Abstract
Sex differences in stress responses can be found at all stages of life and are related to both the organizational and activational effects of gonadal hormones and to genes on the sex chromosomes. As stress dysregulation is the most common feature across neuropsychiatric diseases, sex differences in how these pathways develop and mature may predict sex-specific periods of vulnerability to disruption and increased disease risk or resilience across the lifespan. The aging brain is also at risk to the effects of stress, where the rapid decline of gonadal hormones in women combined with cellular aging processes promote sex biases in stress dysregulation. In this Review, we discuss potential underlying mechanisms driving sex differences in stress responses and their relevance to disease. Although stress is involved in a much broader range of diseases than neuropsychiatric ones, we highlight here this area and its examples across the lifespan.
Maintenance of homeostasis depends on the tight orchestration of factors involved in the response to stress and its recovery, where sex differences exist at all levels. Regulation of the stress axis involves the coordination between multiple brain regions guiding the magnitude of the stress response as well as the negative feedback necessary for its return to baseline. At its most fundamental level, the hypothalamic-pituitary-adrenal (HPA) stress axis is driven by corticotropin-releasing factor (CRF) neurons in the paraventricular nucleus of the hypothalamus (PVN) that upon stress activation release CRF into the hypophyseal portal circulation. CRF activation of corticotrope cells in the anterior pituitary results in a release of adrenocorticotropic hormone (ACTH) into the general circulation to activate melanocortin-2 receptors in the adrenal gland cortex, which activates the synthesis and release of glucocorticoids1,2. These glucocorticoids, largely through binding of the glucocorticoid receptor (GR), promote a cascade of catabolic actions in peripheral tissues, providing the necessary fuel availability to appropriately respond to the environmental perturbation. Finally, glucocorticoids provide the critical negative feedback on the HPA axis to ensure a return to a homeostatic state. At the hypothalamic level, CRF neurons are tightly regulated by a host of neurotransmitter and neuromodulatory systems, including serotonin (5-HT), GABA, glutamate and norepinephrine3,4. Vasopressin release from the PVN can also augment the stress-mediated release of ACTH from the pituitary5. Significant and important differences between males and females are present at all points along the HPA axis, including inputs to the PVN, neuromodulatory regulation of CRF neurons, and the relative size and steroidogenic activity of the adrenal gland cortex6,7. Such differences are largely driven by gonadal hormone changes that occur over dynamic periods of development and maturation, contributing to sex-specific stress responses and vulnerabilities across the lifespan8.
Stress dysregulation programmed during sensitive periods in brain development and maturation can result in future sensitivity, and is associated with an increased disease risk, including for a large number of neuropsychiatric diseases9–11. Therefore, the continued ability across the lifespan to respond appropriately to stress is a necessary component in disease prevention12. Sex differences in stress responses can be found at all stages of life, and these differences are related to both the organizational and activational effects of gonadal hormones and to genes found on the sex chromosomes (Fig. 1)13–18. During gestation, sex differences in placental and embryonic responses to maternal stress and environmental perturbations are well documented in humans, where males are at a greater risk for short-term and long-term negative outcomes19–21. In contrast, during childhood, adversity appears to preferentially increase the risk for affective disorders in women, especially during their reproductive years (Fig. 2)21–25. In aging, ovarian senescence contributes to sex differences in stress responsivity and stress-related neuropsychiatric disease risk26–28.
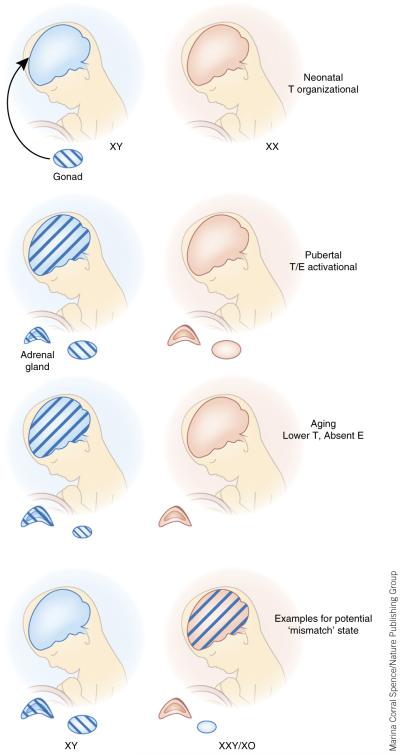
Figure 1.
Programming and maturation of the sexually dimorphic brain and importance in lifelong sex differences in stress circuitry. Exposure of the male (XY) neonatal brain to estrogen (E) via central aromatization of testosterone (T) produced by the testes during this developmental window sets the stage for sex differences throughout life. Perturbations such as maternal stress can disrupt testosterone production and its ability to fully masculinize the brain, resulting in a mismatch between the gonad and brain later in life when activational T levels are present. Genes expressed on the X and Y chromosomes also drive differences in neurodevelopment and stress neurocircuitry that will result in pushing the male and female brain and HPA axis stress response further apart, these may include epigenetic modifiers such as miRNAs abundant on the X chromosome and histone demethylases on the Y. Studies in children with Turner syndrome (XO) and Klinefelter syndrome (XXY) indicate that X-linked gene dosage is important in morphological development of brain regions involved in stress regulation. During puberty, activational gonadal hormones produced in males and females further act on the blueprint established during early life to mature the sexually dimorphic brain. During this stage, T in the male also reduces the adrenal gland size and steroidogenic activity to promote an additional level for sex differences in stress responses. The aging brain receives significantly lower levels of gonadal hormones in women, and this, combined with additional cellular aging processes, produces unique sex differences in stress responsivity at this life stage. For women, there is an additional peak at this stage of life for presentation of stress-related disorders including schizophrenia and depression.
Figure 2.
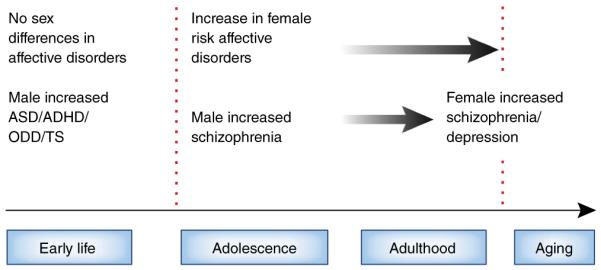
Sex differences in stress-related neuropsychiatric disease across the lifespan. Sex differences in response to prenatal and early life stress put males at an increased risk to present with neurodevelopmental disorders including ASD, ADHD, oppositional defiant disorder (ODD) and Tourette syndrome (TS). Males are also at greater risk of early onset schizophrenia. Although there are no sex differences in affective disorders before puberty, females show an increased risk in adolescence and throughout adulthood, especially if they have experience stress-related early life adversity or trauma. During peri-menopause and menopausal transition, women are at greater risk for presenting with schizophrenia, and affective disorders including depression.
Dysregulation of stress neurocircuitry is the most common feature across neuropsychiatric diseases, with both hyper- and hypo-reactivity of the HPA stress axis being reported17,29–31. Closer examination of sex differences in stress responsivity may provide unique insight as to the possible points of vulnerability in brain development and maturation in which disruptions in stress pathways can occur32. In rodents, females display a significantly greater physiological stress response than males as noted by elevated corticosterone levels after exposure to a variety of stressors7. This is largely due to an overall suppression of the HPA axis in males by activational testosterone after puberty. Assessing the impact of sex on the human HPA-axis response to an acute stressor is more complicated and is modified by age, oral contraceptive use, exercise and overall health33,34. At a molecular level, studies have identified effects of gonadal hormones on sex differences in stress responsivity via regulation of neurotransmitter systems including 5-HT, norepinephrine, and CRF receptor expression and internalization6,7,35–42. Further, similar sex differences exist in behavioral stress responses in which female rodents use more passive strategies in response to stress, such as increased immobile time in the forced-swim and tail-suspension tests compared to males43. In humans, such passive coping responses to stress are associated with the presentation of depressive symptoms and are more common in women44. To this point, functional magnetic resonance imaging (MRI) analyses in women have reported fluctuations across the menstrual cycle in response to emotional stimuli in the orbito-frontal cortex, a brain region important in affect determination and stress regulation45,46.
In addition to the organizational effects of gonadal hormones, sex chromosomes contribute to the developmental programming of the sexually dimorphic brain and its response to the activational effects of reproductive hormones later in life. In humans, observations of the brain and behavioral impact of sex chromosome abnormalities aid our understanding of the specific contributions of the X and Y chromosomes to sex differences. Brain studies comparing children in early puberty with Turner syndrome (XO) and Klinefelter syndrome (XXY) to age matched controls revealed a dose-dependency of the X chromosome to decreased parieto-occipital and increased temporo-insular gray matter volumes, suggesting an involvement of X-linked genes in structural brain development47,48. Another study reported smaller hippocampal volumes in XXY boys compared to age-matched controls49. Boys with XYY karyotypes do not present with a detectable phenotype, have normal behavior and cognitive abilities, and normal serum testosterone levels. Together, these findings suggest that while the dosage of X-linked genes is critical in normal development, this is less clear for Y-linked genes. However, it should be noted that it is inherently difficult to completely dissect the sex hormone and sex chromosome effects on the developing brain as both XO and XXY children are also typically deficient in gonadal hormone production. Further, the testis-determining factor, SRY, is a Y chromosome gene and a necessary transcription factor for the bipotential gonad to develop into a testis, a necessity for testosterone production during neonatal and pubertal developmental organizational and maturational periods50. Therefore, if SRY is absent, the testes are as well, and no testosterone is produced during neonatal development to masculinize the male brain.
Prenatal environment: stress and sex
Stress experienced during gestation is a risk factor in neurodevelopmental and neuropsychiatric disorders, including depression, anxiety, schizophrenia and autism spectrum disorder (ASD)17,30. The mechanisms through which fetal antecedents such as exposure to maternal psychosocial stress contribute to disease development involve complex interactions between dynamic changes within the maternal environment, the placenta and the developing embryo. Sex differences are important to consider in this equation of gene × environment × developmental period = programming outcomes at all levels. Certainly, numerous neurodevelopmental disorders exhibit strong sex biases, including ASD with an overall sex ratio of 4:1 for boys:girls (as reviewed in refs. 51–53) as well as attention deficit hyperactivity disorder (ADHD) with a male-to-female sex ratio of 3.2:1 (reviewed in ref. 54).
The timing of maternal stress experience over the course of pregnancy and its long-term impact on neurodevelopmental disorder risk is also sex-specific. For example, women exposed during their second trimester of pregnancy to the stress of the 1940 invasion of The Netherlands had male, but not female, children with an increased risk of schizophrenia55. A significant association between maternal psychosocial stress experienced during the first trimester of pregnancy has also been reported to significantly increase the risk of schizophrenia, again only in male children56. Further, exposure to maternal stress before 32 weeks of gestation is a known contributing factor in ASD, also a male-biased neurodevelopmental disorder57. A recent report detected a significant effect of maternal depression during pregnancy on offspring postnatal anxiety development, an effect that was again significant only in boys58. These studies support the importance of fetal sex in the association between maternal stress and offspring long-term disease risk. Although there are multiple factors likely contributing to sex differences in disease, these studies support the premise that sex-specific responses to fetal antecedents may promote a divergence in neurodevelopmental trajectories. For example, MRI in male and female patients with schizophrenia confirmed a disruption of the normal brain sexual dimorphisms (in which specific brain regions are known to be larger or smaller in one sex), including the orbitofrontal cortex:amygdala ratio, where male schizophrenic patients showed a phenotypically more female-like pattern in these brain regions15,59–62. These outcomes support the hypothesis that fetal antecedents, such as maternal stress, can disrupt normal sex-specific organizational trajectories and patterns. Such studies also reinforce our understanding that sex differences begin in utero.
Disease risk is considered then to be a product of a potential mismatch between this hormonal organizational programming involved in determining the sexually dimorphic brain (as discussed below and shown in Fig. 1) and the activational hormones then produced beginning in puberty. For example, if maternal stress disrupts a male fetus’s testosterone production during late gestation, that male’s brain will not be fully masculinized and thus as an adult may appear to functionally be less male-like (which is not the same as being more female). Then, during puberty, when the testes begin producing adult levels of testosterone, the activational mediators and functional endpoints in the brain having not been appropriately programmed, do not match, and the potential for dysregulation occurs between what an XY brain should look and function like.
Animal models of maternal stress have provided important opportunities to examine mechanisms underlying the programming of long-term offspring outcomes relevant to neurodevelopmental disorders. Offspring phenotypes vary depending on the stressors used, outcomes examined and timing of the stress event during pregnancy63–66. However, maternal stress has been clearly shown in mice, rats, guinea pigs and nonhuman primates to alter the development and sensitivity of offspring HPA stress axis, behavioral stress reactivity and cognitive deficits, all of which are endophenotypes relevant to neurodevelopmental disorders43,65,67–73. Similar to findings in humans, these animal models have reported findings from maternal stress that demonstrate increased stress responsivity and cognitive deficits specific to the gestational timing of stress exposure as well as to the sex of the offspring, where again, stress earlier in pregnancy produced significant effects in male offspring70,71,73–75. The early postnatal period in mice and rats has also demonstrated this remarkable sex specificity in the developing brain’s response to stress, revealing how maternal behaviors including fragmented care can disrupt these pathways, resulting in adults with depleted hippocampal development and dysregulation of their HPA stress axis76–83.
The mammalian brain develops in the face of combined and opposing forces contributing to resiliency or vulnerability. The sexually dimorphic developing brain is organized in large part by developmental hormone exposure, with males experiencing elevated testosterone levels during the process of normal testes development (Fig. 1). Aromatization of this testosterone to estradiol in the brain of the developing organism drives masculinization, an active process that affects cell differentiation and connectivity in the brain13,84. Animal models have established the importance of the estrogenic involvement in cell death and cell birth in the developing nervous system as a critical component in organizing the sexually dimorphic brain, as is the newly discovered epigenetic process of DNA methylation in feminization of the female brain13,16,18. Certainly, evidence in humans also suggests a strong correlation between fetal testosterone levels and steroidogenic activity with neurodevelopmental disease risk and adult cognitive and behavioral stress reactivity, supporting the importance of the processes involved in establishing the sexually dimorphic brain, as discussed above52,85–87. Programming of important stress regulatory brain regions, including the limbic circuitry and the neuroendocrine hypothalamus, via hormone effects on cell migration patterns during early development also contributes to sex differences in how the individual responds to stress experiences throughout life (Fig. 1)15.
In rodent models, studies have manipulated this critical organizational window to mechanistically examine its importance in sex differences in long-term stress programming. For example, normal adult sex differences in hippocampal expression of the glucocorticoid receptor, critical for the negative feedback and modulation of the HPA axis, were disrupted in females masculinized at birth by a single injection of testosterone (thus mimicking the normal male masculinization of brain organization and producing a mismatch in the adult XX female brain), supporting the importance of the male testosterone surge in normal wiring of sex differences in stress pathways6,38,88. In addition to this early critical period of programming, the rise in gonadal hormones beginning in puberty interacts with these organizational changes and exerts modulatory actions on neurotransmitter systems critical in regulation of sex differences in stress responsivity. Ultimately then, there is an important coordination that must occur between organizational and activational periods of hormone exposure with additional factors from the sex chromosomes to orchestrate a ‘normal’ stress phenotype for each sex, avoiding a mismatch that would indicate an elevated disease risk. Therefore, neurodevelopmental disorder predisposition, such as for schizophrenia or ASD, may result from an underlying mismatch between these processes beginning with a perturbation during early development (Fig. 1)27,89. Further, the male sex bias in these disorders may result from a mismatch between the prenatal and postnatal environment being more detrimental to males than to females. For example, the process involved in masculinization may be more susceptible to prenatal disturbances by a mechanism yet to be determined.
Puberty: stress and the adolescent brain
Before the onset of puberty, there are limited differences between males and females in their physiological stress response90,91. Similarly, before puberty, boys and girls have an equivalent presentation rate of affective disorders, for both major depression and anxiety92. However, brain maturation that occurs during the adolescent window results in a significant blunting of the male HPA stress axis, predominantly related to the rise in testosterone and its interaction with the organizational program established by neonatal testosterone93. After adolescence and into adulthood, there is an increased presentation of affective disorders in women compared to men (Fig. 2). These clinical findings again support an important involvement of gonadal hormones in guiding brain plasticity within key stress regulatory regions. In both humans and rodents, brain regions involved in stress regulation and mood, including the prefrontal cortex, amygdala, hypothalamus and hippocampus continue to mature throughout this pubertal period. Such a dynamic developmental state may increase the vulnerability to external perturbations, thereby promoting a potential sex-specific window for stress dysregulation91,94.
During puberty, the stress circuitry matures as a result of a major intersection of gonadal and adrenal axes, where in primates adrenarche precedes gonadarche95. For instance, in adrenal gland development where sex differences are an important factor, the zona fasciculate cortical region diverges during puberty with a significant decrease in its growth rate in males as a direct result of testosterone production. It is not surprising that the timing of production of this testosterone and adrenal development is associated with the point in puberty when sex differences in HPA responses also arise, supporting the importance of this activational stage of gonadal hormones in programming stress responsivity96.
Sex differences in the maturation of stress circuitry are, in part, a result of changes in the broader transcriptome via gonadal hormone influences on epigenetic and other chromatin regulatory mechanisms97. During puberty, limbic brain regions, including the amygdala and hippocampus, which express large numbers of androgen and estrogen steroid hormone receptors, grow excessively in volume in both rodents and humans98. It is surprising that puberty is a time of considerable brain reorganization and maturation. In humans there is a peak and subsequent decline in cortical gray matter and a continual, though sexually dimorphic, increase in cortical white matter volume, in both the frontal and parietal lobes99,100. Studies in rodents have established that new cells are added in a sex-specific manner to the hypothalamus and amygdala during puberty, and gonadectomy before puberty prevents this effect101. These sex differences in the number of newly added cells correspond to similar differences in adult brain volumes, suggesting that the changes programmed during puberty are long lasting. Studies in sheep have similarly described sex-specific changes in the morphology of specific limbic system brain nuclei during puberty102. Together, these data support that key aspects of normal brain development, across species, are critically dependent on gonadal hormone levels, and represent an important organizational effect of the gonadal hormone onset during puberty. Thus, the connectivity and function of networks important in the regulation of emotion and stress are poised for modification by this pubertal rise in hormones. Likewise, any alterations in the normal trajectory of brain development as a result of gestational adversity, such as to stress, will serve as the substrate for these activational effects of pubertal hormone production.
Studies identifying sex-specific molecular changes that occur during puberty that may be important to stress neurocircuitry have largely focused on steroid hormones and their receptors, including gonadal hormone and glucocorticoid receptors. For instance, in rodent studies, levels of hippocampal GRs gradually increase during development, and a peak during puberty, which is concurrent with the emergence of receptor autoregulation103,104. The negative feedback inhibition of the HPA stress axis also reaches normal adult sensitivity during adolescence103. Both androgens, estrogens and their respective metabolites act through classic receptor mechanisms inside and surrounding the PVN to modulate the stress response of the CRF neurons105. This is where testosterone’s actions to suppress stress reactivity in males are thought to occur, influencing the GABAergic inhibitory tone of the PVN.
Estrogen and progesterone cyclicity in females is also a means by which stress responsivity and arousal circuitry are dynamically regulated in women across menstrual cycle stages. Cortisol responses to a laboratory social stressor are blunted in luteal compared to follicular phase females, whereas the response among users of oral contraceptive pills is similar to that of follicular phase females. Likewise, observations of sex differences in HPA axis response depend on the menstrual cycle phase of the female participants. Typically sex differences in salivary cortisol response to stress are noted when women are in the low-estrogen state of the follicular phase or using oral contraceptives, and is most similar between males and luteal phase females34, a finding of interest given this would be when males and females are most hormonally divergent. In contrast, fMRI results indicate that the largest sex differences in blood oxygen level–dependent (BOLD) signal within the stress neurocircuitry is observed when the female participants were in the high estradiol, mid-late follicular phase106.
As the brain continues to mature, the potential influences of stress and the environment on the brain are appreciable during adolescence. The incidence of affective disorders dramatically increases in puberty100,107. In males, the maximal levels to which stress-induced corticosteroids rise decreases with progression through puberty90. Studies examining the long-term effects of adverse early childhood experiences suggest that adults exposed to the stress of child abuse and/or neglect are at a significantly greater risk for lifetime affective disorder presentation23,24,108–110. This is especially true for women, where trauma during this period of brain development remains the highest predictor for affective disorders across the lifespan. In clinical studies, there is clear evidence for the interaction of neurobiological, neuroendocrine and immune alterations after exposure to adverse events during the peripubertal period in brain development111. For example, plasma stress hormone levels in response to relatively mild stressors are markedly increased in individuals who have experienced early life trauma, including sexual or physical abuse22,25. Further, brain imaging studies demonstrate that timing of adversity with respect to age and in relation to puberty is an important factor in the overall impact on the functioning of specific brain regions94,108. In rodents, chronic stress experienced during puberty produces sex-specific outcomes, with stressed females, but not males, exhibiting changes in HPA stress axis sensitivity and a blunting of neurogenesis in the dentate gyrus112. Likewise, similar to outcomes reported in animal models of early life stress, recent epigenetic studies in humans indicate that exposure to early life trauma differentially regulates the expression and level of methylation of the GR promoter (NR3C1)113. In humans, whether these alterations in brain structure and function or in GR methylation vary by sex in response to stress exposure has not be fully examined, although one study focusing on the CRF receptor-1 (CRFR1) genetic polymorphism suggests that there may be a sex × childhood trauma × sex effect on risk for lifetime depression studies22.
Together, studies in rodents and humans point to an increased susceptibility to stress in females during the peripubertal and pubertal window of maturation for programming of long-term risk for stress-related affective disorders (Fig. 2). This is distinct from the apparent increased vulnerability of males to fetal antecedents as discussed above. Such differences in timing support the importance of unique patterns of brain development and maturation, mechanisms for which appear to involve an interaction of X- and Y-linked genes and gonadal hormones (Fig. 1).
Stress and sex differences in the aging brain
The transition to reproductive senescence is a critical part of the aging process, particularly for women, as they experience uncharacteristically erratic ovarian hormone fluctuations followed by hypogonadism during the late fifth and early sixth decades of life. For males, this process of reproductive senescence is far less dramatic and occurs at a much later age, if at all. However, aromatization of testosterone to estradiol in the male brain during aging can be variable and dependent on a number of factors114. Given the impact of reproductive hormones on stress neural circuitry and HPA axis function, it is not surprising that these aspects of aging lead to alterations in stress responsivity within and between the sexes, and risk for neuropsychiatric conditions is manifest. Women are two to three times more likely to experience a first episode of depression during the perimenopausal period, and the onset of schizophrenia after age 40 is two times higher in women than in men (Fig. 2)115,116. Psychotic disorders similarly demonstrate an impact of sex, age and life stress117–121.
Animal models have identified interactions of these considerable gonadal hormone changes during aging with the regulation of genes important in stress responsivity, including many of the same targets as discussed above such as 5-HT, CRF and GABA signaling122–130. As gonadal hormones have a profound impact on brain structure and function, morphology and neurochemistry in brain regions that are critical for executive function, learning and memory, and stress regulation, the reduction in hormones or their metabolites can clearly promote sex-specific outcomes during aging131. For example, post-menopausal estrogen treatment sensitizes women to the adverse effects of stress on mood and symptoms of confusion under the context of a laboratory social-evaluative stressor132,133. Premenopausal women appear to have the opposite response to estradiol exposure, a finding that supporting a differential effect of estradiol on brain function and stress reactivity dependent on age134.
In studies focused on aging and gender effects on HPA axis function, older women demonstrate an accentuated cortisol response compared to age-matched men, and younger men and women in response to a cognitive challenge135–137. A meta-analysis similarly comparing cortisol levels between young versus old healthy volunteers exposed to a pharmacological or psychological challenge reported that older subjects produced a significantly higher cortisol response to challenge or less inhibition during a suppression test138. In this large-scale analysis of 45 parallel group studies, it was again found that the effect of aging to increase stress-mediated cortisol release was significantly stronger in women.
Despite the fact that stress can precede a major depression episode, exacerbate ongoing anxiety disorders, and is required for the diagnosis of post-traumatic stress disorder in both men and women, women still represent the majority of individuals diagnosed with stress-related affective disorders from puberty to reproductive senescence92,139. In the perimenopausal state, the risk for depression in women increases two- to fivefold depending on previous psychiatric history, demographic factors, education and adverse childhood experiences140,141. However, in the post-menopausal state, the risk for affective disorders among women diminishes to match that of their male counterparts141. These studies suggest that the dynamic change in hormonal status rather than the absolute level is a key determinant in sex differences in stress responses and disease risk.
Animal models for examining mechanisms involved in sex differences in stress and aging have revealed similar outcomes as found in humans, where aged female rats have higher basal corticosterone levels compared to same age males142. As mentioned earlier, there is a vast literature on the modulation of activational gonadal hormones, both androgens and estrogens, to influence the HPA stress axis and stress-related behaviors. In rodents, it is clear that testosterone promotes a suppression of overall corticosterone levels in response to a stressor, and can act at many levels within the brain, including the hypothalamus, to affect vasopressin and CRF synthesis and release96. This may in part be due to the androgen metabolite, 3B-diol, which in rodents has been shown to act within the hypothalamus to directly inhibit vasopressin and CRF PVN neurons and effectively reduce activity of the HPA stress axis105. Therefore, a reduction in testosterone or its metabolites in the brain in aging males would result in an increased stress response. Although such studies support the significant increase in basal free cortisol levels between young and aged men, they do not explain the much more dramatic rise in stress-challenge cortisol levels in aged women, particularly because the majority would be presumably hypogonadal135. A shortcoming of the literature on human studies is that studies examining age and hormonal effects on stress responsiveness outcomes have typically administered estradiol to postmenopausal women who are considerably distant from their final menstrual period. However, one study tested for the presence of sex differences in stress responsivity in the gonadal suppressed state in young men and women and found that in the absence of gonadal hormones, men produced higher ACTH and cortisol levels in response to a CRF stimulation or exercise compared to women143. Testosterone replacement in gonadally suppressed men dampens the normally enhanced HPA axis response to challenge, and the same is seen in gonadectomized versus hormone-replaced rodents144. These studies support the important involvement of gonadal hormones in modulation of stress responsivity across the lifespan, and further suggest the influence of rapidly declining hormone levels in aging on how the brain perceives and responds to stress, especially in women.
Mechanistically, estrogen is vital to neural plasticity, as demonstrated in studies where hippocampal and hypothalamic synaptogenesis and neuronal membrane firing potential are affected by estradiol145. Therefore, the dynamic change in hormonal status in women during the menopause transition may promote a dysregulation of cellular processes involved in HPA axis activation and feedback. Less is known as to the effects of aging and the decline of estrogen on GRs and how negative feedback of the stress circuitry is impacted, but rodent studies have clearly demonstrated that the aging hippocampus is significantly more vulnerable to stress-mediated glutamate neurotoxicity and the damaging effects of glucocorticoids26,146. This increased susceptibility may be result in part from decreased synapse spine plasticity in the aging brain, especially in stress-responsive regions such as the hippocampus and prefrontal cortex. As estrogen plays a vital role in the brain’s resilience to these effects, women may be at a greater risk to hippocampal damage during the menopause transition, resulting in a cycle of a less responsive negative feedback and greater stress reactivity.
Conclusions
The mechanisms by which sex differences in stress responsivity arise and promote sex biases in disease risk or resilience are complex but appear to involve an interaction of sex chromosome genes with periods of dynamic hormonal changes that may be compounding across the lifespan. For instance, disruptions during key periods in development and maturation can alter the sexually dimorphic brain, changing how the organism responds to or copes with stress due to a mismatch between the gonadal sex and brain sex. Animal models and human studies support an increased risk in males for behavioral or neurodevelopmental disorders such as ASD, schizophrenia, and ADHD in association with maternal adversity during pregnancy. However, females appear to be at greater risk to early life and peripubertal adversity to present with affective disorders throughout life. The aging brain also shows sex-specific changes in stress reactivity, which in part result from dynamic hormonal reductions in women and other aging-related cellular processes in limbic brain regions.
The brain’s continuous ability across the lifespan to perceive and appropriately respond to stress is necessary for homeostasis and survival. Therefore, the continued focus and appreciation for how sex differences in stress responses may predict disease risk and resiliency is critical for developing preventive strategies and treatments. Why these sex differences exist and continue to present across the lifespan is a key question in understanding individual health and disease, and suggests that an early adaptation made it advantageous for males and females to respond differently to stress. Studies that include sex as a factor continues to be a major need across the lifespan as our best effort in mental health research.
ACKNOWLEDGMENTS
The work discussed in this Review was funded in part by US National Institutes of Health grants MH073030 (T.L.B.), MH091258 (T.L.B.), MH087597 (T.L.B.), MH104184 (T.L.B.), MH108286 (T.L.B.), MH099910 (C.N.E. and T.L.B.), AG048839 (C.N.E.), DA030301 (C.N.E.). We thank T. Tiliakos for editorial assistance with the manuscript.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 2.Rivier C, Vale W. Effects of corticotropin-releasing factor, neurohypophyseal peptides, and catecholamines on pituitary function. Fed. Proc. 1985;44:189–195. [PubMed] [Google Scholar]
- 3.Sawchenko PE, et al. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found. Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- 4.Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 5.Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–327. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- 6.Goel N, Bale TL. Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology. 2008;149:6399–6405. doi: 10.1210/en.2008-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 8.Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- 9.Brydges NM, Wood ER, Holmes MC, Hall J. Prepubertal stress and hippocampal function: sex-specific effects. Hippocampus. 2014;24:684–692. doi: 10.1002/hipo.22259. [DOI] [PubMed] [Google Scholar]
- 10.Ege MA, Messias E, Thapa PB, Krain LP. Adverse childhood experiences and geriatric depression: results from the 2010 BRFSS. Am. J. Geriatr. Psychiatry. 2015;23:110–114. doi: 10.1016/j.jagp.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan A, et al. Childhood maltreatment, depression, and suicidal ideation: critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Front. Psychiatry. 2015;6:42. doi: 10.3389/fpsyt.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am. J. Psychiatry. 2001;158:587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy MM, Nugent BM. Epigenetic contributions to hormonally mediated sexual differentiation of the brain. J. Neuroendocrinol. 2013;25:1133–1140. doi: 10.1111/jne.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bale TL, et al. Early life programming and neurodevelopmental disorders. Biol. Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan CP, Bale TL. Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol Sex Differ. 2012;3:22. doi: 10.1186/2042-6410-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat. Rev. Neurosci. 2015;16:332–344. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nugent BM, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 2013;75:327–335. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DR, Bale TL, Epperson CN. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr. Psychiatry Rep. 2015;17:5. doi: 10.1007/s11920-014-0546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br. J. Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 22.Heim C, et al. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Front. Behav. Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol. Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 24.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 25.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev. Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 26.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karatsoreos IN, McEwen BS. Resilience and vulnerability: a neurobiological perspective. F1000Prime Rep. 2013;5:13. doi: 10.12703/P5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly SD, Harrell CS, Neigh GN. Chronic stress modulates regional cerebral glucose transporter expression in an age-specific and sexually-dimorphic manner. Physiol. Behav. 2014;126:39–49. doi: 10.1016/j.physbeh.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Clin. Lab. Med. 2010;30:865–891. doi: 10.1016/j.cll.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers AB, Bale TL. Germ cell origins of posttraumatic stress disorder risk: the transgenerational impact of parental stress experience. Biol. Psychiatry. 2015;78:307–314. doi: 10.1016/j.biopsych.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 32.Kornstein SG. Gender differences in depression: implications for treatment. J. Clin. Psychiatry. 1997;58:12–18. [PubMed] [Google Scholar]
- 33.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom. Med. 2001;63:966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goel N, Bale TL. Identifying early behavioral and molecular markers of future stress sensitivity. Endocrinology. 2007;148:4585–4591. doi: 10.1210/en.2007-0479. [DOI] [PubMed] [Google Scholar]
- 37.Goel N, Bale TL. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 2010;151:1784–1794. doi: 10.1210/en.2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel N, Plyler KS, Daniels D, Bale TL. Androgenic influence on serotonergic activation of the HPA stress axis. Endocrinology. 2011;152:2001–2010. doi: 10.1210/en.2010-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol. Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiser MJ, Goel N, Sandau US, Bale TL, Handa RJ. Androgen regulation of corticotropin-releasing hormone receptor 2 (CRHR2) mRNA expression and receptor binding in the rat brain. Exp. Neurol. 2008;214:62–68. doi: 10.1016/j.expneurol.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanninen V, Aro H. Sex differences in coping and depression among young adults. Soc. Sci. Med. 1996;43:1453–1460. doi: 10.1016/0277-9536(96)00045-7. [DOI] [PubMed] [Google Scholar]
- 45.Protopopescu X, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc. Natl. Acad. Sci. USA. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. Neuroimage. 2006;32:457–464. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Hong DS, et al. Influence of the X-chromosome on neuroanatomy: evidence from Turner and Klinefelter syndromes. J. Neurosci. 2014;34:3509–3516. doi: 10.1523/JNEUROSCI.2790-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong DS, Bray S, Haas BW, Hoeft F, Reiss AL. Aberrant neurocognitive processing of fear in young girls with Turner syndrome. Soc. Cogn. Affect. Neurosci. 2014;9:255–264. doi: 10.1093/scan/nss133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose AB, et al. Effects of hormones and sex chromosomes on stress-influenced regions of the developing pediatric brain. Ann. NY Acad. Sci. 2004;1032:231–233. doi: 10.1196/annals.1314.027. [DOI] [PubMed] [Google Scholar]
- 50.Capel B. Sex in the 90s: SRY and the switch to the male pathway. Annu. Rev. Physiol. 1998;60:497–523. doi: 10.1146/annurev.physiol.60.1.497. [DOI] [PubMed] [Google Scholar]
- 51.Newschaffer CJ, et al. The epidemiology of autism spectrum disorders. Annu. Rev. Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 52.Gore AC, Martien KM, Gagnidze K, Pfaff D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr. Rev. 2014;35:961–991. doi: 10.1210/er.2013-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis EP, Pfaff D. Sexually dimorphic responses to early adversity: implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology. 2014;49:11–25. doi: 10.1016/j.psyneuen.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erskine HE, et al. Epidemiological modelling of attention-deficit/hyperactivity disorder and conduct disorder for the Global Burden of Disease Study 2010. J. Child Psychol. Psychiatry. 2013;54:1263–1274. doi: 10.1111/jcpp.12144. [DOI] [PubMed] [Google Scholar]
- 55.van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br. J. Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 56.Khashan AS, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch. Gen. Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 57.Beversdorf DQ, et al. Timing of prenatal stressors and autism. J. Autism Dev. Disord. 2005;35:471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- 58.Gerardin P, et al. Depression during pregnancy: is the developmental impact earlier in boys? a prospective case-control study. J. Clin. Psychiatry. 2011;72:378–387. doi: 10.4088/JCP.09m05724blu. [DOI] [PubMed] [Google Scholar]
- 59.Cowell PE, Kostianovsky DJ, Gur RC, Turetsky BI, Gur RE. Sex differences in neuroanatomical and clinical correlations in schizophrenia. Am. J. Psychiatry. 1996;153:799–805. doi: 10.1176/ajp.153.6.799. [DOI] [PubMed] [Google Scholar]
- 60.Gur RE, et al. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biol. Psychiatry. 2004;55:512–517. doi: 10.1016/j.biopsych.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Goldstein JM, Cherkerzian S, Tsuang MT, Petryshen TL. Sex differences in the genetic risk for schizophrenia: history of the evidence for sex-specific and sex-dependent effects. Am. J. Med. Genet. 2013;162:698–710. doi: 10.1002/ajmg.b.32159. [DOI] [PubMed] [Google Scholar]
- 62.Goldstein JM, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch. Gen. Psychiatry. 2002;59:154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- 63.Clarke AS, Schneider ML. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Dev. Psychobiol. 1993;26:293–304. doi: 10.1002/dev.420260506. [DOI] [PubMed] [Google Scholar]
- 64.Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J. Neuroendocrinol. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 65.Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J. Physiol. (Lond.) 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyons DM, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front. Behav. Neurosci. 2009;3:32. doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lyons DM, Parker KJ, Schatzberg AF. Animal models of early life stress: implications for understanding resilience. Dev. Psychobiol. 2010;52:616–624. doi: 10.1002/dev.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- 70.Kapoor A, Kostaki A, Janus C, Matthews SG. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav. Brain Res. 2009;197:144–149. doi: 10.1016/j.bbr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 71.Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol. Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 72.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog. Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 74.Mueller BR, Bale TL. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol. Behav. 2006;88:605–614. doi: 10.1016/j.physbeh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 75.Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- 76.Franklin TB, et al. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 77.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ivy AS, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korosi A, et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J. Neurosci. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 82.Meaney MJ, et al. Individual differences in the hypothalamic-pituitary-adrenal stress response and the hypothalamic CRF system. Ann. NY Acad. Sci. 1993;697:70–85. doi: 10.1111/j.1749-6632.1993.tb49924.x. [DOI] [PubMed] [Google Scholar]
- 83.Barha CK, Pawluski JL, Galea LA. Maternal care affects male and female offspring working memory and stress reactivity. Physiol. Behav. 2007;92:939–950. doi: 10.1016/j.physbeh.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 84.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baron-Cohen S, et al. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry. 2014;20:369–376. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lombardo MV, et al. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J. Neurosci. 2012;32:674–680. doi: 10.1523/JNEUROSCI.4389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruta L, Ingudomnukul E, Taylor K, Chakrabarti B, Baron-Cohen S. Increased serum androstenedione in adults with autism spectrum conditions. Psychoneuroendocrinology. 2011;36:1154–1163. doi: 10.1016/j.psyneuen.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 88.Bingham B, Viau V. Neonatal gonadectomy and adult testosterone replacement suggest an involvement of limbic arginine vasopressin and androgen receptors in the organization of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2008;149:3581–3591. doi: 10.1210/en.2007-1796. [DOI] [PubMed] [Google Scholar]
- 89.Pembrey M, Saffery R, Bygren LO. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J. Med. Genet. 2014;51:563–572. doi: 10.1136/jmedgenet-2014-102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front. Neuroendocrinol. 2010;31:232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Romeo RD, McEwen BS. Stress and the adolescent brain. Ann. NY Acad. Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- 92.Kessler RC. Epidemiology of women and depression. J. Affect. Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 93.Gomez F, Manalo S, Dallman MF. Androgen-sensitive changes in regulation of restraint-induced adrenocorticotropin secretion between early and late puberty in male rats. Endocrinology. 2004;145:59–70. doi: 10.1210/en.2003-0565. [DOI] [PubMed] [Google Scholar]
- 94.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 95.Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum. Reprod. Update. 2004;10:67–77. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- 96.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J. Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 97.Morrison KE, Rodgers AB, Morgan CP, Bale TL. Epigenetic mechanisms in pubertal brain maturation. Neuroscience. 2014;264:17–24. doi: 10.1016/j.neuroscience.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Bellis MD, et al. Sex differences in brain maturation during childhood and adolescence. Cereb. Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 99.Schmitt JE, et al. The dynamic role of genetics on cortical patterning during childhood and adolescence. Proc. Natl. Acad. Sci. USA. 2014;111:6774–6779. doi: 10.1073/pnas.1311630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pfefferbaum A, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 101.Ahmed EI, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nuruddin S, et al. Peri-pubertal gonadotropin-releasing hormone agonist treatment affects sex biased gene expression of amygdala in sheep. Psychoneuroendocrinology. 2013;38:3115–3127. doi: 10.1016/j.psyneuen.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Sapolsky RM, Meaney MJ, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res. 1985;350:169–173. doi: 10.1016/0165-3806(85)90261-5. [DOI] [PubMed] [Google Scholar]
- 104.Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. I. Ontogeny and autoregulation. Brain Res. 1985;350:159–164. doi: 10.1016/0165-3806(85)90259-7. [DOI] [PubMed] [Google Scholar]
- 105.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J. Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Romeo RD. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J. Neuroendocrinol. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- 108.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 110.Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depress. Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 112.Barha CK, Brummelte S, Lieblich SE, Galea LA. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2011;21:1216–1227. doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- 113.van der Knaap LJ, et al. Glucocorticoid receptor gene (NR3C1) methylation following stressful events between birth and adolescence. The TRAILS study. Transl. Psychiatry. 2014;4:e381. doi: 10.1038/tp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Araujo AB, Wittert GA. Endocrinology of the aging male. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:303–319. doi: 10.1016/j.beem.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nemeroff CB. Stress, menopause and vulnerability for psychiatric illness. Expert Rev. Neurother. 2007;7:S11–S13. doi: 10.1586/14737175.7.11s.S11. [DOI] [PubMed] [Google Scholar]
- 116.Freeman EW, Sammel MD, Boorman DW, Zhang R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry. 2014;71:36–43. doi: 10.1001/jamapsychiatry.2013.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Godar SC, Bortolato M. Gene-sex interactions in schizophrenia: focus on dopamine neurotransmission. Front. Behav. Neurosci. 2014;8:71. doi: 10.3389/fnbeh.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front. Neurosci. 2014;8:19. doi: 10.3389/fnins.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rabinowitz J, Levine SZ, Hafner H. A population based elaboration of the role of age of onset on the course of schizophrenia. Schizophr. Res. 2006;88:96–101. doi: 10.1016/j.schres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 120.Harsh V, Schmidt PJ, Rubinow DR. The menopause transition: the next neuroendocrine frontier. Expert Rev. Neurother. 2007;7:S7–S10. doi: 10.1586/14737175.7.11s.S7. [DOI] [PubMed] [Google Scholar]
- 121.Maki PM, et al. Summary of the National Institute on Aging-sponsored conference on depressive symptoms and cognitive complaints in the menopausal transition. Menopause. 2010;17:815–822. doi: 10.1097/gme.0b013e3181d763d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shively CA, et al. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch. Gen. Psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 123.Lima FB, Bethea CL. Ovarian steroids decrease DNA fragmentation in the serotonin neurons of non-injured rhesus macaques. Mol. Psychiatry. 2010;15:657–668. doi: 10.1038/mp.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bethea CL, Reddy AP. Effect of ovarian hormones on genes promoting dendritic spines in laser-captured serotonin neurons from macaques. Mol. Psychiatry. 2010;15:1034–1044. doi: 10.1038/mp.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front. Neuroendocrinol. 2009;30:212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bethea CL, Reddy AP, Pedersen D, Tokuyama Y. Expression profile of differentiating serotonin neurons derived from rhesus embryonic stem cells and comparison to adult serotonin neurons. Gene Expr. Patterns. 2009;9:94–108. doi: 10.1016/j.gep.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J. Appl. Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 128.Suda S, Segi-Nishida E, Newton SS, Duman RS. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol. Psychiatry. 2008;64:311–319. doi: 10.1016/j.biopsych.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav. Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 130.Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol. Behav. 2004;83:505–513. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 131.Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum. Brain Mapp. 2014;35:847–865. doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Newhouse PA, et al. Estrogen treatment impairs cognitive performance after psychosocial stress and monoamine depletion in postmenopausal women. Menopause. 2010;17:860–873. doi: 10.1097/gme.0b013e3181e15df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dumas JA, et al. The effects of age and estrogen on stress responsivity in older women. Am. J. Geriatr. Psychiatry. 2012;20:734–743. doi: 10.1097/JGP.0b013e31825c0a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Albert K, Pruessner J, Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015;59:14–24. doi: 10.1016/j.psyneuen.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 136.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 137.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 138.Otte C, et al. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 139.Kessler RC, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015:1–17. doi: 10.1017/S2045796015000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch. Gen. Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 141.Freeman EW, et al. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch. Gen. Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 142.Bowman RE, Maclusky NJ, Diaz SE, Zrull MC, Luine VN. Aged rats: sex differences and responses to chronic stress. Brain Res. 2006;1126:156–166. doi: 10.1016/j.brainres.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 143.Roca CA, et al. Sex-related differences in stimulated hypothalamic-pituitary-adrenal axis during induced gonadal suppression. J. Clin. Endocrinol. Metab. 2005;90:4224–4231. doi: 10.1210/jc.2004-2525. [DOI] [PubMed] [Google Scholar]
- 144.Rubinow DR, et al. Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology. 2005;30:1906–1912. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 146.Bloss EB, et al. Morphological and molecular changes in aging rat prelimbic prefrontal cortical synapses. Neurobiol. Aging. 2013;34:200–210. doi: 10.1016/j.neurobiolaging.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]