
| DLCO% predicted (mean ± SD) | 43.65 ± 16.91 |
| 6MWT distance (mean ± SD) (m) | 464.89 ± 249.54 |
FVC forced vital capacity, FEV1 forced expiratory volume 1 second, DLCO diffusing capacity for carbon monoxide
Efficacy Assessment
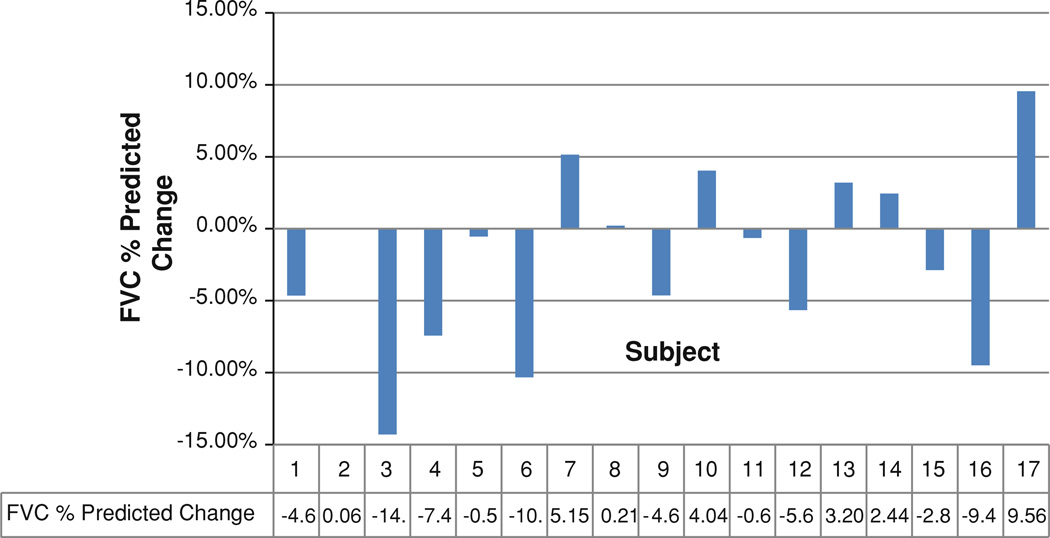
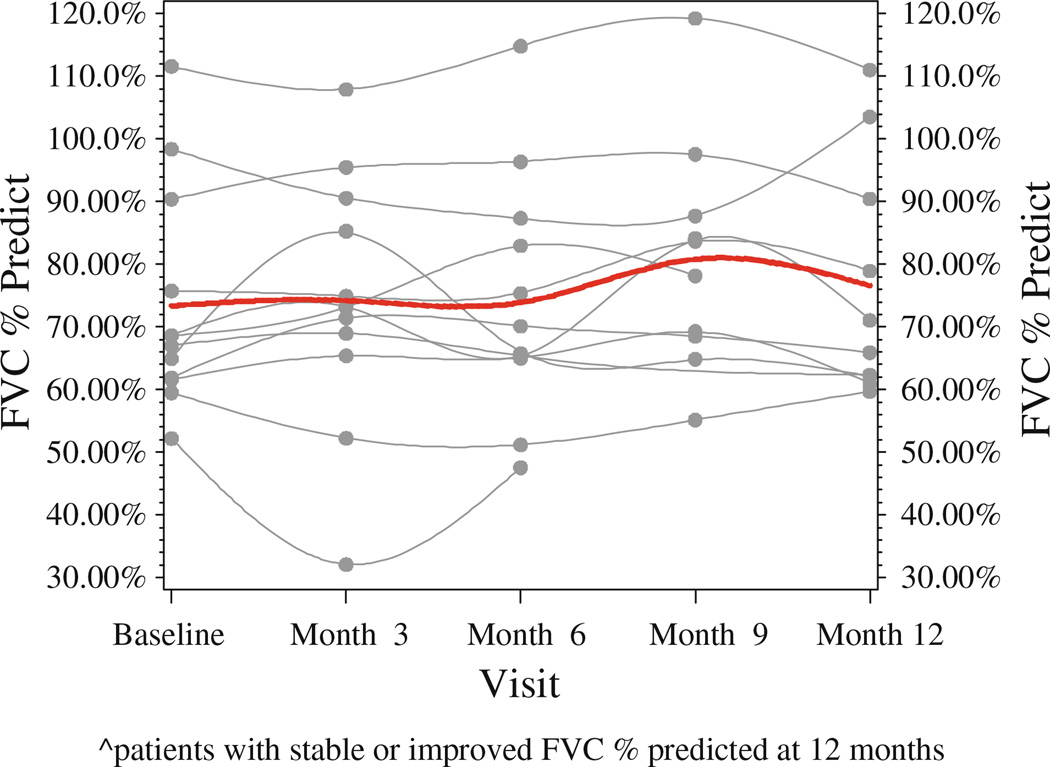
Twelve of 17 patients (71 %) had a stable or improved %FVC after taking losartan for 12 months. Figure 1 depicts the changes in %FVC for all participants. Figure 2 depicts the changes in %FVC for each responder at 3, 6, 9, and 12 months, with the red line showing the mean change in %FVC for all responders over the course of the study. No significant difference was observed between the baseline %FVC of the responders and nonresponders (p = 0.82). Of the five patients who did not respond to losartan therapy, their mean %FVC was 75.34 (±9.70), which was similar to the baseline %FVC of the responders, 73.39 (±17.71).
Fig. 1.
Change in FVC % predicted over 12 months of losartan therapy
Fig. 2.
Change in FVC % predicted at 3, 6, 9, and 12 months of losartan therapy for responders (patients with stable or improved FVC % predicted at 12 months) and their mean FVC % predicted change over time
Similar results were observed for secondary outcomes, which are presented in Table 2: 58, 71, and 65 % of patients had a stable or improved %FEV1, %DLCO, and 6MWT distance, respectively, at 12 months when compared with their baseline values.
Table 2.
Change in secondary markers of lung function over 12 months of losartan therapy
| N (%) | FEV1 | DLCO | 6MWT distance |
|---|---|---|---|
| Stable | 8 (47.06) | 10 (58.82) | 6 (35.29) |
| Improved | 2 (11.76) | 2 (11.76) | 5 (29.41) |
| Deteriorated | 7 (41.18) | 5 (29.42) | 6 (−1.38) |
FEV1 forced expiratory volume 1 second, DLCO diffusing capacity for carbon monoxide
At the end of study, 7 of 17 (29.41 %) patients had a stable or improved transition dyspnea score (less dyspnea) compared to the baseline dyspnea score, while 10 patients (58.82 %) reported more dyspnea (decreased transition dyspnea scores) compared to baseline evaluations.
Safety Assessment
Losartan was well tolerated in this patient population. Of the 17 patients who received losartan for 12 months, none discontinued the study agent because of side effects. The only side effects experienced included a rash in one patient and fatigue in another that resolved shortly after presentation. One patient died after 6 months in the study due to complications of IPF and the death was deemed unrelated to the study agent. There were no episodes of asymptomatic or symptomatic hypotension documented for any patients in the study.
Discussion
Losartan is an innovative antifibrotic agent that had not been used prospectively in a clinical trial prior to this study. Our open-label pilot project prospectively evaluated losartan in 20 patients with a diagnosis of IPF. We chose a dose of 50 mg because of multiple published studies documenting losartan’s successful antifibrotic properties at 50 mg in hepatic, renal, and myocardial fibrosis [19–22, 25, 26]. Losartan may have attenuated the progression of the disease in over 50 % of patients with IPF. The primary objective of FVC as well as other markers of lung function, including FEV1, was in most cases stable in over 50 % of patient after 12 months. Patients with stable or improved lung function also walked a greater distance for the 6MWT as seen with the 5 of 17 patients who walked more than 28 m when compared to baseline and with another 6 of 17 patients who had a stable 6MWT distance after taking losartan for 12 months. We chose the baseline and transition dyspnea indexes to follow patient-reported outcomes of the most common symptom seen in patients with IPF that dramatically affects their quality of life. This index measures a patient’s degree of breathlessness related to their activities of daily living, including their functional impairment and their magnitude of task and effort. Two patients had an improvement in their dyspnea index after 12 months of losartan therapy and 5 patients had a stable dyspnea index. The two patients with improvement in their dyspnea index also had an improvement in their FVC, FEV1, and 6MWT distance at 12 months.
Losartan was well tolerated in our study. With only two patients with documented minimal side effects of rash and fatigue, which were self-limited, and no serious adverse events related to losartan, its safety is comparable to placebo. It was also safe to add to antihypertensive regimens without resulting in episodes of hypotension, which was closely monitored throughout the study.
The results of this prospective pilot project are promising and provide support for a future phase II study, which is necessary to establish losartan’s effect on IPF. While the small number of patients and the lack of controls prevent establishment of statistically significant efficacy, the number of patients with stable or improved lung function is hopeful for this rare progressive disease.
The results of this study are encouraging when compared to those of previous pilot studies that evaluated new antifibrotic agents to treat IPF. Agents such as etanercept, a human tumor necrosis factor receptor, interferon-γ1b, and tyrosine kinase inhibitors such as imatinib showed no difference in their predefined end points involving survival and/or pulmonary function [27–29]. Agents such as bosentan, an endothelin receptor antagonist that did not show superiority over placebo in the primary end point of the 6-minute walk distance up to month 12, still prompted further evaluation in phase III studies [30, 31].
The most recent agent with the potential to treat IPF is pirfenidone. This antifibrotic agent was evaluated in a pilot study in 1999 and it showed the possible trends of a stable FVC in patients with IPF [32]. Therefore, further testing was encouraged and recently completed using the CAPACITY trial. The published results include a reduction in the decline of FVC of −8.5 % in the pirfenidone group compared to a reduction of −11 % in the placebo group [33]. Although research with these novel agents has been completed, at this time the results do not recommend them for treating IPF [34].
IPF remains a fatal disease with significant morbidity and a mortality rate that has not changed over the past decade. Unfortunately, a safe and efficacious agent for treatment has not yet been identified. As we look to the future for novel antifibrotic therapies, losartan is a promising agent that is taken orally once a day. With few side effects and no symptoms that warranted discontinuation of the drug, this study provides further support for the safety of this agent. The potential attenuation of disease progression reported in this article makes losartan a reasonable agent to evaluate in a phase II placebo-controlled multicenter trial.
Acknowledgments
This work was supported by the SunCoast CCOP Research Base (award No. U10CA081920 from the National Cancer Institute). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflict of interest The authors have no conflicts of interest or financial ties to disclose.
Ethical standards The experiment complies with the current laws of the United States of America.
Contributor Information
Marisa Couluris, Email: mcouluri@health.usf.edu, Division of Pulmonology, Department of Pediatrics, University of South Florida College of Medicine, Tampa, FL, USA; Department of Pediatrics, University of South Florida, 3650 Spectrum Blvd., Suite 100, Tampa, FL 33612, USA.
Brent W. Kinder, Division of Pulmonary and Critical Care, Department of Medicine, University of Cincinnati, Cincinnati, OH, USA
Ping Xu, Division of Epidemiology, Department of Pediatrics, University of South Florida College of Medicine, Tampa, FL, USA.
Margaret Gross-King, Division of Epidemiology, Department of Pediatrics, University of South Florida College of Medicine, Tampa, FL, USA.
Jeffrey Krischer, Division of Epidemiology, Department of Pediatrics, University of South Florida College of Medicine, Tampa, FL, USA.
Ralph J. Panos, Division of Pulmonary and Critical Care, Department of Medicine, University of Cincinnati, Cincinnati, OH, USA
References
- 1.ATS/ERS. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, Offord KP. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 3.King TE, Jr, Tooze JA, Schwarz MI. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 4.Marshall RP, Gohlke P, Chambers RC, et al. Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L156–L164. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- 5.Papp M, Li X, Zhuang J, et al. Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am J Physiol Lung Cell Mol Physiol. 2002;282:L714–L718. doi: 10.1152/ajplung.00103.2001. [DOI] [PubMed] [Google Scholar]
- 6.Yao HW, Zhu JP, Zhao MH, Lu Y. Losartan attenuates bleomycin-induced pulmonary fibrosis in rats. Respiration. 2006;73(2):236–242. doi: 10.1159/000090140. [DOI] [PubMed] [Google Scholar]
- 7.Molteni A, Moulder JE, Cohen EF, et al. Control of radiation- induced pneumopathy and lung fibrosis by angiotensinconverting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76(4):523–532. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Rayford H, Uhal BD. Essential roles for angiotensin receptor AT1a in bleomycin-induced apoptosis and lung fibrosis in mice. Am J Pathol. 2003;163:2523–2530. doi: 10.1016/S0002-9440(10)63607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei H, Li D, Lu H, et al. Effects of angiotensin II receptor blockade on hepatic fibrosis in rats. Zhonghua Gan Zang Bing Za Zhi. 2000;8(5):302–304. [PubMed] [Google Scholar]
- 10.Wei HS, Li DG, Lu HM, et al. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl(4) World J Gastroenterol. 2000;6(4):540–545. doi: 10.3748/wjg.v6.i4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei YH, Jun L, Qiang CJ. Effect of losartan, an angiotensin II antagonist, on hepatic fibrosis induced by CCl4 in rats. Dig Dis Sci. 2004;49(10):1589–1594. doi: 10.1023/b:ddas.0000043369.88701.5b. [DOI] [PubMed] [Google Scholar]
- 12.Molina-Molina M, Serrano-Mollar A, Bulbena O, et al. Losartan attenuates bleomycin-induced lung fibrosis by increasing prostaglandin-E2 synthesis. Thorax. 2006;61:604–610. doi: 10.1136/thx.2005.051946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park DH, Baik SK, Choi YH, et al. Inhibitory effect of angiotensin blockade on hepatic fibrosis in common bile ductligated rats. Korean J Hepatol. 2007;13(1):61–69. [PubMed] [Google Scholar]
- 14.Croquet V, Moal F, Veal N, et al. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol. 2002;37(6):773–780. doi: 10.1016/s0168-8278(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 15.Kellner D, Chen J, Richardson I, et al. Angiotensin receptor blockade decreases fibrosis and fibroblast expression in a rat model of unilateral ureteral obstruction. J Urol. 2006;172(2):806–812. doi: 10.1016/j.juro.2006.03.076. [DOI] [PubMed] [Google Scholar]
- 16.Moulder JE, Fish BL, Cohen EP. Angiotensin II receptor antagonist in the treatment and prevention of radiation nephropathy. Int J Radiat Biol. 1998;73(4):415–421. doi: 10.1080/095530098142257. [DOI] [PubMed] [Google Scholar]
- 17.Liang X, Xie X, Yang T, Sun M, Zhao S. Apoptosis, myocardial fibrosis and angiotensin II in the left ventricle of hypertensive rats treated with fosinopril or losartan. Chin Med J (Engl) 2002;115(9):1287–1291. [PubMed] [Google Scholar]
- 18.Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, Marian AJ. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sookoian S, Fernández MA, Castaño G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: a pilot study. World J Gastroenterol. 2005;11(48):7560–7563. doi: 10.3748/wjg.v11.i48.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibasaki Y, Nishiue T, Masaki H, Tamura K, Matsumoto N, Mori Y, et al. Impact of the angiotensin II receptor antagonist, losartan, on myocardial fibrosis in patients with end-stage renal disease: assessment by ultrasonic integrated back-scatter and biochemical markers. Hypertens Res. 2005;28:787–795. doi: 10.1291/hypres.28.787. [DOI] [PubMed] [Google Scholar]
- 21.Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;106(25):12–17. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 22.Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40(5):1222–1225. doi: 10.1002/hep.20420. [DOI] [PubMed] [Google Scholar]
- 23.Enright PL, Beck KC, Sherrill DL. Repeatability of spirometry in 18,000 adult patients. Am J Respir Crit Care Med. 2004;169:235–238. doi: 10.1164/rccm.200204-347OC. [DOI] [PubMed] [Google Scholar]
- 24.Swigris JJ, Wamboldt FS, Behr J, du Bois RM, King TE, Raghu G, Brown K. The 6 minute walk in idiopathic pulmonary fibrosis: longitudinal changes and minimum important difference. Thorax. 2010;65:173–177. doi: 10.1136/thx.2009.113498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 26.López G, Querejeta R, Larman M, Díez J. Myocardial COX-2 overexpression and fibrosis in hypertensive heart disease. Am J Hypertens. 2005;18(5 Pt 2):A223–A233. [Google Scholar]
- 27.Raghu G, Brown KK, Bradford WZ, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;250(2):125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 28.Raghu G, Brown KK, Costabel U, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178(9):948–955. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 29.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181(6):604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 30.King TE, Jr, Behr J, Brown KK, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(1):75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 31.King TE, Jr, Brown KK, Raghu G, duBois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, Behr J. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 32.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open label phase II study. Am J Respir Crit Care Med. 1999;159:1061–1069. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 33.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 34.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]