Abstract
Background: Maternal overweight and obesity affect two-thirds of women of childbearing age and may increase the risk of impaired child cognition.
Objective: Our objective was to test the hypothesis that high/low gestational weight gain (GWG) and high/low prepregnancy BMI were associated with offspring intelligence quotient (IQ) and executive function at age 10.
Methods: Mother–infant dyads (n = 763) enrolled in a birth cohort study were followed from early pregnancy to 10 y postpartum. IQ was assessed by trained examiners with the use of the Stanford Binet Intelligence Scale–4th edition. Executive function was assessed by the number of perseverative errors on the Wisconsin Card Sorting Test and time to complete Part B on the Trail Making Test. Self-reported total GWG was converted to gestational-age–standardized GWG z score. Multivariable linear regression and negative binomial regression were used to estimate independent and joint effects of GWG and BMI on outcomes while adjusting for covariates.
Results: At enrollment, the majority of women in the Maternal Health Practices and Child Development cohort were unmarried and unemployed, and more than one-half reported their race as black. The mean ± SD GWG z score was −0.5 ± 1.8, and 27% of women had a pregravid BMI ≥25. The median (IQR) number of perseverative errors was 23 (17, 29), the mean ± SD time on Part B was 103 ± 42.6 s, and 44% of children had a low average IQ (≤89). Maternal obesity was associated with 3.2 lower IQ points (95% CI: −5.6, −0.8) and a slower time to complete the executive function scale Part B (adjusted β: 12.7 s; 95% CI: 2.8, 23 s) compared with offspring of normal-weight mothers. Offspring of mothers whose GWG was >+1 SD, compared with −1 to +1 SD, performed 15 s slower on the executive function task (95% CI: 1.8, 28 s). There was no association between GWG z score and offspring composite IQ score (adjusted β: −0.32; 95% CI: −0.72, 0.10). Prepregnancy BMI did not modify these associations.
Conclusions: Although GWG may be important for executive function, maternal BMI has a stronger relation than GWG to both offspring intelligence and executive function. Our findings contribute to evidence linking maternal obesity to long-term child outcomes.
Keywords: gestational weight gain, body mass index, obesity, child, cognition, intelligence
Introduction
Maternal overweight and obesity affect two-thirds of women of childbearing age in the United States (1) and increase the risk of a number of adverse offspring health outcomes, such as preterm birth, infant mortality, offspring obesity (2), insulin resistance (3), and asthma (4). Recent research suggests that offspring of overweight and obese mothers may also have impaired brain development (5, 6). It has been posited that the inflammatory and hormonal (leptin and insulin) milieu of obesity (7, 8) may lead to an over- and underactivation of a number of fetal neurodevelopmental processes (9, 10), including neuron proliferation and differentiation (11), myelination, and synapse formation (11). Studies from 3 large European cohorts reported that obese mothers had children with lower general intelligence at 3–4 y of age (5) and lower cognitive performance in infancy (6) than did normal-weight mothers.
Gaining the optimal amount of weight during pregnancy may attenuate the risk of adverse birth outcomes associated with obesity (12), but whether this is true for offspring cognition is not known. Furthermore, gestational weight gain (GWG)12 itself may be independently associated with cognitive outcomes. The data examining the association between GWG and child cognitive development in humans is limited (13). When the Institute of Medicine revised the GWG guidelines in 2009, the committee stressed the importance of filling this knowledge gap. Subsequently, one study in a large European cohort reported a modest decrease in offspring intelligence with increasing GWG (14), and 2 studies reported a null finding on the independent relation between maternal GWG and offspring cognition (15, 16).
Understanding how maternal weight and weight gain contribute to offspring cognitive development is important, but key knowledge gaps remain. Intelligence is not the only domain of offspring cognition that may be negatively associated with maternal obesity. Executive function is the coordinating system of the brain, and although it is interrelated with intelligence, it is employed for self-regulation to achieve goal-directed behavior (17, 18). Additionally, most previous work assessed general offspring cognitive development in infancy or early childhood, but infants are too young to assess domain-specific disruptions. Therefore, assessments in infancy are less predictive of adult intelligence than assessments in late childhood, which can be domain-specific (19). Importantly, a lack of adequate adjustment for maternal intelligence or stimulation at home may have biased previous findings. Our objective was to assess associations between both prepregnancy BMI and GWG and offspring intelligence and executive function at age 10.
Methods
Study participants.
The Maternal Health Practices and Child Development (MHPCD) cohort was designed to study the effects of prenatal substance use on long-term offspring outcomes (20–23). Recruitment for the MHPCD took place at a prenatal care clinic at Magee-Womens Hospital in Pittsburgh, Pennsylvania, from 1983–1986. Women ≥18 y of age and <21 wk gestation were approached and interviewed in a private setting; 1360 women were screened for eligibility (15% refusal rate). To address the primary study question, women were selected for inclusion based on their first trimester alcohol and marijuana use. The alcohol cohort was composed of all women who drank ≥3 drinks/wk and an equal sample of women who drank <3 drinks/wk [1 drink = 14 g of absolute alcohol (24)]. The marijuana cohort was composed of all women who smoked ≥2 joints/mo and an equal sample of women who smoked <2 joints/mo. The cohorts were combined for this analysis (n = 829 combined). There was a 48% overlap in the combined cohorts. At delivery, 763 mothers remained in the study and most were light substance users, including 508 (67%) who drank <3 drinks/wk and 516 (67%) who smoked <2 joints/mo in the first trimester.
Enrollment and the first study visit occurred at a median of 18.7 wk (IQR: 17.1, 20.7). The second study visit and delivery visit occurred at a median of 31.3 wk (IQR: 29.4, 33.1) and a median of 39 wk (IQR: 38, 40), respectively. Mother–child pairs were followed for 10 y. At each postpartum visit, sociodemographic status, substance use, maternal psychological status, and offspring cognitive development were assessed. Women provided informed written consent and the study was approved by the University of Pittsburgh Institutional Review Board (No. PRO14020264).
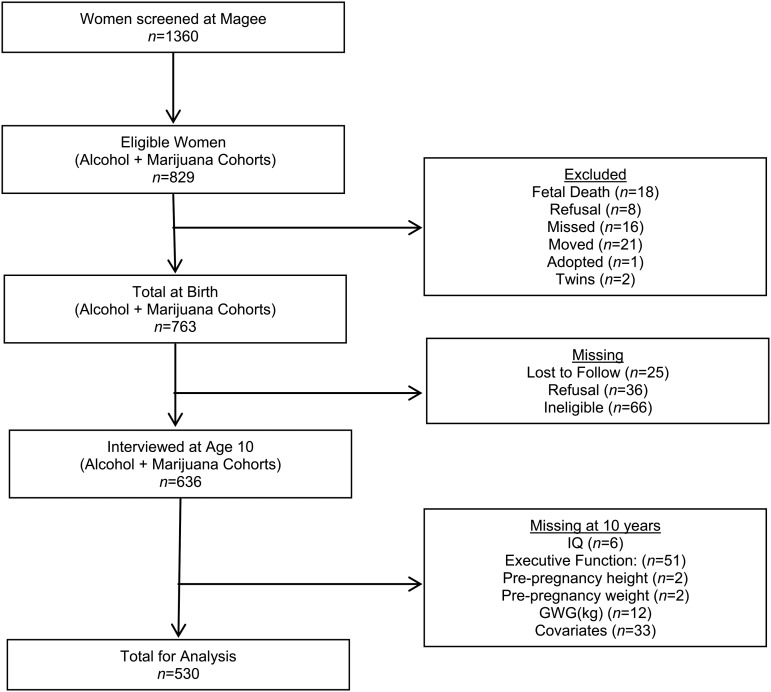
At the 10 y study visit, there were 636 mother–child dyads interviewed (83% of the birth cohort) (Figure 1). We further excluded mother–offspring pairs with incomplete data on BMI (n = 4), GWG (n = 12), intelligence (n = 6), or executive function (n = 51) assessments at age 10, or other covariates (n = 33). A total of 530 pairs were used in the final analysis.
FIGURE 1.
Participant flow diagram for the maternal health practices and child development study, Pittsburgh, 1983–1986. GWG, gestational weight gain; IQ, intelligence quotient.
Description of exposure: Prepregnancy BMI and maternal GWG.
Prepregnancy weight and height were self-reported at the first study visit. Prepregnancy BMI (weight in kilograms divided by height in meters squared) was categorized with the use of criteria from the WHO (25). We classified self-reported total GWG according to gestational age–standardized z scores, a measure of GWG that by design is uncorrelated with gestational age (26). Z score charts were developed from serial prenatal weight measurements in a random sample of normal-weight term pregnancies without complications from Magee-Womens Hospital (1998–2008) (26). Z scores were calculated with the use of charts for normal weight women to allow us to evaluate whether the association between GWG z scores and offspring intelligence and executive function varied depending on prepregnancy BMI.
Description of outcome: Intelligence and executive function.
Trained interviewers blinded to maternal prenatal and current substance use administered all neuropsychological assessments to children at age 10. Offspring intelligence quotient (IQ) was assessed with the use of the Stanford Binet Intelligence Scale 4th Edition (SBIS) (27). The SBIS has a high internal consistency (0.96) and test–retest reliability (0.95) (28). The scale has 4 subtests, which are combined to create the composite score to indicate general intellectual ability (27). We studied IQ as both a continuous and dichotomous variable [low average IQ (≤89) compared with average or above IQ (>89) based on the SBIS defined ranges (27)].
Offspring executive function was assessed with the use of the Wisconsin Card Sorting Test (WCST) and Trail Making Test Part B (TMT-B), which are both reliable and commonly used tools (28). The WCST assesses the ability of the subject to use computer feedback to shift and inhibit unwanted responses (28), measured by the number of perseverative errors. A greater number of errors indicates lower executive function. The TMT-B assesses mental flexibility, visual attention, and motor impulsivity (28), indicated by the ability to update working memory and shift to the appropriate response (29), and is measured by the time in seconds to complete Part B. A longer time to complete the scale indicates lower executive function ability.
Other covariates.
At the first study visit, trained interviewers collected information on sociodemographic characteristics (i.e., maternal age, race, parity, employment, education, income, and marital status), and women indicated the quantity and frequency of substance use during the year before pregnancy and during specific segments of the first trimester. Self-reported substance use over a 3 mo period was validated as an accurate depiction of first trimester use (30). To quantify the amount and pattern of prenatal substance use, detailed assessments of alcohol, marijuana, cigarette, and cocaine use were collected by interviewers at both prenatal visits and delivery and were summarized as average daily drinks, average daily joints, cigarettes per day, and cocaine use per trimester (yes or no), respectively. We categorized each substance into nonusers, users only during the first trimester when many women do not know they are pregnant, and use throughout pregnancy. Maternal depressive symptoms (31) and anxiety were measured at the first study visit (32). Maternal intelligence was assessed at 10 y postpartum (33). The Home Observation for Measurement of the Environment–Short Form (HOME-SF) was administered to mothers or caretakers at 10 y postpartum to assess the quality and quantity of support for cognitive and social development in the home environment. We included the HOME-SF as a continuous variable in models and as a dichotomized variable for descriptive purposes [lower stimulation (<16) compared with higher stimulation (≥16)] (34).
Data analysis.
Student’s t tests and 1-factor ANOVA were used to determine differences in child cognitive outcomes, GWG, and BMI by maternal characteristics. Pearson correlation coefficients were used to assess the strength of association between scales. Multivariable linear regression models were used to estimate β coefficients and their corresponding 95% CIs for the association between prepregnancy BMI and offspring intelligence (SBIS scale), as well as executive function (TMT-B). We estimated incidence rate ratios and 95% CIs for the relation between prepregnancy BMI and WCST executive function scale (count of errors) with the use of negative binomial regression because of a skewed and overdispersed distribution. Differences were considered significant at α < 0.05. Effect modification by maternal race was tested by including a statistical interaction term between race and BMI (continuous) in fully adjusted models. Similar models were built for GWG z scores as the main exposure, with effect modification by prepregnancy BMI tested by including a statistical interaction term between BMI and GWG z score (tested as both continuous and categorical variables) in fully adjusted models. Effect modification was present when α = 0.05 based on a Wald P-value (linear regression) or likelihood ratio test (negative binomial regression). A sample size of 530 achieved 80% and 90% power to detect an R2 of 0.01 and 0.02, respectively, attributed to 1 independent variable with the use of an F test with a significance level of 0.05. The variables tested were adjusted for an additional 7 independent variables with an R2 of 0.3 on the IQ test and 0.05–0.1 on the executive function scales (35).
We examined the nonlinear relation between each child outcome and maternal prepregnancy BMI and GWG z score with the use of restricted cubic spline terms with 3 knots at the 10th, 50th, and 90th percentiles and as a linear spline term with one knot. We compared the spline models with the use of Akaike Information Criterion and Bayesian Information Criterion model comparison criteria to select the best variable specification (36). After model estimation, we used linear combinations to calculate coefficients and 95% CIs for selected BMI values compared with 22 kg/m2 as the referent, which was selected based on the observed point of inflection. For ease of interpretation, we categorized GWG as <−1 SD, −1 SD to +1 SD, and >+1 SD from the mean because the relation between GWG and outcomes did not deviate from linearity. We selected potential confounders based on directed acyclic graph theory, a tool to identify variables that must be controlled to obtain unbiased estimates (37, 38): maternal intelligence, home environment stimulation, maternal race, parity, income, employment status, marital status, education, maternal depressive symptoms, maternal anxiety, prenatal substance use, child gender, and prepregnancy BMI (GWG models only). We did not control for factors that we believed to lie on the causal pathway between our main exposures (prepregnancy BMI and GWG) and cognitive function including child birthweight, gestational age, or child weight at age 10 (37). Parsimonious models were built by adjusting for confounders that, if removed from the model, changed the effect estimate of the primary exposure by >10% (39). Maternal race, child sex, parity, income, maternal intelligence, prepregnancy BMI (GWG models only), and the home environment met confounder inclusion criteria for all models. Prenatal substance use variables were forced into models based on a priori decisions. Adjusted predicted IQ and executive function scores and 95% CIs were plotted vs. prepregnancy BMI with covariates set to population means.
Although substance use was common in this cohort, women were recruited from an antenatal clinic serving the general obstetric population and were able to attend routine study visits and fill out assessment materials. We performed a sensitivity analysis to address the potential bias of the high amount of substance use by excluding high marijuana (>1 joint/d) (40), alcohol (>1 drink/d) (41), cigarette (≥20 cigarettes/d) (42), cocaine (any use), and illicit drug (any use) users during the first or third trimester. Analyses were conducted with the use of Stata software, version 13.0 (43). Values are reported as means ± SDs unless otherwise indicated.
Results
There were no differences in GWG, prepregnancy BMI, maternal race, substance use, offspring IQ, or executive function between those with and without missing data at 10 y (Supplemental Table 1). At enrollment, the majority of women in the MHPCD cohort were unmarried and unemployed, and had a family income of <$500/mo [<$1400/mo in 2014 dollars (44)] (Table 1). Over one-half of the women reported their race as black and nearly one-half as white. Most of the women reported moderate depressive symptoms during the first trimester, but did not use illicit drugs or marijuana prenatally. At 10 y postpartum, mothers tended to provide a low stimulating home environment and to have a low average IQ. Total GWG was 14 ± 5.8 kg and prepregnancy BMI was 23 ± 5.7 kg/m2.
TABLE 1.
Maternal characteristics at enrollment or 10 y postpartum1
| Values | |
| Enrollment | |
| Maternal race | |
| White | 254 (47.9) |
| Black | 276 (52.1) |
| Marital status | |
| Never married | 360 (67.9) |
| Married | 170 (32.1) |
| Maternal employment2 | |
| No | 389 (73.4) |
| Yes | 141 (26.6) |
| Family income, $/mo | |
| <500 | 321 (60.6) |
| ≥500 | 209 (39.4) |
| Prenatal alcohol use, any | |
| Never used | 131 (24.7) |
| Drank first trimester only | 157 (29.6) |
| Drank ≥2 trimesters | 242 (45.7) |
| Prenatal marijuana use, any | |
| Never used | 263 (49.6) |
| Smoked first trimester only | 126 (23.8) |
| Smoked ≥2 trimesters | 141 (26.6) |
| Prenatal cigarette use, any | |
| Never used | 197 (37.2) |
| Smoked first trimester only | 44 (8.3) |
| Smoked ≥2 trimesters | 289 (54.5) |
| Prenatal illicit drug use | |
| No | 467 (88.1) |
| Yes | 63 (11.9) |
| Maternal depressive symptoms | |
| Moderately depressed (≥16) | 383 (72.2) |
| Not depressed (<16) | 147 (27.8) |
| 10 y postpartum | |
| HOME stimulation scale | |
| Lower stimulation (<16) | 457 (86.3) |
| Higher stimulation (≥16) | 73 (13.8) |
| Maternal IQ | |
| Low average (≤89) | 319 (60.2) |
| Average or above (>89) | 211 (39.8) |
Values are n (%); overall n = 530. HOME, Home Observation for Measurement of the Environment. IQ, intelligence quotient.
Includes school attendance.
The prevalence of children with low average IQ (≤89) was 44%, with an IQ score of 92 ± 12. On the executive function scales, the median (IQR) number of perseverative errors was 23 (17, 29) and the time to complete the TMT-B scale was 103 ± 43 s. The Pearson correlation coefficient comparing the 2 executive function scales was 0.18. The correlations ranged from 0.29 to 0.41, comparing each executive function scale with the IQ scale.
Offspring IQ and executive function were higher in children of white mothers, married women, and mothers with higher income, average or above IQ, or a home environment that provided higher stimulation (Table 2). Child IQ was also significantly higher in children of mothers using illicit drugs (i.e., cocaine, heroin etc.), which is likely explained by the fact that illicit drug users were disproportionately white women (70%). Neither offspring IQ nor executive function differed by maternal prenatal alcohol, marijuana, or cigarette use.
TABLE 2.
Offspring intelligence and executive function scores by maternal characteristics at enrollment or 10 y postpartum1
| IQ | Executive function perseverative errors | Executive function time to complete TMT-B, s | |
| Enrollment | |||
| Maternal race | |||
| White | 95.9 ± 11.3* | 21 (14, 29)* | 93.7 ± 38.9* |
| Black | 87.6 ± 10.3 | 24 (19, 30) | 112 ± 44.1 |
| Marital status | |||
| Never married | 90.1 ± 11.2* | 23 (18, 29) | 104 ± 42.7 |
| Married | 94.7 ± 11.8 | 21 (15, 29) | 102 ± 42.7 |
| Maternal employment2 | |||
| No | 91.0 ± 11.6 | 23 (17, 30) | 105 ± 43.7 |
| Yes | 93.2 ± 11.5 | 22 (16, 29) | 99.6 ± 39.4 |
| Family income, $/mo | |||
| <500 | 90.0 ± 10.9* | 23 (17, 30) | 107 ± 44.8* |
| ≥500 | 94.0 ± 12.0 | 22 (15, 29) | 97.2 ± 38.5 |
| Alcohol use during pregnancy, any | |||
| Never used | 91.2 ± 11.6 | 23 (17, 29) | 103 ± 41.6 |
| Drank first trimester | 91.3 ± 11.4 | 22 (16, 29) | 103 ± 45.7 |
| Drank ≥2 trimesters | 91.9 ± 11.7 | 23 (17, 30) | 104 ± 41.3 |
| Marijuana use during pregnancy, any | |||
| Never used | 92.2 ± 11.6 | 22 (15, 29) | 103 ± 42.3 |
| Smoked first trimester | 91.6 ± 11.8 | 24 (18, 30) | 101 ± 40.2 |
| Smoked ≥2 trimesters | 90.4 ± 11.4 | 22 (18, 30) | 106 ± 45.5 |
| Cigarette use during pregnancy, any | |||
| Never used | 90.9 ± 11.3 | 23 (16, 30) | 106 ± 42.9 |
| Smoked first trimester | 91.3 ± 12.9 | 23 (19, 29) | 104 ± 41.2 |
| Smoked ≥2 trimesters | 92.1 ± 11.6 | 22 (17, 29) | 101 ± 42.8 |
| Illicit drug use | |||
| No | 90.1 ± 11.4* | 23 (17, 30) | 105 ± 43.4 |
| Yes | 96.2 ± 11.6 | 21 (14, 28) | 93.6 ± 35.6 |
| Maternal depressive symptoms | |||
| Moderately depressed (≥16) | 91.2 ± 11.5 | 24 (17, 29) | 105 ± 44.3 |
| Not depressed (<16) | 92.6 ± 11.6 | 22 (15, 29) | 98.8 ± 37.7 |
| 10 y postpartum | |||
| HOME stimulation scale | |||
| Lower Stimulation (<16) | 90.7 ± 11.3* | 23 (17, 30)* | 106 ± 42.8* |
| Higher Stimulation (≥16) | 97.5 ± 11.7 | 21 (13, 27) | 86.8 ± 38.1 |
| Maternal IQ | |||
| Low average (≤89) | 87.9 ± 10.7* | 24 (19, 31)* | 109 ± 44.1* |
| Average or above (>89) | 97.2 ± 10.7 | 20 (14, 27) | 94.0 ± 38.7 |
Values are means ± SDs or medians (IQRs); overall n = 530. *Categories differ from one another (t test or Wilcoxon–Mann-Whitney) P < 0.05. HOME, Home Observation for Measurement of the Environment; IQ, intelligence quotient; TMT-B, Trail Making Test Part B.
Includes school attendance.
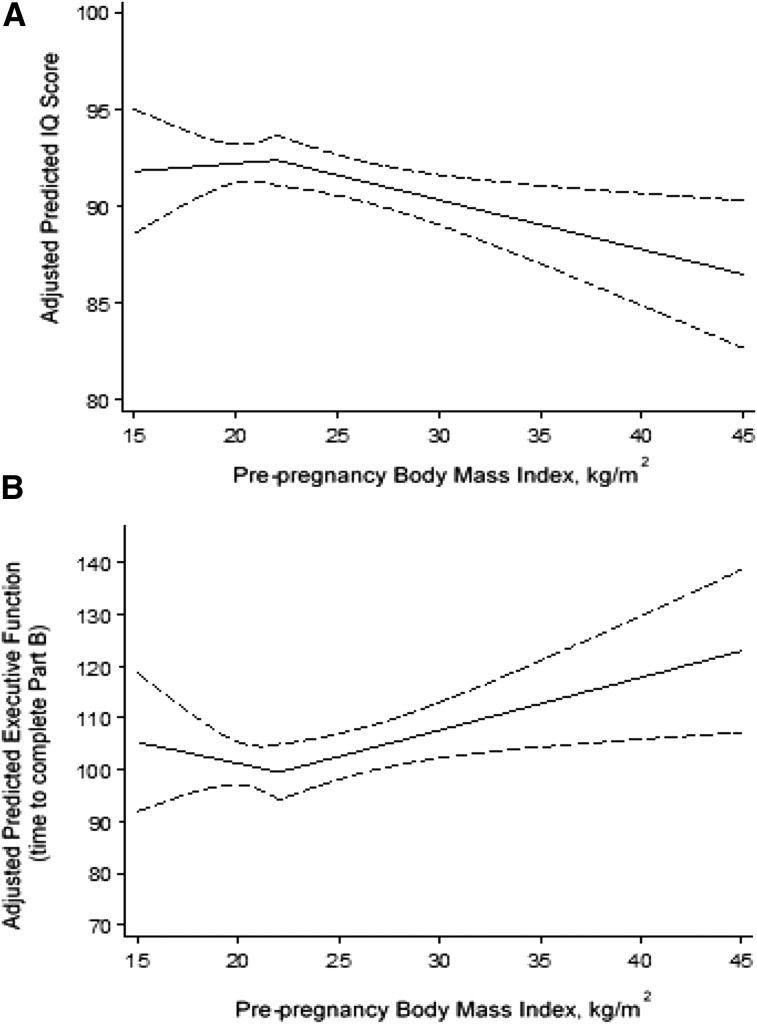
There was no difference in offspring IQ or executive function by prepregnancy BMI or GWG before confounder adjustment (Supplemental Table 2). After adjusting for maternal race, child sex, parity, income, maternal intelligence, home environment, and prenatal substance use, there was a significant nonlinear relation between prepregnancy BMI and offspring IQ (Table 3 and Figure 2A). Offspring IQ was relatively constant when prepregnancy BMI was below 22 kg/m2. In contrast, offspring IQ was 1.1 points (adjusted β: −1.1; 95% CI: −1.8, −0.3) lower among women with a BMI of 26 kg/m2 and 2.5 points (adjusted β: −2.5; 95% CI: −4.5, −0.6) lower among women with a BMI of 32 kg/m2 compared with women with a BMI of 22 kg/m2. This same trend was observed for the IQ quantitative subscale (Supplemental Table 3). Similarly, on the Trails B, offspring executive function time-to-complete was longer as prepregnancy BMI increased beyond 22 kg/m2 (Table 4 and Figure 2B). Offspring time to complete the scale was 4.1 s slower (adjusted β: 4.1; 95% CI: 0.9, 7.3) when their mothers had a BMI of 26 kg/m2 than with mothers who had a BMI of 22 kg/m2. There was no association between maternal BMI and the number of executive function perseverative errors.
TABLE 3.
Multivariable linear and negative binomial regression estimates of the association between prepregnancy BMI and offspring intelligence and executive function1
| Prepregnancy BMI,2 kg/m2 | IQ3 | Executive function perseverative errors3 | Executive function time to complete TMT-B,3 s |
| 18 | –0.3 (−2.6, 1.9) | 0.93 [0.9, 1.0] | 3.3 (−6.0, 13) |
| 20 | −0.2 (−1.3, 0.9) | 0.97 [0.9, 1.0] | 1.7 (−3.1, 6.4) |
| 22 | 0.0 | 1.0 | 0.0 |
| 24 | −0.5 (−0.9, −0.1) | 1.0 [1.0, 1.4] | 2.0 (0.4, 3.6) |
| 26 | −1.1 (−1.8, −0.3) | 1.0 [0.9, 1.1] | 4.1 (0.9, 7.3) |
| 28 | −1.5 (−2.6, −0.4) | 1.0 [0.9, 1.1] | 6.0 (1.3, 11) |
| 30 | −2.1 (−3.6, −0.5) | 0.9 [0.9, 1.1] | 8.1 (1.8, 15) |
| 32 | −2.5 (−4.5, −0.6) | 0.9 [0.9, 1.1] | 10.1 (2.2, 18) |
| 34 | −3.2 (−5.6, −0.8) | 0.9 [0.8, 1.1] | 12.7 (2.8, 23) |
Values are adjusted β coefficients (95% CIs) or adjusted incidence rate ratios [95% CIs]; n = 530. The 95% CI values that do not contain the null value (0.0 or 1.0) are statistically significant at P < 0.05. IQ, intelligence quotient; TMT-B, Trail Making Test Part B.
Modeled with the use of a linear spline with a single knot specified at a BMI of 22 kg/m2.
Adjusted for maternal race, child sex, parity, income, maternal intelligence, the home environment, maternal depressive symptoms, and prenatal substance use (marijuana, alcohol, cigarettes, and illicit drugs).
FIGURE 2.
Predicted association between prepregnancy BMI and offspring intelligence (A) and offspring executive function (B). The solid lines represent the point estimate and dashed lines represent 95% CIs. Predicted scores were estimated with the use of linear regression and were set at the population average for maternal race, child sex, parity, income, maternal intelligence, the home environment, maternal depressive symptoms, and prenatal substance use (marijuana, alcohol, cigarette, and illicit drugs). Prepregnancy BMI was modeled with the use of a single-knot (BMI = 22 kg/m2) linear spline. IQ, intelligence quotient.
TABLE 4.
Multivariable linear and negative binomial regression estimates of the association between GWG z score and offspring intelligence and executive function1
| GWG z score2 | IQ3 | Executive function perseverative errors3 | Executive function time to complete TMT-B,3 s |
| <−1 SD | 1.7 (−0.27, 3.7) | 1.1 [0.96, 1.2] | −0.33 (−8.8, 8.1) |
| −1 SD to +1 SD | 0.0 | 1.0 | 0.0 |
| >+1 SD | −1.1 (−4.2, 2.2) | 0.94 [0.82, 1.1] | 15 (1.8, 28) |
Values are adjusted β coefficients (95% CIs) or adjusted incidence rate ratios [95% CIs]; n = 530. The 95% CI values that do not contain the null value (0.0 or 1.0) are statistically significant at P < 0.05. GWG, gestational weight gain; IQ, intelligence quotient; TMT-B, Trail Making Test Part B.
<−1 SD, n = 131; −1 SD to +1 SD, n = 354; >+1 SD, n = 45.
Adjusted for maternal race, child sex, parity, income, maternal intelligence, the home environment, maternal depressive symptoms, and prenatal substance use (marijuana, alcohol, cigarettes, and illicit drugs).
High and low maternal GWG z scores were not associated with offspring SBIS composite IQ score (Table 4) or subscales (Supplemental Table 4). Compared with children of mothers who gained from −1 SD to +1 SD, offspring of mothers who had GWG >+1 SD from the mean had lower executive function performance (adjusted β: 15; 95% CI: 1.8, 28), indicated by a longer time to complete the TMT-B scale, after adjustment for confounders (Table 4). GWG was not associated with the number of perseverative errors on the WCST. These results did not vary by prepregnancy BMI.
None of the associations varied by maternal race, and the addition of other potential confounders such as maternal income, employment, and education 10 y postpartum had no meaningful impact on the results (Supplemental Table 5). Results were not meaningfully different after excluding high substance users (Supplemental Tables 6 and 7).
Discussion
In a longitudinal cohort of mother–child pairs followed for 10 y, we observed that offspring IQ score and executive function performance on 1 of 2 scales decreased as prepregnancy BMI rose above 22 kg/m2. Mothers with GWG >+1 SD from the mean (>23 kg at 40 wk) were more likely to have a child with lower executive function than women who gained less weight, but there were no differences in offspring intelligence. These associations were observed after adjusting for a number of important confounders, including maternal intelligence, the home environment, maternal depressive symptoms, maternal race, child sex, parity, income, and prenatal substance use.
Executive function is used every day to plan, organize, and adjust to novel situations (45), yet it is complex and remains difficult to define and measure. One accepted model suggests that 3 main executive function constructs exist: “inhibition” (suppressing responses), “shifting” (flexibly moving between tasks), and “updating” (monitoring of mental content) (46). The TMT-B assessed the “shifting” and “updating” constructs and the Wisconsin Card Sorting Test assessed “shifting” and “inhibition” constructs. Our results suggest that the “updating” dimension of executive function may be susceptible to excessive maternal weight gain or prepregnancy BMI, whereas “inhibition” and “shifting” may be more resilient. We are aware of only one other study that has assessed offspring executive function and the independent relation with prepregnancy BMI and GWG. Buss et al. (47) measured the “inhibition” construct of executive function with the use of the Go/No-Go Task in a sample of 174 mother–child pairs (47). Consistent with our findings, this study observed no association between GWG and the “inhibition” construct of executive function (47). In contrast with our findings, that study observed lower offspring “inhibition” performance in children of obese mothers. However, the study is limited by the use of total GWG (which cannot untangle effects of weight gain from effects of shortened gestational age) and adjustment for factors on the causal pathway (e.g., gestational age and birthweight), which may bias the findings. Although executive function on one scale appears to be associated with maternal obesity, the null finding for “inhibition” may be explained by the challenge of measuring construct-specific functioning.
Our results are consistent with the majority of literature that has reported lower intelligence in children of mothers with a higher BMI, even after adjusting for important confounders (5, 6, 14, 48–50). Bliddal et al. (51) and Huang et al. (52) identified a similar nonlinear association between maternal BMI and offspring intelligence, with offspring intelligence peaking at a maternal BMI of 20–22 kg/m2 (51, 52). Huang et al. (52) reported a 2 point deficit in IQ scores in children of obese mothers compared with those of normal-weight mothers, which is similar to the 3 point difference reported in our study. Although a 2–3 point difference in IQ is modest, this difference may be more drastic in offspring of severely obese women—a group that is rapidly increasing (1). We had too few severely obese women to evaluate this relation directly. Additionally, although other factors such as genetics may have a stronger impact on offspring IQ than maternal obesity, prepregnancy BMI has the advantage of being modifiable.
Few studies have examined the association between GWG and IQ, and the findings are mixed. Gage et al. (14) reported a 0.02–0.07 point increase in child IQ with increasing trimester-specific GWG (14), with first- and second-trimester GWG having the strongest relation with IQ. Despite the statistical significance, this increase in IQ may be too small to have clinical implications. Consistent with our findings, the remainder of studies reported no association between GWG and offspring IQ (15, 16, 53). Although the majority of evidence suggests no independent relation between GWG and offspring intelligence, GWG may still be important for offspring intelligence when modified by prepregnancy BMI. However, the cohort used in our study and cohorts in previous studies may be too small to detect effect modification by BMI.
Results may not generalize to today’s obstetric population because of differences in sample characteristics, including higher substance use than the national norm, less obesity, and lower socioeconomic status. With our smaller sample size, we could not assess severe maternal obesity. However, there is no evidence that cognitive development or its association with obesity or substance use has changed over time. Although prenatal substance use may directly affect offspring cognition, when we examined the impact of excluding the high substance-using women in our results, our estimates remained unchanged. Our findings also contribute information to the literature on low-income women, an under-represented group in the current literature who are potentially at higher risk of offspring impairment. The compounding stressors associated with a lower socioeconomic status may contribute to a more susceptible environment for excess maternal weight to affect offspring cognitive development.
A potential limitation of this study was the use of a single questionnaire, the HOME-SF, to assess the quality of the home environment. Although this questionnaire is a widely employed and reliable tool, it might not adequately cover all areas of cognitive and environmental stimulation. Therefore, our results may be biased away from the null. Additionally, we were limited by a reliance on self-reported prepregnancy weight, height, and total GWG. Although some data suggest that BMI is correctly classified in 85% of women, other studies have shown that the accuracy of self-reported weight, height, and BMI varies by how heavy the mother is and other maternal characteristics, including race/ethnicity and education (54). Unfortunately, we do not have information on the validity of self-reported weight and weight gain in this population. Lastly, the longitudinal nature of this study lends itself to attrition bias. However, the retention rate at 10 y was 83%, and there was no difference in GWG, BMI, maternal race, or substance use between those with and without missing data at age 10, making it unlikely that this bias is of concern.
Our study had several unique strengths. The longitudinal nature of this study allowed us to assess offspring cognition at 10 y, a time point when domain-specific dysfunction can be measured. The objective nature and high construct validity and reliability of the cognitive assessment tools instill confidence that children are correctly classified. In addition, we controlled for a number of important confounders, including socioeconomic status, maternal intelligence, and home environment. We also evaluated nonlinear relations between BMI, GWG, and offspring cognition, because our goal was to most accurately describe underlying associations. Lastly, this study used a measure to assess GWG that is independent of gestational age, which is important when studying outcomes such as cognitive development that are associated with early delivery (55).
This study provides valuable insight into the relation between maternal obesity and long-term offspring intelligence and executive function in children at 10 y of age. In general, prepregnancy BMI remained more strongly associated with cognitive outcomes than GWG. The observed decrease in offspring intelligence and executive function in children of obese mothers compared with children of normal-weight mothers may not be meaningful at an individual level, but may have a substantial impact on child cognition in the population.
Our findings bolster the notion that offspring of obese mothers may be at an increased risk of impaired cognition. However, future research into the mechanisms underpinning the relation between child cognition and maternal weight and weight gain is needed. Unfortunately, we lacked biospecimens to evaluate markers of the inflammatory or hormonal milieu. Future research should expand on our findings by examining constructs of executive function and other domains of cognition potentially affected by maternal obesity, and evaluate whether there is merit to screening children of obese mothers for cognitive delays.
Acknowledgments
SJP, GAR, JAH, KPH, MMB, and LMB designed the research; GAR and NLD provided essential materials; SJP analyzed the data, wrote the paper, and had primary responsibility for the final content. All authors critically reviewed the drafts and read and approved the final manuscript.
Footnotes
Abbreviations used: GWG, gestational weight gain; HOME-SF, Home Observation for Measurement of the Environment–Short Form; IQ, intelligence quotient; MHPCD, Maternal Health Practices and Child Development; SBIS, Stanford Binet Intelligence Scale 4th Edition; TMT-B, Trail Making Test Part B; WCST, Wisconsin Card Sorting Test.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 2.Vasudevan C, Renfrew M, McGuire W. Fetal and perinatal consequences of maternal obesity. Arch Dis Child Fetal Neonatal Ed 2011;96:F378–82. [DOI] [PubMed] [Google Scholar]
- 3.Mingrone G, Manco M, Mora ME, Guidone C, Iaconelli A, Gniuli D, Leccesi L, Chiellini C, Ghirlanda G. Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care 2008;31:1872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe A, Braback L, Ekeus C, Hjern A, Forsberg B: Maternal obesity during pregnancy as a risk for early-life asthma. J Allergy Clin Immunol 2011;128:1107–9. [DOI] [PubMed] [Google Scholar]
- 5.Brion M-J, Zeegers M, Jaddoe V, Verhulst F, Tiemeier H, Lawlor DA, Smith GD. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 2011;127:e202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casas M, Chatzi L, Carsin AE, Amiano P, Guxens M, Kogevinas M, Koutra K, Lertxundi N, Murcia M, Rebagliato M, et al. Maternal prepregnancy overweight and obesity, and child neuropsychological development: two Southern European birth cohort studies. Int J Epidemiol 2013;42:506–17. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan EL, Nousen EK, Chamlou KA. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol Behav 2014;123:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta SH, Kerver JM, Sokol RJ, Keating DP, Paneth N. The association between maternal obesity and neurodevelopmental outcomes of offspring. J Pediatr 2014;165:891–6. [DOI] [PubMed] [Google Scholar]
- 9.Tozuka Y, Kumon M, Wada E, Onodera M, Mochizuki H, Wada K. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem Int 2010;57:235–47. [DOI] [PubMed] [Google Scholar]
- 10.Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci 2009;27:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouret SG. Neurodevelopmental actions of leptin. Brain Res 2010;1350:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cedergren MI. Optimal gestational weight gain for body mass index categories. Obstet Gynecol 2007;110:759–64. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen KY. A., editors: Weight gain during pregnancy: reexamining the guidelines. Washington (DC): Institute of Medicine and National Research Council of the National Academies; 2009. [PubMed] [Google Scholar]
- 14.Gage SH, Lawlor DA, Tilling K, Fraser A. Associations of maternal weight gain in pregnancy with offspring cognition in childhood and adolescence: findings from the Avon Longitudinal Study of Parents and Children. Am J Epidemiol 2013;177:402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keim SA, Pruitt NT. Gestational weight gain and child cognitive development. Int J Epidemiol 2012;41:414–22. [DOI] [PubMed] [Google Scholar]
- 16.Tanda R, Salsberry PJ, Reagan PB, Fang MZ. The impact of prepregnancy obesity on children’s cognitive test scores. Matern Child Health J 2013;17:222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brydges CR, Reid CL, Fox AM, Anderson M. A unitary executive function predicts intelligence in children. Intelligence 2012;40:458–69. [Google Scholar]
- 18.Mahone EM, Hagelthorn KM, Cutting LE, Schuerholz LJ, Pelletier SF, Rawlins C, Singer HS, Denckla MB. Effects of IQ on executive function measures in children with ADHD. Child Neuropsychol 2002;8:52–65. [DOI] [PubMed] [Google Scholar]
- 19.Domsch H, Lohaus A, Thomas H. Prediction of childhood cognitive abilities from a set of early indicators of information processing capabilities. Infant Behav Dev 2009;32:91–102. [DOI] [PubMed] [Google Scholar]
- 20.Day NL, Richardson GA, Geva D, Robles N. Alcohol, marijuana, and tobacco: Effects of prenatal exposure on offspring growth and morphology at age six. Alcohol Clin Exp Res 1994;18:786–94. [DOI] [PubMed] [Google Scholar]
- 21.Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol 2002;24:309–20. [DOI] [PubMed] [Google Scholar]
- 22.Goldschmidt L, Richardson GA, Willford J, Day NL. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry 2008;47:254–63. [DOI] [PubMed] [Google Scholar]
- 23.Goldschmidt L, Richardson GA, Willford JA, Severtson SG, Day NL. School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol Teratol 2012;34:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A pocket guide for alcohol screening and brief intervention [Internet]. [cited 2015 Aug 15.] Available from: http://pubs.niaaa.nih.gov/publications/Practitioner/PocketGuide/pocket.pdf.
- 25.World Health Organization. Physical status: The use and interpretation of anthropometry. World Health Orgn Tech Rep Ser 1995. Report No.: 854. [PubMed] [Google Scholar]
- 26.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr 2013;97:1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorndike R, Hagen E, Sattler J. The Stanford-Binet Intelligence Scale. 4th ed. Chicago: Riverside Publishing; 1986. [Google Scholar]
- 28.Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford University Press; 2006. [Google Scholar]
- 29.Hedden T, Yoon C. Individual differences in executive processing predict susceptibility to interference in verbal working memory. Neuropsychology 2006;20:511–28. [DOI] [PubMed] [Google Scholar]
- 30.Robles N, Day NL. Recall of alcohol consumption during pregnancy. J Stud Alcohol 1990;51:403–7. [DOI] [PubMed] [Google Scholar]
- 31.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 32.Speilberger CD. Preliminary manual for the State-Trait Personality Inventory (STPI). Tampa (FL): University of South Florida; 1979. [Google Scholar]
- 33.Brooker BH, Cyr JJ. Tables for clinicians to use to convert WAIS-R short forms. J Clin Psychol 1986;42:982–6. [Google Scholar]
- 34.Baker P, Mott F: National longitudinal study of youth child handbook. 1989. [Google Scholar]
- 35.Hintze J. PASS 2008 [Internet]. NCSS, LLC. Kaysville (UT) [cited 2015 Jul 5.]. Available from: www.ncss.com.
- 36.Kuha J. AIC and BIC: Comparisons of assumptions and performance. Sociol Methods Res 2004;33:188–229. [Google Scholar]
- 37.Hernán MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002;155:176–84. [DOI] [PubMed] [Google Scholar]
- 38.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- 39.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989;79:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day NL, Richardson GA, Goldschmidt L, Robles N, Taylor PM, Stoffer DS, Cornelius MD, Geva D. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol 1994;16:169–75. [DOI] [PubMed] [Google Scholar]
- 41.Day NL, Richardson GA. An analysis of the effects of prenatal alcohol exposure on growth: A teratologic model. Am J Med Genet C Semin Med Genet 2004;127C:28–34. [DOI] [PubMed] [Google Scholar]
- 42.Cornelius MD, Goldschmidt L, DeGenna N, Day NL. Smoking during teenage pregnancies: Effects on behavioral problems in offspring. Nicotine Tob Res 2007;9:739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.StataCorp: Stata Statistical Software: Release 13. College Station (TX): StataCorp LP; 2013. [Google Scholar]
- 44.Consumer Price Index Calculator [Internet]. [cited 2015 Jul 13.] Available from: http://data.bls.gov/cgi-bin/cpicalc.pl.
- 45.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry 2005;57:1336–46. [DOI] [PubMed] [Google Scholar]
- 46.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognit Psychol 2000;41:49–100. [DOI] [PubMed] [Google Scholar]
- 47.Buss C, Entringer S, Davis EP, Hobel CJ, Swanson JM, Wadhwa PD, Sandman CA. Impaired executive function mediates the association between maternal prepregnancy body mass index and child ADHD symptoms. PLoS One 2012;7:e37758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basatemur E, Gardiner J, Williams C, Melhuish E, Barnes J, Sutcliffe A. Maternal prepregnancy BMI and child cognition: a longitudinal cohort study. Pediatrics 2013;131:56–63. [DOI] [PubMed] [Google Scholar]
- 49.Craig WY, Palomaki GE, Neveux LM, Haddow JE. Maternal body mass index during pregnancy and offspring neurocognitive development. Obstetric Medicine 2013;6:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinkle SN, Schieve LA, Stein AD, Swan DW, Ramakrishnan U, Sharma AJ. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int J Obes (Lond) 2012;36:1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bliddal M, Olsen J, Stovring H, Eriksen HL, Kesmodel US, Sorensen TI, Nohr EA. Maternal prepregnancy BMI and intelligence quotient (IQ) in 5-year-old children: a cohort based study. PLoS One 2014;9:e94498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang L, Yu X, Keim S, Li L, Zhang L, Zhang J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Int J Epidemiol 2014;43:783–92. [DOI] [PubMed] [Google Scholar]
- 53.Neggers YH, Goldenberg RL, Ramey SL, Cliver SP. Maternal prepregnancy body mass index and psychomotor development in children. Acta Obstet Gynecol Scand 2003;82:235–40. [DOI] [PubMed] [Google Scholar]
- 54.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J 2007;11:137–44. [DOI] [PubMed] [Google Scholar]
- 55.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol 2011;174:1062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]