Abstract
BACKGROUND
Unlike most chronic diseases, approved osteoporosis treatments are generally limited to a single drug at a fixed dose and frequency. Nonetheless, no approved therapy is able to restore skeletal integrity in most osteoporotic patients and the long-term use of most osteoporosis drugs remains controversial due to efficacy and safety concerns. Thus, many patients are treated with the sequential use of two or more therapies. Discontinuing teriparatide and denosumab, two of our most potent agents, results in rapidly declining bone mineral density (BMD). It is unknown if switching from one therapy to another prevents this decline or further increases BMD.
METHODS
This study is a pre-planned extension of the Denosumab and Teriparatide Administration study (DATA), a 24-month study in which 94 postmenopausal osteoporotic women were randomized to receive 24-months of teriparatide (20-μg-daily), denosumab (60-mg-every-6-months), or both drugs. In this extension trial, women originally assigned to 24-months of teriparatide received 24-months of denosumab whereas subjects originally randomized to 24-months of denosumab received 24-months of teriparatide. Subjects who originally received both drugs, received an additional 24-months of denosumab alone. BMD at the hip, spine and wrist were measured 6,12,18, and 24 months after the drug transitions as were biochemical markers of bone turnover.
FINDINGS
Transitioning from teriparatide or combination therapy to denosumab further increases BMD whereas switching from denosumab to teriparatide results in transient bone loss at the spine and hip and progressive loss at the radius shaft. After 48-months, spine BMD increased by18.3±8.5%, 14.0±6.7%, and 16.0±4.1% in the teriparatide-to-denosumab, denosumab-to-teriparatide, and combination-to-denosumab groups, respectively (P=NS for between-group comparisons). Conversely, total hip BMD increased most in the combination-to-denosumab group (8.6±3.0%), intermediately in the teriparatide-to-denosumab group (6.6±3.3%) and least in the denosumab-to-teriparatide group (2.7±3.3%), (P<0.05 for all between-group comparisons). Femoral neck BMD changes resembled those at the total hip. After 48-months, radius BMD was unchanged in the teriparatide-to-denosumab group (0.0±2.9%), decreased by −1.8±5.9% in the denosumab-toteriparatide group, and increased by 2.8±3.2% in the combination-to-denosumab group (P<0.01 combination-to-denosumab versus both other groups).
INTERPRETATION
In postmenopausal osteoporotic women switching from teriparatide to denosumab, BMD continued to increase whereas switching from denosumab to teriparatide results in progressive or transient bone loss. Combination denosumab/teriparatide therapy followed by denosumab alone results in the largest 4-year increases in hip and wrist BMD. These results should be considered when choosing the initial and subsequent management of postmenopausal osteoporotic patients.
Introduction
Osteoporotic fractures, over 75% of which occur in women, are a major cause of death, disability and worldwide healthcare expenditure.1,2 Unlike the vast majority of chronic diseases, approved osteoporosis treatments are generally limited to the use of a single drug at a fixed dose and dosing frequency. And while the therapeutic options in osteoporosis treatment have expanded greatly over the past two decades, no currently approved therapy is able to restore skeletal integrity in most patients with established disease.
Current medications approved to treat postmenopausal osteoporosis can be separated into two categories. The most commonly used drugs are the anti resorptive medications, a class of drugs that includes the nitrogen-containing bisphosphonates and the RANK-ligand inhibitor, denosumab. Less commonly used and generally reserved for patients with severe and established osteoporosis, is the anabolic agent teriparatide (PTH-1-34).Given that current recommendations discourage the long-term use of potent antiresorptive osteoporotic medications and teriparatide's use is limited to 18-24 months by regulatory bodies,3,4 the treatment of patients with established or severe osteoporosis often requires the sequential use of multiple drugs.
While denosumab and teriparatide are two of the most potent therapies currently available to physicians,5 both are associated with abrupt and rapid bone loss when discontinued.6,7 It is currently not known if switching from denosumab to teriparatide or from teriparatide to denosumab can prevent this decline in bone mineral density (BMD) or further increase bone mass.In the Denosumab And Teriparatide Administration study of postmenopausal osteoporotic women(DATA), we reported that concurrent denosumab and teriparatide administration increases spine and hip BMD more than either drug alone and to a greater degree than has been achieved with any currently available agent.8,9 Less positive results have been reported for combinations of teriparatide and bisphosphonates.10-12 In the prospectively planned DATA-Switch study, we nowtest the hypothesis that the transition from teriparatide or combined teriparatide/denosumab to denosumab monotherapy and the transition from denosumab to teriparatide monotherapy will further increase BMD in postmenopausal osteoporotic women. In so doing, we aimed to provide physicians the evidence necessary to formulate a rational approach to the sequential and combined use of these medications.
Methods
Participants
Postmenopausal women aged 45 or older were recruited through targeted mailings, advertisements, and physician referrals between September2009 and January 2011. Subjects were required to be at least 36 months since last menses (or since hysterectomy if FSH level > 40 U/L) and at high fracture risk. High fracture risk was defined as a BMD T score ≤ −2.5 at the spine, hip, or femoral neck; T score ≤ −2.0 with at least one BMD-independent risk factor (fracture after age 50, parental hip fracture after age 50, prior hyperthyroidism, inability to rise from a chair with arms elevated, or current smoking),13 or T score ≤ −1.0 with a history of a fragility fracture. These entry criteria resulted in mean 10-year fracture risks (based on the World Health Organization Fracture Risk Assessment Tool, https://www.shef.ac.uk/FRAX/index.aspx) of 14.4% and 2.6% for major osteoporotic fracture and hip fracture, respectively. Fifty-nine percent of subjects had at least one T-score of ≤ −2.5.Subjects were excluded if they had evidence of hyperparathyroidism, vitamin D deficiency (serum level less than 20 ng/mL), other congenital or acquired bone disease, history of malignancy (with the exception of non-melanoma skin cancer), history of ionizing radiation therapy, significant cardiopulmonary, liver, or renal disease, major psychiatric disease, or excessive alcohol intake. Subjects were also excluded if they had ever taken parenteral bisphosphonates, teriparatide, or strontium ranelate. Additionally, subjects were excluded if they had taken glucocorticoids or oral bisphosphonates within six months of enrollment or if they had taken estrogen, selective estrogen receptor modulators, or calcitonin within three months of enrollment. All provided written informed consent. The study was approved by the Partners Healthcare Institutional Review Board and is registered with ClinicalTrials.gov, number NCT00926380.
Randomization and Masking
This study was performed at Massachusetts General Hospital in Boston, MA. The original DATA study was 24-month, open label, randomized controlled trial. Subjects were originally randomized on a 1:1:1 basis to receive teriparatide 20-μg subcutaneously daily (Forteo, Eli Lilly, Inc., Indianapolis, IN), denosumab 60-mg subcutaneously every six months (Prolia, Amgen, Inc., Thousand Oaks, CA), or both medications. Randomization was performed in random blocks of three or six created with a computer algorithm. Before randomization, women were stratified for age (younger than 65 years vs. 65 years or older) and previous bisphosphonate use. Subjects completing the 24-month trial were then offered enrollment in the DATA-Switch study as long they continued to meet 1 of the following 3 criteria:
DXA spine or hip T-score ≤−1.5
DXA spine or hip T-score ≤−1.0 plus 1+ of the following risk factors for fracture: fracture after age 50, parental hip fracture after age 50, prior hyperthyroidism, inability to rise from a chair with one's arms elevated, current tobacco smoker.
History of > 1 adult low-trauma* fracture with any BMD (*low-trauma fracture = fracture after no trauma; or fracture after falling < 6 inches when stationary or moving slower than a run).
In DATA-Switch, women originally assigned to 24-months of teriparatide received 24-months of denosumab whereas those subjects who were originally randomized to 24-months of denosumab received 24-months of teriparatide. Subjects who originally received both drugs, received an additional 24-months of denosumab alone(Fig 1).Switching subjects from combination therapy to teriparatide was not an option, as teriparatide administration is limited to 2-years by regulatory bodies.After the medication transition, subjects were seen 1 month later (month 25), and then again at month 30, 36, 42, and 48. At the 25-month visit subjects underwent blood sampling only whereas at all the other visits blood sampling and dual energy x-ray absorptiometry (DXA) were performed. All blood sampling was done prior to teriparatide administration (i.e. 24-hours after the last teriparatide dose) and physicians interpreting BMD assessments were blinded to treatment group. All subjects were given calcium carbonate and vitamin D supplements if needed to achieve a total daily intake of 1200-mg of elemental calcium and to maintain a serum 25-hydroxy vitamin D level of at least 20 ng/mL. Adherence to teriparatide was assessed by medication diary.
Figure 1.
DATA-Switch study design
Study Assessments
Bone Mineral Density Measurements
Areal BMD of the posterior-anterior lumbar spine, total hip, femoral neck, and distal 1/3 radius shaft was measured by DXA using a Hologic QDR 4500A densitometer (Hologic, Waltham, MA). All scans of an individual subject were performed on the same densitometer. Quality control measurements were performed daily with a Hologic anthropomorphic spine phantom. Our standard deviations of in vivo same-day reproducibility are 0.005, 0.006, and 0.007 g/cm2 for PA spine, total hip, and femoral neck BMD measurements, respectively.
Biochemical Measurements
Fasting morning blood samples (collected 24 hours after last injection if taking teriparatide) were obtained at each visit. Serum osteocalcin, a marker of bone formation, was measured via electrochemiluminescent immunoassay (Meso Scale Discovery, Rockville, Maryland) with inter-and intra-assay coefficients of variation (CVs) of 10 and 8%, respectively. Serum β-c-terminal telopeptide of type one collagen (CTX), a marker of bone resorption,was measured via a fully automated electrochemiluminescent immunoassay (Roche Diagnostics, Indianapolis, IN) with an inter-assay CV of <5%. The limit of detection for serum CTX was 0.01 ng/mL and the reportable range was 0.01 to 5.99 ng/mL. Biochemical markers of bone turnover were only measured in subjects completing 48-months of therapy. For each marker, all blood samples from a participant were analyzed together in the same assay run.
Safety and Tolerability
Study physicians assessed the safety and tolerability of the medications at each visit. At the time of reporting, a physician also determined whether each adverse event was related to the study drug.
Statistical analysis
Sample size considerations were reported previously.9 Statistical analysis was carried out using SAS version 9.2. Between-group baseline characteristics of DATA-Switch enrollees were compared by one-way analysis of variance (ANOVA). The predetermined primary end-point was the percent change in PA spine BMD over four years. Secondary endpoints included the percent change in total hip, femoral neck, and radius shaft BMD as well as the percent change in serum osteocalcin and CTX. For BMD, we used a modified intention-to-treat analysis, which included all data from subjects completing at least one additional bone density measurement after switching therapies (month 30). Between-group differences in the mean change in BMD from baseline to 48-months were examined by one-way ANOVA and subsequent between-group differences confirmed by independent samples t-test. Between-group differences in the percent change in BMD from 24 to 48 months were also examined by one-way ANOVA and if significant by subsequent between-group differences confirmed by independent samples t-test. Biochemical markers of bone turnover measurements were restricted to subjects who completed all visits (valid completers). As the changes in these marker were not normally distributed, the medians and 25th-75th percentiles were used for data summary and the between group differences in each marker at each time point was examined by Wilcoxon's Rank Sum test. Two-sided P-values of ≤0.05 were considered statistically significant.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or thewriting of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
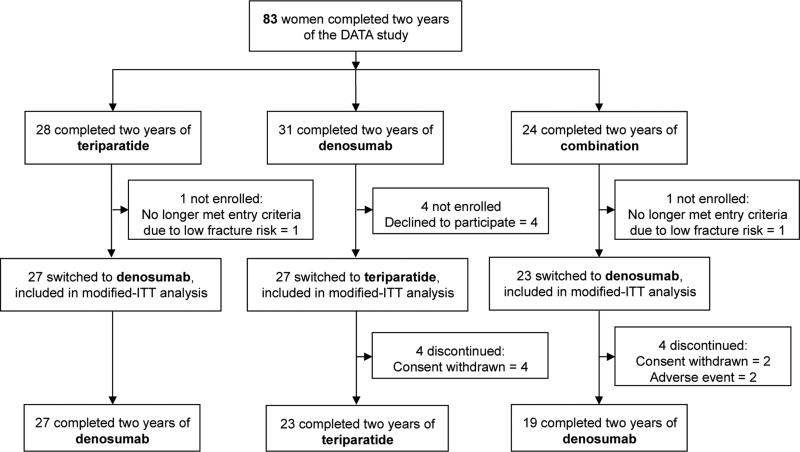
Of the 94 women enrolled in the DATA study, 83 completed 24-months of treatment and were considered for participation in DATA-Switch. Of the 83 potential enrollees, 77 subjects completed at least 1 post-baseline visit (modified intention to treat population) and 69 completed all visits through 48-months (Fig 2).
Figure 2.
Subject disposition.
There were no significant differences in the baseline characteristics among the three treatment groups in patients enrolled in DATA-Switch (Table 1). Additionally, there were no significant differences in the baseline characteristics of women in the DATA-Switch cohort as compared to those original DATA population (either within each group or the population as a whole).
Table 1.
Baseline clinical characteristics of the study subjects.
| Characteristic | Teriparatide (N=27) | Denosumab (N=27) | Combination (N=23) | P value |
|---|---|---|---|---|
| Age (year) | 66.1 ± 7.9 | 65.1 ± 6.2 | 65.3 ± 8.0 | 0.88 |
| Body mass index (kg/m2) | 25.5 ± 3.7 | 23.8 ± 4.1 | 25.9 ± 5.2 | 0.20 |
| Percent White, non-Hispanic(no, %) | 27 (100%) | 24 (89%) | 20 (87%) | 0.17 |
| Clinical fracture at age >45 (no, %) | 14 (52%) | 10 (37%) | 8 (35%) | 0.40 |
| Previous oral bisphosphonate use (no, %) | 12 (44%) | 9 (33%) | 9 (39%) | 0.70 |
| Duration of use (months) | 45 ± 23 | 45 ± 26 | 25 ± 21 | 0.15 |
| Time since discontinuation (months) | 27 ± 20 | 35 ± 24 | 41 ± 18 | 0.31 |
| Serum 25-hydroxyvitamin D level (ng/mL) | 32.2 ±8.5 | 35.9 ± 11.0 | 34.8 ± 12.8 | 0.44 |
| Osteocalcin (ng/mL) | 46.3 ± 26.1 | 43.9 ± 20.2 | 55.0 ± 32.6 | 0.31 |
| CTX (ng/mL) | 0.34 ± 0.15 | 0.41 ± 0.22 | 0.44 ± 0.17 | 0.20 |
| DXA BMD (g/cm2) | ||||
| Posterior-anterior spine | 0.815 ± 0.109 | 0.863 ± 0.096 | 0.847 ± 0.130 | 0.31 |
| Femoral neck | 0.642 ± 0.064 | 0.639 ± 0.090 | 0.638 ± 0.054 | 0.98 |
| Total hip | 0.756 ± 0.072 | 0.759 ± 0.102 | 0.750 ± 0.068 | 0.93 |
| One third radius | 0.618 ± 0.072 | 0.608 ± 0.088 | 0.614 ± 0.072 | 0.92 |
Values are mean ±SD unless otherwise noted.
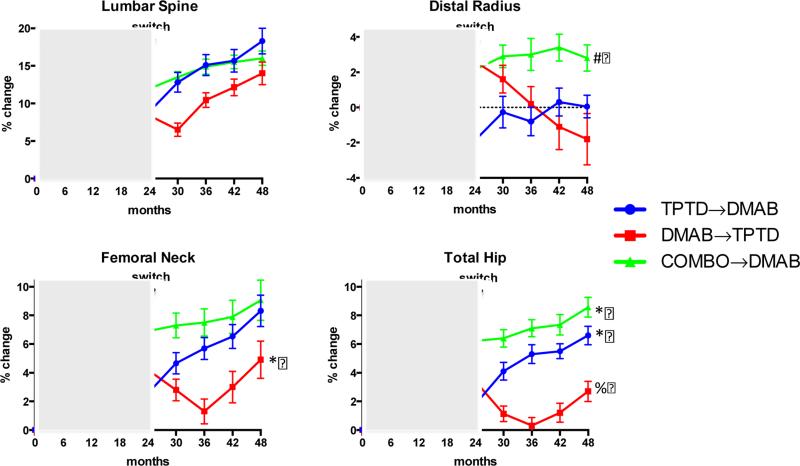
Figure 3 shows the changes in the mean DXA-derived areal BMD over the 48-month treatment period. Table 2 shows the mean percent changes in BMD in both the 0-48 and 24-48 time periods.
Figure 3.
Mean percent change (±SEM) in BMD from baseline to 48-months in the lumbar spine, 1/3 distal radius, femoral neck, and total hip. *P<0.05 versus both other groups. #P<0.01 versus both other groups. %P<0.0005 versus both other groups.
Table 2.
Change in BMD between 0-48 (a) and 24-48 (b) months.
| a. | |||
|---|---|---|---|
| Percent Change 0-48 months | |||
| TPTD→DMAB | DMAB→TPTD | COMBO→DMAB | |
| Lumbar Spine | 18.3 (14.9-21.8) | 14.0 (10.9- 17.2) | 16.0 (14.0- 18.0) |
| Femoral Neck | 8.3 (6.1- 10.5) | 4.9 (2.2- 7.5) | 9.1 (6.1- 12.0) |
| Total Hip | 6.6 (5.3- 7.9) | 2.8 (1.3- 4.2) | 8.6 (7.1- 10.0) |
| Distal Radius | 0.0 (−1.3- 1.4) | −1.8 (−5.0- 1.3) | 2.8 (1.2- 4.4) |
| b. | |||
|---|---|---|---|
| Percent Change 24-48 months | |||
| TPTD→DMAB | DMAB→TPTD | COMBO→DMAB | |
| Lumbar Spine | 8.6 (6.6- 10.6) | 4.8 (2.2- 7.4) | 3.4 (1.7- 5.2) |
| Femoral Neck | 5.6 (3.9- 7.2) | 1.2 (−1.0- 3.4) | 2.1 (−0.2- 4.5) |
| Total Hip | 4.7 (3.7- 5.8) | −0.7 (−2.0- 0.7) | 2.2 (1.3- 3.1) |
| Distal Radius | 2.3 (0.5- 4.1) | −5.0 (−7.5- −2.6) | 0.5 (−0.6- 1.6) |
Values are % change (95% confidence intervals)
As reported previously, after 24-months of the originally assigned medication(s), mean lumbar spine BMD had increased significantly in all treatment groups relative to baseline with the greatest increases in women treated with both medications together.8 In women switching from teriparatide to denosumab, mean (± standard deviation) lumbar spine BMD continued to increase resulting in 48-month increases of 18.3±8.5%. In women switching from combination therapy to denosumab, the net 48-month increase in BMD was 16.0±4.1%. Conversely, in women who after 24-months of denosumab were treated with 24-months teriparatide, lumbar spine BMD decreased over the first 6-months followed by increases resulting in a mean net 48-month increase of 14.0±6.7%.There was no significant difference in the 48-month increase in lumbar spine BMD among any of the treatment group (primary endpoint). BMD increased more after the treatment transition (between months 24 and 48) in the teriparatide-to-denosumab group (8.6±5.0) than either the denosumab-to-teriparatide group (4.8±5.6, between group P=0.0203) or the combination-to denosumab group (3.4±3.5, between-group P=0.0005).
Also as reported previously, after 24-months of the originally assigned therapy, mean total hip BMD had increased significantly in all treatment groups relative to baseline with the greatest increases in women treated with both medications.8 In women switching from teriparatide to denosumab, total hip BMD continued to increase resulting in 48-month increases of 6.6±3.3%. In women switching from combination therapy to denosumab, total hip BMD also increased resulting in 48-month net increases of 8.6±3.0%. Conversely, in women who were treated with 24-months of denosumab followed by 24-months of teriparatide, total hip BMD progressively decreased from 24 to 36 months before beginning to increase between 36 and 42 months. At the conclusion of DATA-Switch (month 48), total hip BMD had increased more in the combination-to-denosumab group than in either the teriparatide-to-denosumab group (between-group P=0.0446) or the denosumab-to-teriparatide group (between-group P<0.0001). At the conclusion of DATA-Switch, total hip BMD also increased more in the teriparatide-to-denosumab group than in the denosumab-teriparatide group (between-group P=0.0002). When the analysis is restricted to changes occurring after the treatment transitions (months 24-48), total hip BMD increased more in the teriparatide-to-denosumab group (4.7±2.6%) than both the combination-to-denosumab group (2.2±1.8%, P=0.0008) and the denosumab-to-teriparatide group (−0.7±3.1, P<0.0001).
Changes in femoral neck BMD after 24-months of the originally assigned therapy showed a pattern similar to total hip, with similar transient bone loss occurring between months 24-36 in women treated with denosumab followed by teriparatide. From 0-48 months, femoral neck BMD increased by 8.3±5.6%, 4.9±6.0%, and 9.1±6.1% in the teriparatide-to-denosumab, denosumab-to-teriparatide, and combination-to-denosumab groups, respectively (P=0.0447 denosumab-to-teriparatide versus teriparatide-to-denosumab, P=0.0336 denosumab-to-teriparatide versus combination-to-denosumab). However, when the analysis is restricted to changes occurring after the treatment transitions (months 24-48), the increases in the teriparatide-to-denosumab group (5.6±4.5%) were larger than in either the combination-to-denosumab group (2.1±4.9%, P=0.0156) or the denosumab-to-teriparatide group (1.2±4.9, P=0.0019).
After 24-months of the originally-assigned therapy, mean BMD at the distal radius had increased in the denosumab and combination groups whereas it decreased in the teriparatide group.8 When patients originally treated with denosumab switched to teriparatide, radius BMD progressively decreased resulting in net 0-48 month decrease of −1.8±5.9%. At the conclusion of DATA-Switch, radius shaft BMD had increased by 2.8±3.2% in the combination-to-denosumab group and reverted to the original baseline in the teriparatide-to-denosumab group (0.0±2.9%). The 0-48 month BMD increases at the distal 1/3 radius in the combination-to-denosumab group were significantly larger than either the teriparatide-to-denosumab group (P=0.0075) or the denosumab-to-teriparatide group (P=0.0099).
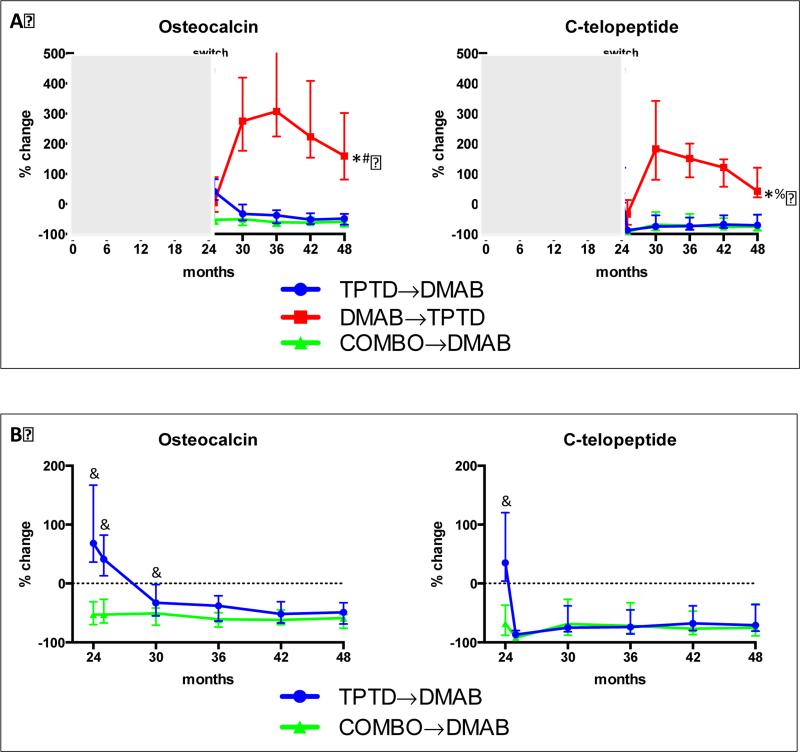
Figure 4 a shows the median percent change in serum osteocalcinand CTX in all treated groups during the 48-month treatment period. For visual clarity, Figure 4b shows the median percent change in these markers between 24 and 48 months in women in the teriparatide-to-denosumab and combination-to-denosumab groups only. In women treated with denosumab followed by teriparatide, median osteocalcin increased by 275% above the original baseline after 6-months of teriparatide (month 30) and remained 159% over the original baseline even after 24-months of teriparatide (month 48). In these same women, CTX increased by 183% at month 30 and were 42% above baseline at month 48. In women treated with teriparatide followed by denosumab, the changes in bone resorption and formation showed different patterns. Bone resorption (CTX) was maximally suppressed after 1-month of denosumab, whereas bone formation (osteocalcin) was not maximally suppressed until 12-24 months of denosumab treatment. In women treated with combination therapy followed by denosumab monotherapy, however, both markers were maximally suppressed at all post-switch time points. When comparing women in the teriparatide-todenosumab group with women in the combination-to-denosumab group (both of which are receiving the same treatment from month 24-48), serum osteocalcin was significantly higher in those switching from teriparatide monotherapy to denosumab monotherapy than in those switching from combination therapy to denosumab monotherapy at 1 and 6 months after the transition (months 25 and 30) (P<0.0001 at month 25 and P<0.0023 at month 30). Conversely, CTX in these two groups did not differ at any time point after the treatment transitions.
Figure 4.
(A) Median percent change (±interquartile range) in osteocalcin and C-telopeptide from baseline to 48-months. *P<0.0001 versus combination-to-denosumab at months 24, 25, 30,36,42,48. #P<0.0001 versus teriparatide-to-denosumab at months 24,30,36,42,48, P=NS at month 25. %P<0.0001 versus teriparatide-to-denosumab at months 25,30,36,42,48, P=NS at month 24.
(B) Median percent change (interquartile range) in osteocalcin and C-telopeptide from baseline to 24-48 months in the teriparatide-to-denosumab group and combination-to-denosumab group. &P<0.05 compared to combination-to-denosumab at the indicated time points.
Significant hypercalcemia (blood calcium >10.8 mg/dL confirmed on repeat testing) was identified in one patient during months 24-48 (denosumab-to-teriparatide group). Serious adverse events were reported in 6 subjects in the teriparatide-to-denosumab group (ductal carcinoma in situ of the breast, syncope, chronic obstructive pulmonary disease exacerbation, elective cervical laminectomy, fundoplication procedure, non-ST elevation myocardial infarction), 4 subjects in the denosumab-to-teriparatide group (appendicitis, laryngitis/pharyngitis, nephrolithiasis without hypercalcemia, anemia due to a gastric ulcer), and 3 subjects in the combination-to-denosumab group (breast cancer, atrial fibrillation, atrial fibrillation with stroke). With the exception of the patient with nephrolithiasis, which was classified as possibly related to treatment (teriparatide), the other serious adverse events were classified as unrelated to therapy by the study investigators and an independent safety monitoring board.
Discussion
In this study, we have demonstrated that in postmenopausal osteoporosis, switching therapy from teriparatide to denosumab further increases bone mineral density at all measured sites whereas switching therapy from denosumab to teriparatide results in transient bone loss at the hip and spine and progressive bone loss at the radius shaft. Additionally, we have demonstrated that 24-months of combined therapy followed by 24-months of denosumab alone is associated with largest cumulative BMD increases at the hip and radius, increases that are greater than have been reported with any currently available therapy taken for a similar duration.14-17
There are several important clinical ramifications of these findings. First, this study clearly illustrates the importance of the order of anabolic versus antiresorptive therapy with denosumab. The bone loss that occurs in patients switching from denosumab to teriparatide was an unexpected finding. Studies investigating the effects of teriparatide after bisphosphonates report further increases in BMD, though generally smaller increases than those observed when teriparatide is given to a patient who has not received prior bisphosphonate therapy.18-23 It was hypothesized that because bisphosphonates are present in the bone matrix for years after administration,24 teriparatide-induced increases in bone turnover were being inhibited in a manner similar to that observed when bisphosphonates are administered concurrently with teriparatide or parathyroid hormone.10-12 If this hypothesis had been correct, one might have expected that the administration of teriparatide after denosumab would not be associated with this blunting and would allow for the full anabolic effect of teriparatide to proceed. Indeed, our data do demonstrate that there is no blunting of teriparatide-induced stimulation of bone turnover after denosumab therapy. On the contrary, bone resorption and formation increased more after switching from denosumab to teriparatide than when the DATA patients were treated with teriparatide de novo. Bone resorption, as measured by median CTX, increased by 183% over the original baseline 6 months after switching from denosumab to teriparatide and bone formation, as measured by median osteocalcin, increased by 275%. Notably, this degree of stimulation of bone metabolism is much greater than in the same women treated with teriparatide de novo, who in the DATA study experienced a 6-month median CTX increase of 86% and a 6-month median osteocalcin increase of 112% (Figure 4b). It is also significantly greater than the magnitude of the reported “overshoot” in bone turnover observedin patients stopping denosumab after 24 months of treatment.7
The mechanism by which teriparatide exerts such a large effect on bone metabolism in patients discontinuing denosumab is unknown but may relate to teriparatide stimulating a large pool of dormant osteoclast precursors in patients in whom RANKL inhibition has been sustained for two years.
The substantial increase in BMD among women switching from teriparatide to denosumab is consistent with studies reporting that bisphosphonates further increase BMD when given after parathyroid hormone or teriparatide.25,26 In the current study, it should be noted that the increases in BMD in women treated with teriparatide monotherapy followed by denosumab were even greater than those treated with combination teriparatide/denosumab therapy followed by denosumab alone. The mechanism underlying the greater 24-48 month increases in the teriparatide-to-denosumab group may relate to the altered relationship between bone formation and bone resorption in these patients. Specifically, bone resorption, as measured by CTX, is more quickly suppressed after transitioning from teriparatide-to-denosumab than is bone formation, as measured by osteocalcin. This discrepancy likely allows for a several-month period of relative unlinking of bone formation and resorption which favors the accrual of bone mass. It is,notably,a very similar unlinking that we hypothesized mechanistically explains the larger increases in BMD achieved by combination teriparatide/denosumab therapyin the initial 12-months of osteoporosis treatment.9 It is also notable that despite the “catch up” in BMD gains achieved in women transitioning from teriparatide to denosumab, women treated initially with combined therapy followed by denosumab experienced the most favorable 48-month BMD changes at the total hip and distal radius, the two measured sites with the highest proportion of cortical bone. Given the importance of cortical bone mass in maintaining skeletal integrity, the observed persistent benefit at these anatomic sites would be expected to confer significantly greater bone strength to these patients.27,28 Moreover, while studies performed in different populations cannot be precisely compared, it is notable that the total BMD increases achieved in both the combination-to-denosumab and teriparatide-to-denosumab groups (16.0-18.3% at the spine and 8.3-9.1% at the femoral neck) are larger than those observed with any single agent administered for a similar period of treatment. Specifically, the reported 4-year BMD gains with either denosumab or zoledronic acid areless than 12% at the spine and less than 6% at the femoral neck.16,17
There are several limitations to our study. First, the size of the study precludes an assessment of the relative safety or anti-fracture efficacy of the three assigned treatment regimens. Bone mineral density, however, has proven to be a reliable, though imperfect, predictor of anti-fracture efficacy in patients treated with osteoporosis medications, including denosumab and teriparatide.29,30 Additionally, the specific clinical impact of the transient bone loss that occurs in women switching from denosumab to teriparatide cannot be precisely estimated. It is notable, however, that several studies have reported that both elevated markers of bone turnover as well as higher rates of bone loss are associated with increased fracture risk.31-35 Consistent with these studies, it has also been reported that postmenopausal women who discontinue estrogen (and hence have increased bone turnover) have a higher risk of hip fracture than women who never used estrogen.36 Thus, even without specific fracture data, we feel that the practicing physician must consider these factors before recommending medication changes or initiating therapy in their osteoporotic patients.
The open label design is a potential limitation. The potential for bias is limited, however, in that the physicians interpreting the DXA measurements and the laboratory performing the bone marker assays were blinded to treatment. Additionally, the women who entered the DATA-Switch were only a subset of those originally randomized in the parent DATA study. That said, our retention in this 24-month extension of a 24-month randomized controlled trial is quite strong and there are no significant differences in any demographic or clinical parameter among those subjects who participated in DATA-Switch and the original DATA cohort. Finally, it should be noted that our study populations is at somewhat lower risk of fracture than those for whom this type of intensive therapy might be recommended and thus should be considered as a “proof-of-concept” study, supporting a more definitive trial with a fracture-reduction endpoint.
Conclusions
In postmenopausal osteoporosis, the order in which denosumab and teriparatide are used has a significant impact on overall treatment efficacy. Specifically, teriparatide does not adequately prevent bone loss after denosumab whereas denosumab stabilizes and further increases BMD when used after teriparatide or combination therapy. Furthermore, the largest increases in BMD at the hip and wrist, and the largest BMD increases possible in any clinical context, are achieved in women treated with 24-months of combined teriparatide/denosumab followed by 24-months of denosumab monotherapy. These results should significantly impact the approach to the initial and sequential treatment of osteoporotic women, particularly those with established disease who are at an acutely high risk of fragility fracture.
Acknowledgments
Dr. Leder serves as a consultant for Eli Lilly, Amgen, Merck and Radius Health and receives research support from Lilly and Amgen. Dr. Neeris a consultant to Eli Lilly and Radius Health Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:Dr. Leder had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Leder, Tsai, Uihlein, Burnett-Bowie, Neer, Lee
Acquisition, analysis, interpretation of data: Leder, Tsai, Uihlein, Burnett-Bowie, Lee, Wallace.
Drafting of the manuscript: Leder, Tsai, Wallace.
Critical revision of the manuscript for important intellectual content: Leder, Tsai, Uihlein, Burnett-Bowie, Neer, Lee, Wallace.
Statistical analysis: Lee.
Obtained funding: Leder.
Trial Registration: clinicaltrials.gov Identifier: NCT00926380
Conflict of Interest Disclosures: All other authors report no conflict of interest.
REFERENCES
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004;15(11):897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis--where do we go from here? N Engl J Med. 2012;366(22):2048–51. doi: 10.1056/NEJMp1202619. [DOI] [PubMed] [Google Scholar]
- 4.Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long? N Engl J Med. 2012;366(22):2051–3. doi: 10.1056/NEJMp1202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161(10):711–23. doi: 10.7326/M14-0317. [DOI] [PubMed] [Google Scholar]
- 6.Leder BZ, Neer RM, Wyland JJ, Lee HW, Burnett-Bowie SM, Finkelstein JS. Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab. 2009;94(8):2915–21. doi: 10.1210/jc.2008-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–9. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–700. doi: 10.1210/jc.2013-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382(9886):50–6. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26(3):503–11. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- 11.Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349(13):1207–15. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1838–45. doi: 10.1210/jc.2009-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black DM, Steinbuch M, Palermo L, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporos Int. 2001;12(7):519–28. doi: 10.1007/s001980170072. [DOI] [PubMed] [Google Scholar]
- 14.Ensrud KE, Barrett-Connor EL, Schwartz A, et al. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res. 2004;19(8):1259–69. doi: 10.1359/JBMR.040326. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen OH, Crawford GM, Mulder H, et al. Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone. 2003;32(2):120–6. doi: 10.1016/s8756-3282(02)00946-8. [DOI] [PubMed] [Google Scholar]
- 16.Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27(2):243–54. doi: 10.1002/jbmr.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papapoulos S, Chapurlat R, Libanati C, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27(3):694–701. doi: 10.1002/jbmr.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosman F, Wermers RA, Recknor C, et al. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab. 2009;94(10):3772–80. doi: 10.1210/jc.2008-2719. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19(5):745–51. doi: 10.1359/JBMR.040117. [DOI] [PubMed] [Google Scholar]
- 20.Eastell R, Nickelsen T, Marin F, et al. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS). J Bone Miner Res. 2009;24(4):726–36. doi: 10.1359/jbmr.081215. [DOI] [PubMed] [Google Scholar]
- 21.Obermayer-Pietsch BM, Marin F, McCloskey EV, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23(10):1591–600. doi: 10.1359/jbmr.080506. [DOI] [PubMed] [Google Scholar]
- 22.Boonen S, Marin F, Obermayer-Pietsch B, et al. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2008;93(3):852–60. doi: 10.1210/jc.2007-0711. [DOI] [PubMed] [Google Scholar]
- 23.Miller PD, Delmas PD, Lindsay R, et al. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93(10):3785–93. doi: 10.1210/jc.2008-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38(5):617–27. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353(6):555–65. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 26.Rittmaster RS, Bolognese M, Ettinger MP, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85(6):2129–34. doi: 10.1210/jcem.85.6.6614. [DOI] [PubMed] [Google Scholar]
- 27.Zebaze R, Seeman E. Cortical bone: a challenging geography. J Bone Miner Res. 2015;30(1):24–9. doi: 10.1002/jbmr.2419. [DOI] [PubMed] [Google Scholar]
- 28.Bala Y, Zebaze R, Ghasem-Zadeh A, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res. 2014;29(6):1356–62. doi: 10.1002/jbmr.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH. Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21(11):1785–90. doi: 10.1359/jbmr.060802. [DOI] [PubMed] [Google Scholar]
- 30.Austin M, Yang YC, Vittinghoff E, et al. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res. 2012;27(3):687–93. doi: 10.1002/jbmr.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 32.Sornay-Rendu E, Munoz F, Duboeuf F, Delmas PD. Rate of forearm bone loss is associated with an increased risk of fracture independently of bone mass in postmenopausal women: the OFELY study. J Bone Miner Res. 2005;20(11):1929–35. doi: 10.1359/JBMR.050704. [DOI] [PubMed] [Google Scholar]
- 33.Berger C, Langsetmo L, Joseph L, et al. Association between change in BMD and fragility fracture in women and men. J Bone Miner Res. 2009;24(2):361–70. doi: 10.1359/jbmr.081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen TV, Center JR, Eisman JA. Femoral neck bone loss predicts fracture risk independent of baseline BMD. J Bone Miner Res. 2005;20(7):1195–201. doi: 10.1359/JBMR.050215. [DOI] [PubMed] [Google Scholar]
- 35.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15(8):1526–36. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 36.Yates J, Barrett-Connor E, Barlas S, Chen YT, Miller PD, Siris ES. Rapid loss of hip fracture protection after estrogen cessation: evidence from the National Osteoporosis Risk Assessment. Obstet Gynecol. 2004;103(3):440–6. doi: 10.1097/01.AOG.0000114986.14806.37. [DOI] [PubMed] [Google Scholar]