Abstract
Purpose.
To characterize visual factors among those who continue to drive and those who restrict night driving in the elderly population.
Methods.
The Salisbury Eye Evaluation Driving Study (SEEDS) is a study of vision, cognition, and driving behaviors of older drivers living in the greater Salisbury, Maryland, metropolitan area. Patients were recruited from listings in the Department of Motor Vehicle Administration. Data are reported from two visits conducted 2 years apart. Night driving was assessed using a real-time driving assessment tool, the Driving Monitor System. Night driving was defined by the presence of at least one episode of driving at night during a 5-day time period (seasonally adjusted). Participants also underwent a battery of cognitive and visual function testing including distance acuity, contrast sensitivity, and visual fields. Logistic regression was used to model factors associated with night driving.
Results.
Complete data were available for 990 of the 1080 participants (92%) attending both visits; 41% of participants were driving at night in each visit. Those who were younger (P < 0.001), male (P < 0.001), and had better measures of cognitive (P = 0.007) and visual function were observed driving at night, whereas those who were older, female, and had poorer measures of cognitive and visual function restricted their night driving behavior. An association was observed between depressive symptoms and less night driving in females (P = 0.003). In multivariate analysis, better contrast sensitivity (odds ratio [OR] 1.18, 95% confidence interval [CI] 1.02–1.36, P = 0.02) and visual field detection (OR 1.21, 95% CI 1.00–1.47, P = 0.05) were associated with driving at night. Visual acuity was not found to be significantly related to night driving (OR 1.08, 95% CI 0.95–1.18, P = 0.12).
Conclusions.
Restricting driving at night is a multifactorial behavior that has a vision component, notably poor contrast sensitivity, and some loss of visual fields.
Elderly drivers with poorer contrast sensitivity and visual field loss tend to restrict their night driving behavior.
Introduction
The elderly constitute the fastest growing subpopulation in the United States.1 Due to advances in medicine, technology, and lifestyle, people are living longer and leading more active lives. Consequently, there has been an increase in the number of older adults continuing to drive well into their 80s and 90s. According to the National Highway Traffic Safety Administration, as many as 15% of drivers are older than 65 years of age.2
Driving is a complex task that requires visual, cognitive, and physical input. Multiple measures of visual function appear to be related to driving performance; these include visual acuity, contrast sensitivity, and visual field detection.3 Degradation of vision may become even more critical during the dark hours of the night when luminance is low, making the discrimination of signs, pedestrians, and road hazards more arduous. The elderly cite visual dysfunction as a major component to restricting driving at night, in bad weather, on long trips, in heavy traffic, on high-speed roads, and other high-risk conditions, suggesting some self-regulation in the face of declining function.4–6
Prospective studies have also shown that participants with loss of contrast sensitivity and binocular visual fields tend to report restricting their night time driving activity.7 In at least one study using self-report data, depression was associated with reduced night-driving patterns, particularly in males.8
Previous studies on elderly night drivers were based on self-report of restricting night driving; there is an absence of data using an independent, objective measure of restricting driving to daylight hours, and characteristics of those who do so. We report on the characteristics of older persons who continue to drive and those who restrict their night driving based on driving data collected from a real-time Driving Monitor System (DMS). We hypothesized that those with better contrast sensitivity and less evidence of visual field loss would be more likely to drive at night, controlling for other factors, compared with those who do not drive at night.
Methods
Population
The Salisbury Eye Evaluation Driving Study (SEEDS) is a study of vision, cognition, and driving behaviors of older drivers living in the greater Salisbury, Maryland metropolitan area with two rounds of data collection. Methods for selection and recruitment have been previously described and are summarized here.3,7,9–16
Participants were recruited from a complete listing of all Maryland Department of Motor Vehicle Administration (DMV), licensees, 67–87 years of age as of May 1, 2005 and those newly 67 years of age as of March 1, 2006. Licensees were residents in zip codes within Salisbury, Maryland. As required by the DMV, letters describing the study were sent to all eligible drivers with an enclosed, stamped, and preaddressed postcard on which recipients could indicate their interest in the study. Those who did not return postcards or returned postcards indicating that they were not interested could not be further contacted. At least three mailings were sent. Numerous seminars, meetings at churches, community centers, and other informational sessions were conducted throughout Salisbury.
From an initial sample of 4050 potential subjects we recruited 1425 participants. We report on baseline data from visit 1 (May 2005 to August 2006) and visit 2 (July 2007 to August 2008). Both of these visits provided data collected by questionnaires, by clinical examination for vision and cognition, and by use of our Driving Monitor System (DMS) described in the following text.
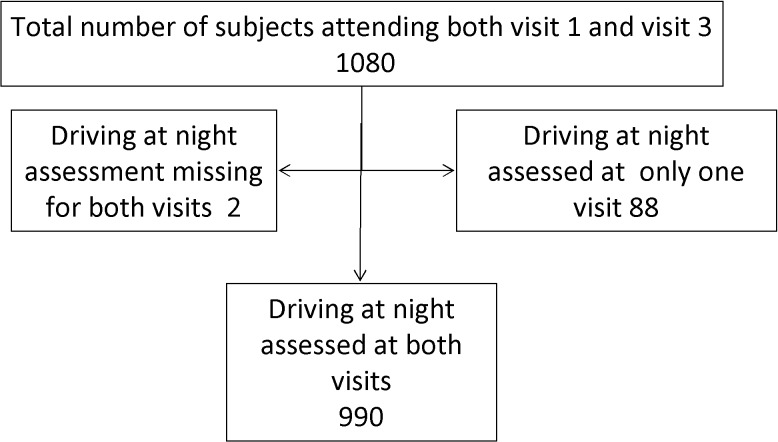
There were 1425 participants at baseline; 1080 attended both visits. Of the 1080, driving data were not available at one visit for 88 participants and at both visits for two participants. The primary reason for lack of data was failure of the video system in the vehicle, a system failure that was largely rectified by round 2. Thus, a total of 990 participants had complete DMS driving records at both visits and were ultimately included in the final analysis (Fig. 1). Institutional Review Board approval at the Johns Hopkins Medical Institutions was obtained prior to the initiation of this work and all participants provided written, informed consent.
Figure 1.
Study participants.
Interview
Interviews were conducted by trained interviewers and included questions on access to other drivers or alternative forms of transportation. Questions did not include asking about night driving because these data were collected in real time. Medical history and medication lists were obtained.
Clinical Testing
The Trail Making Test (TMT) was used to measure visuomotor skills (part A) and executive function (part B). In the TMT A, participants were asked to manually connect circles numbered from 1 to 25 in order; TMT B required participants to manually connect circles alternating between numbers 1 and 13 and letters A through L. Scores were recorded in seconds to completion of each task.17
Trained examiners administered the Brief Test of Attention, a test of auditory attention and working memory for which participants listened to an audiotaped series of lists of numbers and letters increasing in length; they were scored according to their ability to recall the number of letters in each sequence. Depressive symptoms were assessed with the Geriatric Depression Scale (GDS), which is an examiner-administered 30-question survey with increasing scores indicating increasing depressive symptoms. The Beery–Buktenica Developmental Test of Visual–Motor Integration was conducted to test visuoconstruction abilities by requiring participants to copy 24 figures of progressive difficulty that were graded using a standard format.18
The participants also undertook a test of attentional visual field that was assessed using a custom-written program that comprised a computer, keyboard, touch-screen monitor, and mouse, and is described in detail elsewhere.10 For a response to be correct, two numbers in the central and peripheral targets had to be correctly identified as well as the location of the peripheral target. The data were recorded as the widest angle out to 20° for which the participant had correct responses.
Vision testing included visual acuity, contrast sensitivity, and visual field assessment. Binocular visual acuity was measured with an Early Treatment Diabetic Retinopathy Study chart at a distance of 3 m in standard illumination, using a forced-choice procedure, and coded as LogMAR (logarithm of the minimal angle of resolution) acuity. Contrast sensitivity testing was done for each eye individually with the Pelli–Robson contrast sensitivity chart. The chart consisted of eight lines of letters of varying contrasts. The first three letters of each line had more contrast than the last three (reading from left to right). Contrast also decreased going down the lines. Scores for the better eye were used. Monocular visual fields were assessed by using an 81-point, quantify defect screening test strategy on a field perimeter (Humphrey Field Analyzer [HFA]; Carl Zeiss Meditec, Inc., Dublin, CA). The visual field results of both eyes were combined using the Nelson-Quigg et al.19 algorithm to create a binocular visual field that consisted of 96 points. The number of points missed on the binocular field test was used in the analysis.
Driving Monitor System (DMS)
The DMS was a custom-built device that captured a host of driving parameters while installed in each participant's vehicle for a 5-day period. Customized programs were written to analyze specific features of the driving data. Many of the driving characteristics have been previously published.14,16,20 The color camera took images of the road, whereas the monochrome camera took images of the driver. All video data were time stamped and registered with accelerometer and Global Positioning System data. Night-driving times were defined according to season: Summer (May–August) night-driving times were from 9 PM until 5 AM; Spring (March–April)/Fall (September–October) night-driving times were from 7 PM until 6 AM; Winter (November–February) night-driving times were from 6 PM until 7 AM. We defined night driving as having at least one video image time stamped or viewed that indicated that the driving episode occurred at night. We did not prespecify any length of time for the episode.
Data Analysis
Differences in baseline characteristics between participants eligible for the analyses and those excluded were evaluated using the χ2 test/t-test as appropriate. Cross-sectional associations between the primary outcome, night driving, and the putative risk factors are presented for each visit, and testing for significance includes adjustment for age, season, miles driven, and visit period. The data suggested variation in persons who drove or did not drive at night from one visit to the next; therefore, we modeled driving at night using the data from the two visits. When combining information the primary outcome was modeled using a repeated-measurement approach, where each subject contributed two observations, one for each visit, with adjustment for the repeated measures. Logistic regression models, including the time period as a covariate, were then constructed. The generalized estimation equation approach was used to correct the SEs of the estimates. A backward-elimination approach was used to select the demographic and cognition factors to be included in the final multivariate models. Three final models examining the association with each vision test are presented. All analyses were done using a commercial analytical software program (SAS 9.3; SAS Institute Inc., Cary, NC).
Results
Of 1425 participants at baseline, 1080 participants were eligible for this analysis because they returned for the second visit. Driving data were absent on one or both visits for 90 participants who were excluded from the final analysis (Fig. 1). Characteristics of those included and for the 90 participants (8.3%) without driving data are described in Table 1. African Americans and those with poorer cognition, visual acuity, and contrast sensitivity were more likely to be in the group without driving data, but there was no difference in age, miles driven, or rural versus urban residence.
Table 1.
Characteristics of Eligible Subjects by Inclusion in the Study and Those Excluded Due to the Absence of Driving Data
|
Characteristic |
Included |
Excluded |
P
Value* |
| Number of subjects | 990 | 90 | |
| Age (y), mean (SD) | 75.7 (5.2) | 75.8 (4.6) | 0.96 |
| % Female | 49.7 | 41.1 | 0.12 |
| % Black | 10.8 | 24.4 | <0.001 |
| Education (y), mean (SD) |
13.6 (2.5) | 13.0 (3.0) | 0.048 |
| Depression score, mean (SD) |
3.4 (3.3) | 4.2 (4.3) | 0.10 |
| Pain score, mean (SD) |
0.85 (1.0) | 0.88 (1.1) | 0.81 |
| Cognition | |||
| Brief test of attention score, mean (SD) |
6.7 (2.4) | 6.3 (2.7) | 0.11 |
| Trail Making Test, part A (s), mean (SD) |
47.4 (19.8) | 57.1 (29.6) | 0.03 |
| Trail Making Test, part B (s), mean (SD) |
120.6 (65.5) | 142.1 (80.7) | 0.018 |
| Test of visual– motor integration score, mean (SD) |
18.5 (3.4) | 17.7 (3.2) | 0.016 |
| Visual attention (angle in degrees), mean (SD) |
13.0 (5.0) | 11.6 (6.0) | 0.04 |
| Visual Function | |||
| Visual acuity (logMAR scale), mean (SD) |
−0.016 (0.11) | 0.016 (0.12) | 0.01 |
| Best contrast sensitivity (letters read), mean (SD) |
35.4 (2.2) | 34.7 (2.5) | 0.006 |
| Binocular visual fields (points missing), mean (SD) |
1.8 (4.2) | 3.2 (7.1) | 0.08 |
| Difficulty in getting someone to drive you places | |||
| % No difficulty | 64.9 | 75.6 | 0.12 |
| % Some difficulty | 20.0 | 14.4 | |
| % A lot of difficulty/walk |
15.1 | 10.0 | |
| % Rural residence | 35.0 | 27.8 | 0.17 |
| Miles driven, mean (SD) |
120.2 (105.5) | 121.6 (121.1) | 0.92 |
χ2 test for dichotomous variables; t-test for continuous, Satterthwaite approach used where variances were unequal.
There was variability over the two visits in who was driving at night; at both visits, 41% of participants were driving at night, whereas 59% were not. However, these were not necessarily the same people; 19% of participants went from being night drivers in visit 1 to nonnight drivers in visit 2 and vice versa (Table 2). We used data from both visits to evaluate characteristics of night drivers, using a cross-sectional approach, then combining both visits and adjusting for the correlation between visits.
Table 2.
Correspondence between Percentage of Night Driving at Visits 1 and 2
|
Visit 2 |
No (%) |
Yes (%) |
Total (%) |
| No (%) | 392 (40%) | 191 (19%) | 583 (59%) |
| Yes (%) | 193 (19%) | 214 (22%) | 407 (41%) |
| Total (%) | 585 (59%) | 405 (41%) | 990 (100%) |
The characteristics of participants who drove at night are described in Table 3. A higher proportion of males than females were driving at night at each visit (P < 0.001). Those with better visual acuity (P = 0.02) and contrast sensitivity (P = 0.005) were more likely to drive at night than those with worse vision. About 40% to 45% of participants reported having difficulty getting someone to drive them around, but that did not vary by those who did or did not drive at night (P = 0.78). More of the participants tested in the winter season were found to be driving at night than in the other seasons (P < 0.001). Those who drove the most miles also were more likely to be driving at night (P < 0.001).
Table 3.
Proportion Observed Driving at Night by Visit and Participant's Characteristics
|
Characteristic |
n
= 990 |
Overall P Value† |
|||
|
Visit 1 |
Visit 2 |
||||
|
(Drivers/Eligible) % |
P
Value* |
(Drivers/Eligible) % |
P
Value* |
||
| Age group (y) |
0.15 |
<0.001 |
<0.001‡ |
||
| 68–69 | (47/145) 32.4 | (18/36) 50.0 | |||
| 70–74 | (149/329) 45.3 | (160/322) 49.7 | |||
| 75–79 | (126/278) 45.3 | (113/274) 41.2 | |||
| ≥80 | (86/238) 34.9 | (116/358) 32.4 | |||
| Sex |
<0.001 |
0.001 |
<0.001‡ |
||
| Male | (239/492) 48.6 | (236/492) 48.0 | |||
| Female | (166/498) 33.3 | (171/498) 34.3 | |||
| Race |
0.37 |
0.72 |
0.40‡ |
||
| White | (360/883) 40.8 | (360/883) 40.8 | |||
| Black | (45/107) 42.1 | (47/107) 43.9 | |||
| Education (y) |
0.04 |
0.83 |
0.21‡ |
||
| <12 | (37/89) 41.6 | (38/89) 42.7 | |||
| 12–13 | (175/452) 38.7 | (176/452) | |||
| 14–15 | (43/105) 41.0 | (38/105) 36.2 | |||
| ≥16 | (150/344) 43.6 | (155/344) 45.1 | |||
| Depression scale score |
0.07 |
0.22 |
0.051‡ |
||
| 0 | (81/181) 44.8 | (85/182) 46.7 | |||
| 1–2 | (137/306) 44.8 | (130/293) 44.4 | |||
| 3-4 | (78/210) 37.1 | (76/201) 37.8 | |||
| ≥5 | (109/293) 37.2 | (116/314) 36.9 | |||
| Pain score |
0.20 |
0.32 |
0.98‡ |
||
| 0 | (206/490) 42.0 | (217/541) 40.1 | |||
| >0–1 | (109/266) 41.0 | (112/265) 42.3 | |||
| >1 | (90/234) 38.5 | (78/184) 42.4 | |||
| Trail Making Test, part A (time) |
0.02 |
0.42 |
0.07‡ |
||
| <34 s | (100/215) 46.5 | (111/239) 46.4 | |||
| 34–42 | (107/257) 41.6 | (118/268) 44.0 | |||
| 43–55 | (106/275) 38.6 | (89/252) 35.3 | |||
| ≥56 | (92/243) 37.9 | (89/229) 38.9 | |||
| Trail Making Test, part B (time) |
0.015 |
0.96 |
0.31‡ |
||
| <81 s | (127/251) 50.6 | (117/245) 47.8 | |||
| 81–106 | (97/240) 40.4 | (108/253) 42.7 | |||
| 107–144 | (101/274) 36.9 | (75/227) 33.0 | |||
| ≥144 | (79/219) 36.0 | (106/259) 40.9 | |||
| Visual–Motor Integration Test score |
0.66 |
0.16 |
0.25‡ |
||
| <16 | (76/206) 36.9 | (98/255) 38.4 | |||
| 16–17 | (65/141) 46.1 | (61/160) 38.1 | |||
| 18–20 | (127/301) 42.2 | (127/312) 40.7 | |||
| ≥21 | (135/336) 40.2 | (121/262) 46.2 | |||
| Visual attention |
0.15 |
0.35 |
0.13‡ |
||
| <9 | (88/221) 36.2 | (77/220) 35.0 | |||
| 9–<14 | (119/302) 39.4 | (99/260) 38.1 | |||
| 14–<17 | (95/232) 41.0 | (105/236) 44.5 | |||
| ≥17 | (109/233) 46.8 | (124/263) 47.2 | |||
| Visual acuity (logMAR scale) |
0.17 |
0.02 |
0.02‡ |
||
| < −0.07 | (185/425) 43.5 | (175/354) 49.4 | |||
| –0.07–<0.02 | (52/134) 38.8 | (41/126) 32.5 | |||
| 0.02–<0.08 | (90/249) 36.1 | (120/295) 40.7 | |||
| >0.08 | (78/182) 42.9 | (71/215) 33.0 | |||
| Best contrast sensitivity (per letter) |
0.016 |
0.09 |
0.005‡ |
||
| <34 | (56/173) 32.4 | (73/215) 34.0 | |||
| 34–35 | (94/229) 41.0 | (105/258) 40.7 | |||
| ≥36 | (255/588) 43.4 | (229/517) 44.3 | |||
| Binocular visual fields (points missing) | |||||
| 0 | (245/575) 42.6 | 0.08 | (249/567) 43.9 | 0.52 | 0.08‡ |
| 1–2 | (63/153) 41.2 | (57/133) 42.9 | |||
| >2 | (93/254) 36.6 | (101/298) 35.0 | |||
| Difficulty in getting someone to drive you places | |||||
| No difficulty | (256/643) 39.8 | 0.83 | (273/664) 41.1 | 0.60 | 0.78‡ |
| Some difficulty | (90/198) 45.5 | (70/185) 37.8 | |||
| A lot of difficulty/walk | (53/149) 39.6 | (63/139) 45.3 | |||
| Rural/urban residence | |||||
| Rural | (143/346) 41.3 | 0.37 | (151/346) 43.6 | 0.53 | 0.35‡ |
| Urban | (262/644) 40.7 | (256/644) 39.8 | |||
| Season | |||||
| Winter | (169/298) 56.7 | <0.001 | (139/277) 50.2 | <0.001 | <0.001 |
| Spring–fall | (149/351) 42.5 | (144/366) 39.4 | |||
| Summer | (87/341) 25.5 | (124/347) 35.7 | |||
| Miles driven (odometer) | |||||
| <48 | (42/217) 19.4 | <0.001 | (63/250) 25.2 | <0.001 | <0.001 |
| 48–91 | (99/247) 40.1 | (100/251) 39.8 | |||
| 92–149 | (125/254) 49.2 | (101/238) 42.4 | |||
| ≥150 | (133/249) 53.4 | (142/246) 57.7 | |||
| Total | (405/990) 40.9 | (407/990) 41.1 | |||
Testing differences in night driving at each visit, adjusting for age, season, and miles driven. Covariates modeled as continuous when appropriate.
Testing differences in night driving by participant's characteristics using observations from visits 1 and 2, with a repeated-measures approach; SEs were corrected to account for the correlation of the two observations from the same subject.
Adjusted for season, miles driven, visit period and adjusted for season, miles driven, visit period, and age.
Overall, there was no evidence that night drivers had more or less depressive symptoms than those who did not drive at night, but there was an interaction with sex. Although there was no evidence that depression in males was associated with night driving, females with lower depression scale scores were more likely to drive at night compared with females with high depression scale scores (Table 4, P = 0.003).
Table 4.
Relationship between Symptoms of Depression and Driving at Night by Sex
|
Sex |
Depression Status |
% Driving at Night, Visit 1 |
% Driving at Night, Visit 2 |
P
Value |
| Males | Depression score ≤ 2 |
(135/273) 49.5 | (128/273) 50.6 | Sex– depression interaction, P = 0.003 |
| Depression score > 2 |
(111/237) 46.8 | (117/257) 45.5 | ||
| Females | Depression score ≤ 2 |
(91/237) 38.4 | (93/240) 38.7 | |
| Depression score > 2 |
(86/293) 29.4 | (82/278) 29.5 |
In multivariate analyses with adjustment for confounders, participants who were younger (odds ratio [OR] 1.03, 95% confidence interval [CI] 1.01–1.05) and performed better on TMT A (OR 1.06, 95% CI 1.01–1.11) were more likely to drive at night (Table 5). Females with fewer symptoms of depression were more likely to drive at night than females with increasing depressive symptoms. Separate models were run for the vision variables because of concern for high correlation. No significant association was found with visual acuity, but participants with better contrast sensitivity and binocular visual fields were more likely to drive at night than those with poorer contrast sensitivity and binocular visual fields.
Table 5.
Factors Associated with Driving at Night Multivariate Models*
|
Characteristic |
Odds Ratios (95% Confidence Interval) (P
Value) |
||
|
Model 1 |
Model 2 |
Model 3 |
|
| Age (y) (per year decrease) |
1.03 | 1.03 | 1.03 |
| (1.01–1.05) | (1.01–1.05) | (1.01–1.05) | |
| (P = 0.003) | (P = 0.005) | (P = 0.001) | |
| Sex and depression status |
|||
| Females (depression score > 2) |
1.00 | 1.00 | 1.00 |
| Females (depression score ≤ 2) |
1.40 | 1.43 | 1.44 |
| (1.06–1.86) | (1.08–1.89) | (1.09–1.90) | |
| (P = 0.02) | (P = 0.01) | (P = 0.01) | |
| Males | 1.87 | 1.89 | 1.94 |
| (1.46–2.40) | (1.48–2.41) | (1.52–2.48) | |
| (P < 0.001) | (P < 0.001) | (P < 0.001) | |
| Trail Making Test, part A (per 10-s decrease) |
1.06 | 1.06 | 1.06 |
| (1.01–1.11) | (1.01–1.11) | (1.01–1.11) | |
| (P = 0.05) | (P = 0.08) | (P = 0.007) | |
| Visual Function | |||
| Visual acuity (per line seen) |
1.08 | — | — |
| (0.95–1.18) | |||
| (P = 0.12) | |||
| Contrast sensitivity (per 3 letters seen) |
— | 1.18 | — |
| (1.02–1.36) | |||
| (P = 0.02) | |||
| Binocular visual field (per 10 points seen) |
— | — | 1.21 |
| (1.00–1.47) | |||
| (P = 0.05) | |||
Adjusted for season, miles driven, and visit period; SEs were corrected to account for the correlation of the two observations from the same subject.
Discussion
In this study, we demonstrated that several visual, cognitive, and demographic factors were associated with restricted night-driving behavior in elderly drivers. Our data corroborate previous studies using self-reported data, which have found that increasing age, female sex, and poorer cognition were related to restricting night driving.6,8,11 Among a battery of tests of specific domains of cognitive function, we found that participants with better scores on a specific test of visual search (TMT A) were driving at night.
We also found that better contrast sensitivity and binocular peripheral visual fields were associated with night driving, similar to our previous finding in a different population, where night driving was based on self-report.7
Reduced contrast sensitivity and not driving at night parallel older drivers' reports of difficulty driving at night and during other conditions when luminance is degraded, such as in poor weather.4,5 Although not a night-driving study, Woods et al.21 found that reduced luminance contributed to decreased driver recognition and performance in a small study done on a closed road circuit. It has been shown that in the elderly, loss of contrast discrimination leads to a decline in global motion processing and that performance accuracy and speed are subsequently degraded.22,23 Such loss could subsequently affect the ability to perceive signs, objects, and other hazards at varying speeds that may make elderly drivers feel less confident, particularly during hours when luminance is reduced.21 Thus, even without knowledge that contrast sensitivity specifically is lost, the effect on performance may lead older drivers to restrict night driving.
Explanations for declines in contrast sensitivity in the elderly include loss of neuronal efficiency with age and diseases such as age-related lens changes (i.e., cataract formation), retinal pathology, corneal disease, and glaucoma. Among these, cataract is the most common cause of reduced contrast sensitivity; studies have demonstrated an improvement in contrast sensitivity following cataract extraction and intraocular lens implantation.24 Ophthalmic findings were not recorded in this study, because we were more interested in evaluating visual function changes rather than the ocular pathology. It is possible that participants in this study suffered from ophthalmic disease, which may have explained poor contrast sensitivity, and suggest that reduced night-driving patterns may be a signal for an ophthalmologic evaluation.
The alterations of neurosensory and global motion processing that occur with aging can also explain the association between slower times on tests of visual search (TMT A) and night-driving restriction observed in this study. This finding is consistent with the literature, which shows that cognitive performance is a determinant of driving behaviors, in that skills of psychomotor performance are important for safe and confident driving.6,11 Feelings of reduced safety and confidence are cited as major reasons for driving cessation in surveys conducted in the elderly.4,5
Previous studies have been unable to draw a uniform consensus on the role of visual acuity in predicting self-regulated driving behaviors in the elderly. In our earlier studies of self-report, we found that worse visual acuity was related to driving fewer miles but not to the decision to stop driving at night.3,7 In this study, we also find that visual acuity is not significantly related to an objective determination of night-driving restriction.
Participants with less visual field loss were more likely to be driving at night, adjusting for other factors. We have previously found higher rates of self-reported night driving continuation among participants with less visual field loss.7 Although persons may be largely unaware of their visual field defect, the reason for cessation of night driving with visual field loss may be due to perceived loss of scotopic vision. Visual field testing, particularly in the periphery, is largely testing scotopic vision. In dark conditions, rod photoreceptors involved in scotopic vision are stimulated. Thus, night drivers with more visual field loss, particularly in the periphery, would predictably suffer greater loss of scotopic vision and find night driving to be a greater challenge than daytime driving. Loss of visual fields may also make it more difficult to perceive road obstacles that appear in a driver's periphery, such as road signs and vehicles in neighboring lanes.
Sex also played a significant role in driving restriction. Although nearly half of the male participants were observed driving at night, only a third of the females were seen doing the same. Similar findings have been reported in prior studies, based on self-report of restriction, including ours.4,6,8 This disparity may be explained in part through social and cultural phenomena of the elderly participants being tested. Males of this generation tended to start driving earlier than their female counterparts and continued to drive more frequently and for greater distances, largely for vocational purposes.8 Females in this age group depended less on driving for activities of daily living. Consequently, males may have adopted driving as part of their identity role. Whereas males will continue driving for as long as their health permits, females may give up driving earlier for other reasons.8 Males also are known to be greater risk takers and may, therefore, be more likely than females to drive in risky driving situations such as at night.8 We have previously found that females report a poorer sense of direction, which coupled with fear of night driving, may lead to cessation of night driving.12 Access to an alternate driver was evaluated to determine if this could be an explanation for sex differences in night driving, presuming females may have a larger social network, but no differences were found. It is also possible that females were such infrequent night drivers, that episodes of their night-driving activities were missed during the 5-day DMS study period; if so, there may have been less sex disparity than we report. Even still, females would have been less frequent night drivers than males.
In this study, we observed an association in females between symptoms of depression and night-driving restriction, which was not significant in males. In our analysis, we defined depressive symptoms as a score of >2 on the GDS. Sensitivity analysis using other cutoffs did not change the sex–depression interaction. Interestingly, Brabyn et al.8 found that males, rather than females, reported restricting their night driving in association with symptoms of depression. The different study findings could be due to the same source bias in the Brabyn study, which used self-report as the source of both depression and night-driving data. Our study used different sources of data; the DMS for real-time night-driving data and self-report data of depressive symptoms, and thus our findings do not have this bias. Additional differences include the populations studied; in the Brabyn study, the self-report of driving at night was much higher in both sexes than we observed in Salisbury. Driving cessation for males in California may be rarer and lead to more symptoms of depression than is true in Salisbury, although it does not explain the difference in females. Our findings suggest that, although females are much less likely in general to drive at night compared with males, depression in females puts them at an even higher risk for not driving at night.
A limitation of this study was the definition of night-driving patterns, which changed appropriately according to season. The length of night-time hours was shortest in the summer (8 hours) and longest in the spring/fall (11 hours) and winter (13 hours); therefore, there were more hours in which subjects could potentially drive at night during the winter compared with other seasons. A participant who routinely stops driving around 8 PM would be driving at night in the winter and not driving at night if observed during the summer. This potential misclassification would likely weaken our associations. Since participants were largely studied in the same season each visit due to the timing of our follow-up visits, we did not have the opportunity to observe change by season in the same person. The participants who changed over time truly changed within the season we observed them. Weather conditions are also known to influence driving patterns and may lead to restriction.5 Thus some of the night-driving restriction in the winter may be due to weather-related factors rather than just night-time restriction. However, winters are not severe due to the presence of the Chesapeake Bay near Salisbury, and rainy conditions can prevail in all seasons.
Another possible limitation of this study is that we may have missed drivers who infrequently drove at night and were missed during the 5-day DMS testing period. They would be classified in our study as day-driving only. Misclassification is always an issue in studies, and would be true in other studies that rely on self-report of driving at night as well. A strength of this study is that we could base our classification on direct observation and classify those who drove at night as true night drivers.
In summary, this study of elderly drivers found that persons with worse contrast sensitivity and fewer number of points seen in binocular visual fields were less likely to drive at night. We also found that those with worse scores in a test of visual search were less likely to drive at night. These findings likely reflect self-regulation of older drivers, and have a positive implication, because there is reason to believe driving at night would be more hazardous for persons with such limitations. Nevertheless, about a third of drivers who were unable to read greater than 34 letters on the contrast sensitivity chart were still driving at night in this population, suggesting the multiplicity of factors that are involved in decisions to drive at night apart from potential visual limitations. Further investigation into the reasons that such drivers continue to drive at night despite limitations is warranted. Further implications stem from the finding that depression in older females is associated with not driving at night. Because of the cross-sectional nature of the data, we cannot determine if depression preceded or followed restricting night driving, and both are plausible. However, self-restriction of driving habits, justified or not, handicaps independent function. Elderly femles who have given up night driving should be further evaluated for depression to be certain that a treatable condition is not the source of relinquishing elements of independent function.
Acknowledgments
The authors thank the staff of the Salisbury Eye Evaluation Driving Study and the study participants for their contributions.
Footnotes
Supported in part by National Institute on Aging/National Institutes of Health Grant AG 23110, Senior Scientific Investigator grant from Research to Prevent Blindness (SKW), and an Ernest and Elizabeth Althouse Special Scholar's award from Research to Prevent Blindness (EWG).
Disclosure: M.A. Kaleem, None; B.E. Munoz, None; C.A. Munro, None; E.W. Gower, None; S.K. West, None
References
- 1. United States Census Bureau. The 2012 Statistical Abstract. Washington, DC: Available at http://www.census.gov/compendia/statab/. Accessed October 3, 2011. [Google Scholar]
- 2. National Highway Traffic Safety Administration. Traffic Safety Facts 2009. Washington, DC: Department of Transportation; 2009. [Google Scholar]
- 3. Freeman E, Munoz B, Turano K, West SK. Measures of visual function and time to driving cessation in older adults. Optom Vis Sci. 2005; 82: 763– 773. [DOI] [PubMed] [Google Scholar]
- 4. Betz M, Lowenstein S. Driving patterns of older adults: results from the Second Injury and Risk Survey. J Am Geriatr Soc. 2010; 58: 1931– 1935. [DOI] [PubMed] [Google Scholar]
- 5. Sullivan K, Smith S, Horswill M, Lurie-Beck J. Older adults' safety perceptions of driving situations: towards a new driving self-regulation scale. Accid Anal Prev. 2011; 43: 1003– 1009. [DOI] [PubMed] [Google Scholar]
- 6. Naumann R, Dellinger A, Kresnow MJ. Driving self-restriction in high risk conditions: how do older drivers compare to others? J Safety Res. 2011; 42: 67– 71. [DOI] [PubMed] [Google Scholar]
- 7. Freeman E, Munoz B, Turano K, West SK. Measures of visual function and their association with driving modification in older adults. Invest Ophthalmol Vis Sci. 2006; 47: 514– 520. [DOI] [PubMed] [Google Scholar]
- 8. Brabyn J, Schneck M, Lott L, Haegerstrom-Portnoy G. Night driving self-restriction: vision function and gender differences. Optom Vis Sci. 2005; 82: 755– 764. [DOI] [PubMed] [Google Scholar]
- 9. Zhang L, Baldwin K, Munoz B, et al. Visual and cognitive predictors of performance on brake reaction test: Salisbury Eye Evaluation Driving Study. Ophthalmic Epidemiol. 2007; 14: 216– 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hassan SE, Turano KA, Munoz B, Munro C, Roche K, West SK. Cognitive and vision loss affects the topography of the attentional visual field. Invest Ophthalmol Vis Sci. 2008; 49: 4672– 4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keay L, Munoz B, Turano K, et al. Visual and cognitive deficits predict stopping or restricting driving: the Salisbury Eye Evaluation Driving Study (SEEDS). Invest Ophthalmol Vis Sci. 2009; 50: 107– 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turano K, Munoz B, Hassan S, et al. Poor sense of direction is associated with constricted driving space in older drivers. J Gerontol B Psychol Sci Soc Sci. 2009; 64: 348– 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rubin G, Ng E, Bandeen-Roche K, Keyl P, Freeman E, West SK. A prospective, population-based study of the role of visual impairment in motor vehicle crashes among older drivers: the SEE study. Invest Ophthalmol Vis Sci. 2007; 48: 1483– 1491. [DOI] [PubMed] [Google Scholar]
- 14. Keay L, Jasti S, Munoz B, et al. Urban and rural differences in older drivers' failure to stop at stop signs. Accid Anal Prev. 2009; 41: 995– 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. West SK, Hahn D, Baldwin K, et al. Older drivers and failure to stop at red lights. J Gerontol A Biol Sci Med Sci. 2010; 65: 179– 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munro C, Jefferys J, Gower E, et al. Predictors of lane change errors in older drivers. J Am Geriatr Soc. 2010; 58: 457– 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006; 1: 2277– 2281. [DOI] [PubMed] [Google Scholar]
- 18. Beery KE. The VMI Developmental Test of Visual-Motor Integration. Cleveland, OH: Modern Curriculum Press; 1989. [Google Scholar]
- 19. Nelson-Quigg JM, Cello K, Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000; 41: 2212– 2221. [PubMed] [Google Scholar]
- 20. Baldwin KC, Duncan D, West SK. The driver monitor system: a means of assessing driver performance. Johns Hopkins APL Tech Digest. 2004; 25: 1– 10. [Google Scholar]
- 21. Woods J, Owens A. Standard measures of visual acuity do not predict drivers' recognition performance under day or night conditions. Optom Vis Sci. 2005; 82: 698– 705. [DOI] [PubMed] [Google Scholar]
- 22. Allen H, Hutchinson C, Ledgeway T, Gayle P. The role of contrast sensitivity in global motion processing deficits in the elderly. J Vis. 2010; 10: 1– 10. [DOI] [PubMed] [Google Scholar]
- 23. See A, Anstey K, Wood J. Simulated cataract and low contrast stimuli impair cognitive performance in older adults: implications for neuropsychological assessment and everyday function. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2011; 18: 1– 21. [DOI] [PubMed] [Google Scholar]
- 24. Horswill M, Plooy A. Reducing contrast makes speeds in a video-based driving simulator harder to discriminate as well as making them appear slower. Perception. 2008; 37: 1269– 1275. [DOI] [PubMed] [Google Scholar]