Abstract
Background: The insidious pace of long-term weight gain (∼1 lb/y or 0.45 kg/y) makes it difficult to study in trials; long-term prospective cohorts provide crucial evidence on its key contributors. Most previous studies have evaluated how prevalent lifestyle habits relate to future weight gain rather than to lifestyle changes, which may be more temporally and physiologically relevant.
Objective: Our objective was to evaluate and compare different methodological approaches for investigating diet, physical activity (PA), and long-term weight gain.
Methods: In 3 prospective cohorts (total n = 117,992), we assessed how lifestyle relates to long-term weight change (up to 24 y of follow-up) in 4-y periods by comparing 3 analytic approaches: 1) prevalent diet and PA and 4-y weight change (prevalent analysis); 2) 4-y changes in diet and PA with a 4-y weight change (change analysis); and 3) 4-y change in diet and PA with weight change in the subsequent 4 y (lagged-change analysis). We compared these approaches and evaluated the consistency across cohorts, magnitudes of associations, and biological plausibility of findings.
Results: Across the 3 methods, consistent, robust, and biologically plausible associations were seen only for the change analysis. Results for prevalent or lagged-change analyses were less consistent across cohorts, smaller in magnitude, and biologically implausible. For example, for each serving of a sugar-sweetened beverage, the observed weight gain was 0.01 lb (95% CI: −0.08, 0.10) [0.005 kg (95% CI: −0.04, 0.05)] based on prevalent analysis; 0.99 lb (95% CI: 0.83, 1.16) [0.45 kg (95% CI: 0.38, 0.53)] based on change analysis; and 0.05 lb (95% CI: −0.10, 0.21) [0.02 kg (95% CI: −0.05, 0.10)] based on lagged-change analysis. Findings were similar for other foods and PA.
Conclusions: Robust, consistent, and biologically plausible relations between lifestyle and long-term weight gain are seen when evaluating lifestyle changes and weight changes in discrete periods rather than in prevalent lifestyle or lagged changes. These findings inform the optimal methods for evaluating lifestyle and long-term weight gain and the potential for bias when other methods are used.
Keywords: long-term weight gain, obesity prevention, prospective cohorts, epidemiological methods, dietary intake change, physical activity change, baseline dietary intake, baseline physical activity
Introduction
Maintaining a healthy body weight is crucial for reducing the risk of many chronic diseases. Whereas randomized intervention studies are useful for evaluating strategies for weight loss, such approaches are far less feasible for assessing causes of long-term weight gain in nonobese populations, which occurs very slowly (∼1 lb/y or 0.45 kg/y) over decades (1). Prospective cohort studies can provide crucial evidence in this regard, yet the optimal methods for analyzing how lifestyle influences weight gain in large prospective datasets are not well established.
Potential approaches include a prevalent diet/physical activity (PA) approach, which is typically used in prospective analyses that study the effect of diet on weight change (2). Other analytic strategies could be envisioned, such as a lagged-change dietary/PA analysis in which the dietary and PA changes within a certain period are associated with weight changes during a subsequent period. Last, a dietary/PA change approach could also be used in which changes in diet or PA and concurrent changes in weight are evaluated within the same timespan. This approach was used in previous work in which it was demonstrated that changes in specific dietary and other lifestyle habits were robustly related to long-term weight gain in 120,877 participants who were followed for more than 1,570,808 person-years in the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and Health Professionals Follow-Up Study (HPFS) (3).
The validity and consistency of these methodological approaches remain unclear. Based on modeling analyses, discrete changes in diet and PA produce initial changes in weight that are then counterbalanced over the course 2–3 y by changes in adipose tissue and lean muscle mass, suggesting that lifestyle changes may influence changes in weight within discrete periods of time (4). Only a limited number of studies, however, have evaluated such a “lifestyle change” approach, and typically only for a few dietary factors (e.g., sugar-sweetened beverages, milk, and whole grains) (5–8). Most previous studies have only evaluated prevalent lifestyle habits and subsequent changes in weight, with inconsistent findings across studies (9–11).
Understanding which of these methods provides the most consistent and valid results is crucial for designing future investigations of the causes of long-term weight gain and for appropriately informing clinical guidelines and public policy. Therefore, we evaluated and compared 3 different methodological approaches in 3 separate prospective cohort studies of US women and men. We evaluated our findings according to their consistency among cohorts, the strength of their associations with weight, and their biological plausibility based on our current understanding of the relation between diet and body weight (12). Based on physiologic modeling of how discrete lifestyle changes influence weight gain (4), we hypothesized that discrete changes in diet and PA would most consistently and validly relate to weight gain.
Methods
Study design and population.
We evaluated 3 separate, well-established prospective cohort studies, including the NHS, which comprised 121,701 female registered nurses from 11 states enrolled in 1976; NHS II, which comprised 116,683 younger female registered nurses from 14 states enrolled in 1989; and HPFS, which comprised 51,530 male health professionals from all 50 states enrolled in 1986 (3). In each cohort, participants were asked to complete biennial validated questionnaires concerning medical history, lifestyle, and health practices. For this analysis, the baseline year was the first year for which detailed information was available on diet, PA, and smoking: 1986 for NHS and HPFS and 1991 for NHS II.
As described previously (3), to understand how long-term weight gain is associated with nonobese individuals as well as to minimize reverse causation as a result of chronic disease, pregnancy, or loss of lean muscle mass with aging, we excluded participants who died before 1986 (NHS: n = 7491; HPFS: n = 1234) or 1991 (NHS II: n = 40); who were already obese (BMI ≥ 30 kg/m2) at baseline (NHS: n = 7432; NHS II: n = 8682; HPFS: n = 2810); those with implausible energy intake (i.e., energy intake < 900 kcal/d or >3500 kcal/d), 9 or more blank responses on the diet questionnaire or missing baseline dietary, PA, or weight data (NHS: n = 51,991; NHS II: n = 21,602; HPFS: n = 15,145); and those aged greater than 65 y who became pregnant during follow-up or who had a prevalent chronic disease (cancer, cardiovascular disease, diabetes, renal disease, pulmonary disease, rheumatologic disorders, ulcerative colitis) at baseline (NHS: n = 6338; NHS II: n = 38,328; HPFS: n = 10,869). We also censored participants during follow-up at the age of 65 y or after a diagnosis of cancer, cardiovascular disease, diabetes, renal disease, pulmonary disease, rheumatologic disorders, or ulcerative colitis (with a 6-y lag, i.e., censored 6 y before diagnosis).
A total of 117,992 generally healthy, nonobese participants were included in this analysis, including 48,449 in the NHS, 48,071 in NHS II, and 21,472 in HPFS. Written informed consent was obtained from study participants, and the Harvard TH Chan School of Public Health and Brigham and Women’s Hospital Human Subjects Committee Review Board approved the study protocol.
Lifestyle and dietary assessment.
In each cohort, usual dietary habits were assessed every 4 y using validated FFQs (13, 14). PA was evaluated in metabolic equivalent hours per week calculated as the intensity self-reported activity multiplied by the duration of activity (15). Based on biologically plausible effects on weight gain, dietary factors of interest included fruits, vegetables, nuts, whole-fat dairy (butter, cheese, whole-fat milk), low-fat dairy (reduced-fat milk, yogurt), potato chips, potatoes (French fries and boiled, baked, or mashed potatoes), whole grains, refined grains, sugar-sweetened beverages, 100% fruit juice, diet soda, sweets and desserts, processed meats, and unprocessed red meats (Supplemental Table 1) (3, 12). We also assessed an overall dietary score as previously described (3). Covariates of interest included BMI, sleep duration, smoking status, amount of time spent watching television, alcohol use, fried foods consumed at home and away from home, and trans fat intake.
Participants were asked to report their average intake in the previous year of commonly consumed foods out of 9 possible responses ranging from “never or less than once per month” to “6 or more servings per day.” These responses were converted to continuous values (servings per day) using the midpoint value of each categorical response.
Dietary score.
Intake quintiles of different foods were assigned a score of 1 to 5 in ascending or descending order depending on their putative association with weight gain (3, 12). Whole grains, fruits, vegetables, yogurt, and nuts were assigned a score of 1 to 5, with 5 being the highest intake for the prevalent analysis or the greatest increase in intake for the change analysis. Conversely, trans fat, processed meat, nonprocessed red meat, potato chips, sweets and desserts, refined grains, potatoes, sugar-sweetened beverages, fried foods consumed at home and away from home, and butter and juice intake were assigned a score of 1 to 5, with 5 being the lowest intake for the prevalent analysis or the greatest decrease in intake for the change analysis. The sum of these scores made up the dietary score, with a higher score favoring diets associated with relative weight loss (see Supplemental Methods). For prevalent diet/PA analysis, this score was based on quintiles of absolute dietary intake; for change and lagged-change analyses, this score was based on quintiles of changes in dietary intake.
Physical activity assessment.
Participants were asked to report the number of hours they spend engaged in the following activities: walking, jogging, running, bicycling, swimming, tennis, squash, racquetball, calisthenics, aerobics, yoga, stretching, toning, or other vigorous activity (e.g., mowing the lawn). The self-reported PA has been validated in a subset of NHS II participants (15).
Weight changes and height measurement.
Weight and height were self-reported on the questionnaire: weight was requested every 2 y, and height was collected once for NHS, HPFS, and NHS II in 1976, 1986, and 1991, respectively. BMI was calculated as the product of weight (kilograms) and the inverse of height (meters) squared. A validation study confirmed that self-reported weights correlated closely with actual body weight (r = 0.96) (16).
Statistical analysis.
We assessed the independent relations among diet, PA, and weight change within 4-y periods over 24 y of follow-up in NHS and HPFS and 16 y of follow-up for NHS II based on 3 different approaches. First, we assessed prevalent diet/PA by evaluating how lifestyle habits at the start of each 4-y period related to weight change during that period. Second, we assessed lifestyle change by evaluating how changes in diet and PA during each 4-y period related to weight change during that period. Third, we assessed a lagged change in diet/PA by investigating the association of lifestyle changes over each 4-y period with weight change during the subsequent 4-y period. For all analyses, we assessed the simultaneous association between food and weight changes within 4-y periods over the follow-up period of 24 y in NHS, 16 y in NHS II, and 24 y in HPFS using generalized estimating equations with an unstructured correlation matrix to account for within-individual repeated measures across follow-ups. All analyses were adjusted for age, BMI at the beginning of each 4-y period, sleep duration, and prevalent levels of/changes in (specific to the analysis) physical activity, alcohol use, amount of time spent watching television, smoking, and all foods simultaneously (see Supplemental Methods for model equations).
Changes in foods and beverages were evaluated as continuous variables in serving per day; values were censored at the 0.5 and 99.5th percentiles to minimize the influence of outliers. Missing values (range: 14–46%) were imputed using carried-forward values; sensitivity analyses that excluded participants with missing values demonstrated similar findings (3). PA (absolute or change) was evaluated in indicator categories in quintiles. Other lifestyle factors were evaluated as indicator categorical variables, with missing values included using a missing indicator category. Findings within each cohort were assessed separately and then pooled through the use of an inverse-variance–weighted, random-effects meta-analysis.
The results from the 3 methodological strategies were compared using 3 approaches. First, for each method (prevalent, change, lagged change), we qualitatively assessed the consistency of findings across the 3 cohorts for each dietary factor and PA, considering both the direction and magnitude of associations. Second, we determined whether the overall findings obtained by each method were significantly different from each other by calculating a Wald test based on the point estimate and standard error of each pooled result. Third, we qualitatively assessed the extent to which the findings from the 3 methods were consistent with plausible directions and magnitudes of effect based on the biological effects of the various dietary factors and PA, e.g., on other physiological or intervention studies (12). We have not reported a quantitative statistical test of overall model performance because the quasi-likelihood information criterion used as a measure of model fit for generalized estimating equations is most appropriately used for comparing the predictive value of nested models. The quasi-likelihood information criterion cannot distinguish which model most closely represents a true biological association; therefore, we chose to evaluate our models based on the more qualitative criteria of robustness, consistency, and biological plausibility. P values < 0.05 were considered statistically significant. Data were analyzed using SAS 9.2 (SAS Institute).
Sensitivity analyses.
To explore how the duration of time may have affected how the relations among diet, PA, and weight change were assessed, we evaluated these relations using longer time periods (e.g., 8–12 y). We also assessed the association of prevalent diet/PA and lagged-change diet/PA with a 2-y weight change. For the diet/PA change analyses, we evaluated the separate and combined effects of previous and current change by creating 9 groups based on tertiles of the previous 4-y change in diet or PA and the current 4-y change in diet or PA. Last, we evaluated the association between our food variables and change in weight using standardized variables (i.e., z score).
Results
Participant characteristics.
Baseline characteristics of the 117,992 participants in the 3 cohorts as well as the average changes in diet and lifestyle over the follow-up period are shown in Supplemental Table 2. In general, the reported dietary intakes in the cohorts were consistent with national estimates (3). Within each cohort, the average 4-y change in each dietary factor and in PA was close to zero, but the ranges of individual participants’ changes in diet and PA were relatively broad. Notably, absolute (prevalent) intakes of different dietary factors were highly intercorrelated, reflecting overall healthier or less healthy dietary patterns as previously reported (17). In contrast, in a previous analysis, we found that changes in intakes of dietary factors had very low intercorrelations (Pearson r < 0.05 for nearly all correlations) (3), indicating that changes in different dietary factors were generally independent.
Diet and weight gain.
Based on the 3 different methodological approaches, the multivariable-adjusted associations between dietary habits and long-term weight gain were quite different (Figure 1, Supplemental Tables 3–8). As previously reported (3), 4-y changes in dietary factors were robustly associated with weight gain, with consistent findings across the 3 cohorts. In contrast, analyses that evaluated prevalent dietary habits or lagged change in diet demonstrated small and inconsistent associations. When the 3 approaches were formally compared, the findings from the prevalent and lagged-change analyses were significantly different from those using the change analysis for nearly all dietary factors (Table 1, Supplemental Figure 1). Including standardized variables in our models typically decreased the effect size on weight change in our analyses in accordance with 1 SD for most foods representing less than 1 serving per day. The interpretation of our results did not change. Using a z score limits comparability between foods within our models, between models, and relative to other populations because effect sizes depend upon the distributions of the variables.
FIGURE 1.
Cohort-specific, multivariable-adjusted results for prevalent diet, dietary change, and lagged-change analyses and long-term weight change among 117,992 US women and men in 3 prospective cohorts. Study participants included 48,449 women in the Nurses’ Health Study who were followed for 24 y (1986–2010); 48,071 women in the Nurses’ Health Study II who were followed for 16 y (1991–2007); and 21,472 men in the Health Professionals Follow-Up Study who were followed for 24 y (1986–2010). Data are β coefficients from multivariable linear regression representing weight change (lb/4 y ± 95% CI) for a 1-serving per day increase in foods. Weight changes are reported for each serving per day higher or increased intake of each food or beverage; lower or decreased intake was associated with the inverse weight change. Findings were adjusted for age, baseline BMI at the start of each 4-y period, sleep duration, smoking status, physical activity, amount of time spent watching television, alcohol use, fried food consumed at home and away from home, trans fat intake, and all of the dietary factors shown. Panel A shows the 4-y weight change associated with the prevalent intake of foods; panel B shows the 4-y weight change associated with change in diet over the same 4-y period; and panel C shows the lagged-change diet analysis, i.e., the association between the 4-y change in diet and the weight change over the next 4-y period. To convert pounds to kilograms, multiply by 0.45. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
TABLE 1.
Pooled, multivariable-adjusted results for prevalent diet, dietary change, and lagged-change analyses and long-term weight change among 117,992 US women and men in 3 prospective cohorts1
| Prevalent analysis2 |
Dietary change analysis3 |
Lagged-change analysis4 |
||||
| 1-serving/day increase | Weight change5,6 | P | Weight change5,6 | P | Weight change5,6 | P |
| Fruits | 0.09 (0.00, 0.18) | 0.06 | −0.53 (−0.68, −0.38) | <0.001 | 0.02 (−0.08, 0.11) | 0.72 |
| Vegetables | 0.08 (0.02, 0.13) | 0.009 | −0.22 (−0.35, −0.10) | <0.001 | −0.04 (−0.13, 0.06) | 0.47 |
| Nuts | −0.09 (−0.17, −0.01) | 0.03 | −0.63 (−1.03, −0.23) | 0.002 | −0.21 (−0.61, 0.19) | 0.31 |
| Whole-fat dairy foods7 | −0.04 (−0.07, 0.00) | 0.07 | 0.18 (0.10, 0.25) | <0.001 | −0.16 (−0.22, −0.10) | <0.001 |
| Butter | −0.04 (−0.09, 0.01) | 0.09 | 0.35 (0.20, 0.51) | <0.001 | −0.12 (−0.21, −0.04) | 0.003 |
| Cheese | 0.01 (−0.13, 0.15) | 0.90 | 0.08 (−0.09, 0.24) | 0.38 | −0.06 (−0.23, 0.10) | 0.45 |
| Whole-fat milk | −0.05 (−0.15, 0.05) | 0.29 | 0.05 (−0.12, 0.21) | 0.58 | −0.31 (−0.50, −0.12) | 0.001 |
| Low-fat dairy foods7 | 0.04 (−0.08, 0.15) | 0.51 | 0.01 (−0.06, 0.08) | 0.85 | 0.08 (0.04, 0.12) | <0.001 |
| Low-fat or skim milk | 0.00 (−0.14, 0.13) | 0.97 | 0.10 (−0.01, 0.21) | 0.06 | 0.05 (−0.06, 0.15) | 0.37 |
| Yogurt | 0.15 (−0.05, 0.36) | 0.15 | −1.13 (−1.26, −1.01) | <0.001 | 0.13 (−0.02, 0.29) | 0.08 |
| Potato chips | −0.41 (−0.54, −0.27) | <0.001 | 1.69 (1.27, 2.11) | <0.001 | −0.61 (−0.83, −0.38) | <0.001 |
| Potatoes7 | 0.03 (−0.20, 0.26) | 0.82 | 1.20 (0.79, 1.61) | <0.001 | −0.11 (−0.27, 0.05) | 0.18 |
| French fries | 0.11 (−0.79, 1.02) | 0.81 | 4.41 (3.27, 5.55) | <0.001 | −0.38 (−0.82, 0.06) | 0.09 |
| Boiled, baked, or mashed | 0.06 (−0.19, 0.32) | 0.62 | 0.60 (0.20, 1.01) | 0.004 | −0.08 (−0.27, 0.11) | 0.41 |
| Whole grains8 | −0.05 (−0.14, 0.04) | 0.25 | −0.32 (−0.51, −0.12) | 0.002 | 0.12 (0.03, 0.22) | 0.008 |
| Refined grains | −0.13 (−0.18, −0.07) | <0.001 | 0.36 (0.15, 0.57) | <0.001 | −0.02 (−0.11, 0.06) | 0.56 |
| Sugar-sweetened beverages | 0.01 (−0.08, 0.11) | 0.81 | 0.99 (0.82, 1.16) | <0.001 | 0.06 (−0.10, 0.21) | 0.48 |
| 100% fruit juice | −0.20 (−0.26, −0.13) | <0.001 | 0.49 (0.30, 0.67) | <0.001 | −0.12 (−0.18, −0.06) | <0.001 |
| Diet soda | 0.31 (0.27, 0.35) | <0.001 | −0.10 (−0.22, 0.02) | 0.11 | 0.20 (0.09, 0.32) | <0.001 |
| Sweets or desserts | −0.16 (−0.26, −0.06) | 0.002 | 0.40 (0.19, 0.61) | <0.001 | −0.02 (−0.08, 0.04) | 0.49 |
| Processed meats | 0.20 (−0.11, 0.52) | 0.21 | 1.09 (0.80, 1.37) | <0.001 | −0.02 (−0.65, 0.60) | 0.94 |
| Unprocessed red meats | 0.28 (0.20, 0.36) | <0.001 | 1.12 (0.75, 1.49) | <0.001 | 0.02 (−0.10, 0.15) | 0.69 |
| Trans fat9 | 0.12 (−0.06, 0.31) | 0.20 | 0.68 (0.39, 0.98) | <0.001 | −0.12 (−0.33, 0.09) | 0.27 |
| Fried foods | ||||||
| Consumed at home | −1.18 (−2.07, −0.29) | 0.009 | 2.13 (1.41, 2.85) | <0.001 | −0.41 (−0.66, −0.16) | 0.002 |
| Consumed away from home | 0.15 (−0.43, 0.74) | 0.61 | 1.55 (0.71, 2.39) | <0.001 | −0.11 (−0.38, 0.15) | 0.40 |
Values are β coefficients from multivariable linear regression representing weight change (lb/4 y ± 95% CI) for a 1-serving/day increase in foods. Data are based on 24 y of follow-up (1986–2010) in the Nurses’ Health Study, 16 y of follow-up (1991–2007) in the Nurses’ Health Study II, and 24 y of follow-up (1986–2010) in the Health Professionals Follow-Up Study. The association between dietary change and weight change was assessed using a multivariable linear regression model that accounted for within-individual repeated measures and pooled using inverse-variance–weighted, random-effects meta-analyses. Usual dietary habits and alcohol use were assessed every 4 y with the use of validated, semiquantitative FFQs.
Prevalent diet was measured at the beginning of each 4-y period and associated with weight change within the same 4-y period.
Dietary change was measured for each 4-y period and associated with weight change within the same 4-y period.
Lagged-change diet was measured for each 4-y period and associated with weight change within the subsequent 4-y period.
Weight changes are shown for a 1-serving increase in consumption. To convert pounds to kilograms, multiply by 0.45.
Values were adjusted for age, BMI at the beginning of each 4-y period, sleep duration, and prevalent levels of/changes in (specific to the analysis) physical activity, alcohol use, amount of time spent watching television watching, smoking, and all dietary factors in the table simultaneously.
For the categories of whole-fat dairy foods, low-fat dairy foods, and potatoes, subtypes were evaluated together in the full, multivariable-adjusted model in place of the overall food group; e.g., butter, cheese, and whole-fat milk were evaluated in place of total whole-fat dairy foods.
One serving was considered 30 g of whole grains.
Weight change per 1% increase in percentage energy from trans fatty acid.
Findings were similar for the overall dietary score (Figure 2). Dietary change was strongly related to weight change, with a weight gain of more than 3.92 lb (95% CI: 2.88, 4.97) [1.78 kg (95% CI: 1.31, 2.25)] when comparing the lowest to the highest quintile. In contrast, prevalent diet was not significantly associated with weight gain [−0.47 lb (95% CI: −1.12, 0.18); −0.21 kg (95% CI: −0.51, 0.08)]. Indeed, this nonsignificant trend of prevalent diet toward less weight gain in the first quintile [reflecting 1) higher absolute consumption of refined grains, potatoes, French fries, potato chips, butter, sugar-sweetened beverages, fruit juice, sweets and desserts, processed and red meat, trans fats and fried foods, and 2) lower consumption of fruits, vegetables, whole grains, nuts, and yogurt] compared with the fifth quintile (reflecting the opposite dietary pattern) was also seen in each individual cohort. Analyses of lagged change in diet also showed an inverse, nonsignificant association with weight change: comparing the bottom to the top quintile, −0.26 lb (95% CI: −0.70, 0.18) [−0.12 kg (95% CI: −0.32, 0.08)], with little dose-response across quintiles.
FIGURE 2.
Association between dietary score quintiles using the prevalent diet, dietary change, and lagged-change analyses and long-term weight gain among 117,992 US women and men in 3 prospective cohorts. In a multivariable adjusted analysis, the dietary score from the Nurses’ Health Study (n = 48,449), Nurses’ Health Study II (n = 48,071), and the Health Professionals Follow-Up Study (n = 21,472) was associated with a 4-y weight change over 16–24 y of follow-up. The dietary score was derived by totaling the ordinal values for the quintiles of change or prevalent intake for each dietary habit in ascending order (1 to 5) or descending order (5 to 1) for habits that were inversely or positively associated with weight gain, respectively. The sum of changes (panels B and C) or sum of prevalent levels (panel A) was then calculated to arrive at a dietary score of which a higher value was associated with a diet favoring weight loss. Data are β coefficients from multivariable linear regression representing weight change (lb/4 y ± 95% CI). Panel A shows the association between the prevalent dietary score at the start of each 4-y period and weight change during the same 4-y period; panel B shows the relation between the dietary change score and weight change during the same 4-y period; and panel C shows the association between the dietary change score and weight change during the next 4-y period. Findings were adjusted for age, baseline BMI at the start of each 4-y period, sleep duration, smoking status, physical activity, amount of time spent watching television, and alcohol use. To convert pounds to kilograms, multiply by 0.45. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; ref, reference.
PA and weight gain.
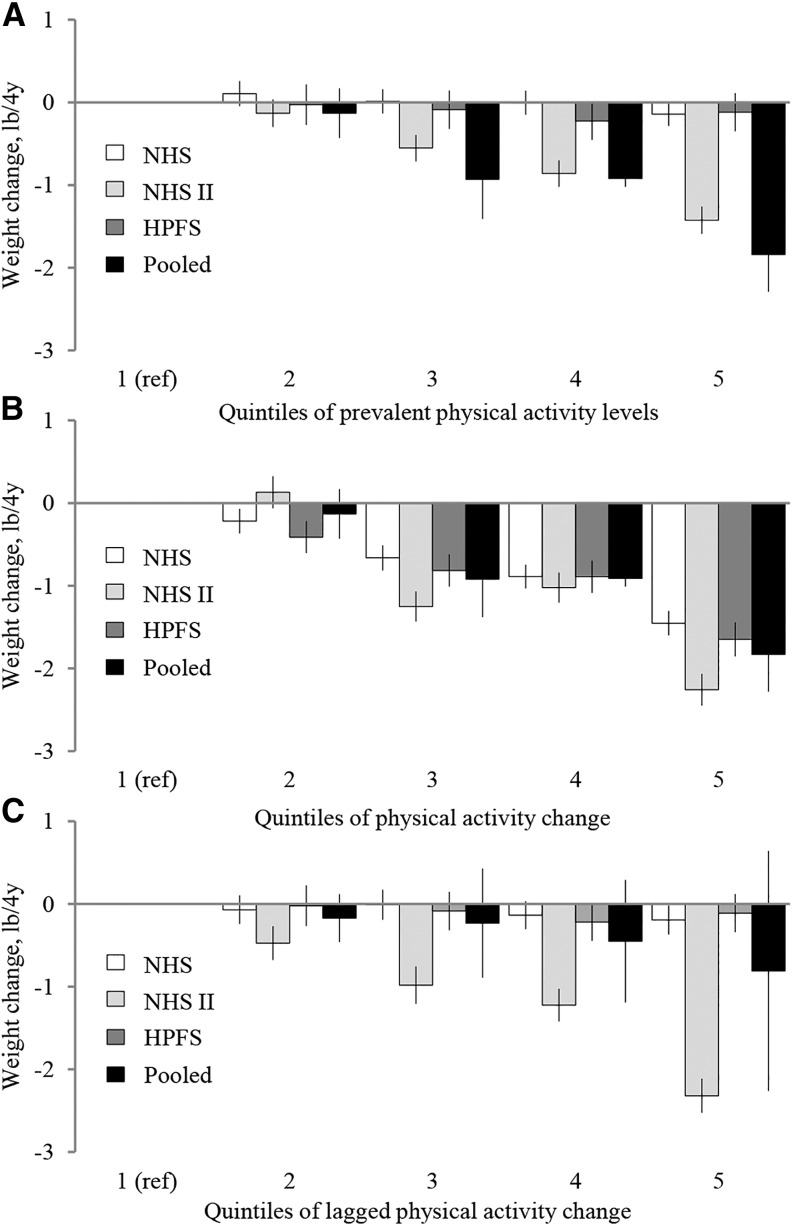
When we evaluated PA using the 3 methodological approaches, PA change was inversely and strongly associated with a 4-y weight gain in all 3 cohorts (Figure 3; see Supplemental Table 9 for the median values of PA and PA change according to quintiles of PA and PA change, respectively). In pooled analyses, participants in the highest quintile of PA change gained 1.83 lb (95% CI: −2.28, −1.39) [−0.83 kg (95% CI: −1.03, −0.63)] less than participants in the lowest quintile of PA change. In contrast, prevalent PA levels were unassociated with weight change in 2 of the cohorts and only associated with less weight gain in the NHS II cohort. Findings were similar for lagged change in PA, with no association between weight change in 2 cohorts (NHS and HPFS) and an inverse association only in NHS II.
FIGURE 3.
Association between quintiles of physical activity (PA) using the prevalent PA, PA change, and lagged-change analyses and long-term weight change among 117,992 US women and men in 3 prospective cohorts. In a multivariable-adjusted analysis, the PA of participants from the Nurses’ Health Study (n = 48,449), the Nurses’ Health Study II (n = 48,071) and the Health Professionals Follow-Up Study (n = 21,472) was associated with a 4-y weight change over 16–24 y of follow-up. Data are β coefficients from multivariable linear regression representing weight change (lb/4 y ± 95% CI). Panel A shows the association between the quintiles of prevalent PA at the start of each 4-y period and weight change during the same 4-y period; panel B shows the relation between quintiles of 4-y PA change and weight change during the same 4-y period; and panel C shows the association between the quintiles of PA and weight change during the next 4-y period. See Supplemental Table 9 for median (minimum, maximum) values of PA and PA change by quintiles of PA and PA change, respectively. All findings were adjusted for age, baseline BMI at the start of each period, sleep duration, smoking status, amount of time spent watching television, alcohol use, change (B and C), or prevalent intake (A) of fruits, vegetables, nuts, whole-fat dairy foods, low-fat dairy foods, potato chips, potatoes, whole grains, refined grains, sugar-sweetened beverages, 100% fruit juice, diet soda, sweets or desserts, processed meats, unprocessed meats, fried foods consumed at home and away from home, and trans fat intake. To convert pounds to kilograms, multiply by 0.45. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; ref, reference.
Sensitivity analyses.
We conducted sensitivity analyses to explore how the duration of the time period influenced the findings. Overall findings were similar, with a progressive, modest attenuation of the effect sizes for most dietary factors when the time period of assessment was increased incrementally from 4 to 12 y (Supplemental Figure 2). Similarly, the associations of prevalent diet and lagged-change diet with weight change were similar when weight change was evaluated only over the next 2 y (Supplemental Figures 3 and 4). When we evaluated the separate and combined effects of dietary change in both the current and previous 4-y period, associations with weight change were driven by current, rather than previous, dietary change (Supplemental Figure 5).
Discussion
Our systematic methodological analyses in 3 separate, well-established, prospective cohorts demonstrate that the method for evaluating the relation between diet or PA and weight gain is tremendously important. Specifically, evaluating concurrent lifestyle and weight changes produced the most robust, consistent, and biologically plausible results in all 3 cohorts. In contrast, findings that used either prevalent diet/PA or lagged-change lifestyle were markedly attenuated, inconsistent across the cohorts, and at times incongruent with our biological understanding of dietary effects on weight gain (e.g., finding sweets, sugary beverages, or potato chips to be most strongly associated with weight loss and no association between sugar-sweetened beverages and weight gain) (12). The heterogeneous associations between foods and weight observed in our change analysis are supported by mechanistic and intervention studies showing that there are differences in how foods affect satiety (18), resting energy expenditure (19), the microbiome (20), liver fat content (21), and other metabolic processes, suggesting that not all calories have equivalent effects on our health and weight and that some calories may preferentially promote weight gain whereas others may promote weight loss. A recent review, for example, supports our findings that adding nuts to the diet does not result in weight gain (22). These results indicate the strong likelihood of misclassification and even reverse causation or bias when evaluating prevalent or lagged-change diet/PA factors. Our findings provide evidence that the specific methods used to assess relations of diet and PA with weight change in prospective cohort studies are crucial for providing valid and unbiased results. These findings have important implications not only for research but also for policy development and obesity prevention.
Two important factors may result in changes in diet and PA and thus better capture the true relation with body weight than prevalent behaviors: 1) the physiology of weight loss and 2) reverse causation. First, because of physiological adaptations of the body during weight loss, including decreases in resting energy expenditure, changes in body composition, and the energetic costs of PA, initial changes to either energy intake (i.e., diet) or energy expenditure (i.e., PA) result in maximum weight loss within approximately 3–4 y, after which a new equilibrium between energy intake/output and body weight is maintained. This has been illustrated by Hall et al. both empirically and theoretically (4). By modeling change rather than prevalence, diet, and PA, we more closely matched the condition seen in both the weight-loss interventions and mathematical modeling. Therefore, as the body achieves a new steady-state over time, changes in diet and PA may be more relevant than prevalent dietary or PA habits.
There is also a strong likelihood for confounding in observational studies, in either direction, when evaluating prevalent diet based on its strong associations with overall diet patterns and other lifestyle factors. In contrast, individuals seem to make changes in different diet factors relatively independently, greatly minimizing confounding and significantly increasing the potential for valid findings. Previously, we found few significant correlations between changes in foods within the same 4-y period, suggesting that although prevalent dietary habits may correlate strongly with one another and create familiar overall dietary habits, individuals make changes to their diet in a relatively independent manner. There were some exceptions; we found that changes in foods that were natural substitutes for one another, or those typically eaten together, correlated. For example, changes in whole-fat dairy and low-fat dairy correlated negatively with one another (Pearson r = −0.08, P < 0.0001), whereas changes in fruits and vegetables correlated positively (Pearson r = 0.21, P < 0.0001) (3); even then, these correlations were relatively small compared with the typical correlations seen between prevalent dietary factors and do not represent a major source of confounding.
The timespan of these effects is also important. The modeling/biology of weight loss suggests that most of the effect of a diet or PA change will occur within 2 y and all within 4 y (4). This is why a 4-y period of diet or PA change model with weight change might be optimal for investigating the effects of changes in diet and PA with weight change. On the other hand, our findings were only slightly attenuated when we considered the association between 8- or 12-y changes in foods and 8- or 12-y changes in weight, respectively. Therefore, the most relevant factor may be simply to have repeated lifestyle and weight measures over time and evaluate concurrent changes rather than a specific time interval. However, evaluating diet or PA change and weight change over just a few months may be too brief to capture the full effect on body weight (4).
Last, we found that the lagged-change analysis, although utilizing changes in diet and PA, was not strongly associated with weight change. This could be because changes to diet in the more distant past are not as important for current weight change as changes to the current diet. This was evident when we looked at weight gain according to current and previous tertiles of dietary score change: only the current tertile of dietary change was associated with weight change, with no influence on current 4-y weight gain based on the previous change in dietary score. Our results for lagged-change PA were nearly identical to those for diet, suggesting that—apart from PA in the NHS II cohort—similar issues exist when studying PA and weight change. Although the NHS II cohort had similar levels and changes in PA compared to the other 2 cohorts, the younger age of NHS II participants could possibly result in physiological differences in the response to exercise (i.e., muscle mass increases) that could explain the differences compared with older individuals (NHS and HPFS cohorts) for the associations between PA and weight change for the prevalent and lagged-change analyses, although this hypothesis requires further testing.
In light of our findings, previous and future studies of prevalent diet, PA, and weight change should be interpreted cautiously. Most prospective studies have evaluated diet and weight gain using prevalent, or baseline, dietary intake, and often these findings are inconsistent among studies. Methodological differences among studies may be responsible for these divergent findings, or, based on our findings, using prevalent food intake as the exposure may be inherently more prone to misclassification and erroneous results. Publication bias may also play a role by overemphasizing significant findings that could have resulted from chance. A systematic review published in 2012 evaluated the associations between dietary factors and long-term weight gain in prospective observational studies and reported that there was inconclusive or only suggestive evidence for an association with weight among most categories of foods (2). Only 5 other studies that have studied the effect of change in food intake with change in weight using the same cohorts (NHS, NHS II, and HPFS) (5–8) have found results consistent with ours. In addition to methodological issues, there are also important policy and public health implications to our findings. Our findings indicate that changes to improve PA and diet have the greatest impact on future weight gain regardless of current diet or PA level.
The large sample size, use of 3 separate cohorts, and consistency of the findings of the change analysis among the 3 cohorts and with our understanding of the biology of weight change make it unlikely the findings resulted simply from chance or error. Both our exposures and outcome were validated. Although the FFQ has been validated for prevalent food intake, it has yet to be validated for change in food intake; therefore, we could not quantify the amount of error present in our dietary and PA change variables. However, measurement error would likely result in attenuation toward the null for our effect sizes. Our 3 cohorts included mainly white, educated, nonobese participants; therefore, the findings may not be generalizable, particularly in regard to aggressive efforts at short-term weight loss in obese adults. However, primary prevention of obesity may be more practical and effective than weight loss after obesity has occurred, and our findings have key implications for preventing weight gain. Although we adjusted for multiple lifestyle factors simultaneously, residual confounding cannot be excluded; however, a strength of the dietary change approach was the minimal intercorrelations of dietary changes, which greatly minimize the potential for confounding.
In summary, these findings provide evidence that changes in diet and PA are most valid and biologically relevant for elucidating the effects on concurrent long-term changes in weight. Prevalent diet/PA or previous changes in lifestyle seem to be far less appropriate for studying the effects on weight gain. These findings have important implications both for investigating the key lifestyle determinants of long-term weight changes and for policies aimed at preventing excess adiposity and related chronic diseases.
Acknowledgments
JDS contributed to the study design, performed the statistical analysis of the data, drafted the article, and had primary responsibility for final content. TH and DS contributed statistical expertise, provided feedback on the study design and methods, and reviewed and approved the final manuscript; FBH, EBR, and WCW were involved in the development, design, and data acquisition for the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study; contributed to the study design; and revised the manuscript for important intellectual content. DM designed and conceptualized the study, supervised and contributed to the drafting of the article, and revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; PA, physical activity.
References
- 1.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–5. [DOI] [PubMed] [Google Scholar]
- 2.Fogelholm M, Anderssen S, Gunnarsdottir I, Lahti-Koski M. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: a systematic literature review. Food Nutr Res 2012;56: DOI:10.3402/fnr.v56i0.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Hou T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr 2003;78:920–7. [DOI] [PubMed] [Google Scholar]
- 6.Rajpathak SN, Rimm EB, Rosner B, Willett WC, Hu FB. Calcium and dairy intake in relation to long-term weight gain in US men. Am J Clin Nutr 2006;83:559–66. [DOI] [PubMed] [Google Scholar]
- 7.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incident type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 8.Koh-Banerjee P, Franz M, Sampson L, Liu S, Jacobs DR Jr, Spiegelman D, Willett W, Rimm E. Change in whole-grains, bran and cereal fiber consumption in relation to 8-y weight gain among men. Am J Clin Nutr 2004;80:1237–45. [DOI] [PubMed] [Google Scholar]
- 9.Langsetmo L, Hitchcock CL, Kingwell EJ, Davison KS, Forsmo S, Zhou W, Kreiger N, Prior JC; Canadian Multicentre Osteoporosis Study Research Group. Physical activity, body mass index and bone mineral density—associations in a prospective population-based cohort of women and men: the Canadian Multicentre Osteoporosis Study (CaMos). Bone 2012;50:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Association between leisure time physical activity and 10-year body mass change among working-aged men and women. Int J Obes Relat Metab Disord 1997;21:288–96. [DOI] [PubMed] [Google Scholar]
- 11.Gordon-Larsen P, Hou N, Sidney S, Sternfeld B, Lewis CE, Jacobs DR Jr, Popkin BM. Fifteen-year longitudinal trends in walking patterns and their impact on weight change. Am J Clin Nutr 2009;89:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The 2015 Dietary Guidelines Advisory Committee. Scientific report of the 2015 Dietary Guidelines Advisory Committee to the Secretaries of the US Departments of Health and Human Services and Agriculture [cited 2015 Sep 2]. Available from: http://www.health.gov/dietaryguidelines/2015-scientific-report.
- 13.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett W. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 14.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 15.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a semi-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 16.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self- reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 17.Hu FB. Globalization of food patterns and cardiovascular disease risk. Circulation 2008;118:1913–4. [DOI] [PubMed] [Google Scholar]
- 18.Lennerz BS, Alsop DC, Holsen LM, Stern E, Rojas R, Ebbeling CB, Goldstein JM, Ludwig DS. Effects of dietary glycemic index on brain regions related to reward and craving in men. Am J Clin Nutr 2013;98:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012;307:2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duca FA, Lam TK. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes Metab 2014;16:68–76. [DOI] [PubMed] [Google Scholar]
- 21.Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr 2011;93:1048–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan SY, Dhillon J, Mattes RD. A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am J Clin Nutr 2014;100:412S–22S. [DOI] [PubMed] [Google Scholar]