Abstract
All around the world a few studies have been found on the effect of guideline implementation on direct medications’ expenditure. The goal of this study was to evaluate cost savings of guideline implementation among patients who had to receive 3 costly medications including albumin, enoxaparin, and pantoprazole in a tertiary hospital in Shiraz, Iran.
An 8-month prospective study was performed in 2 groups; group 1 as an observational group (control group) in 4 months from June to September 2014 and group 2 as an interventional group from October 2014 to January 2015.
For group 1 the pattern of costly medications usage was determined without any intervention. For group 2, after guideline implementation, the economic impact was evaluated by making comparisons between the data achieved from the 2 groups.
A total of 12,680 patients were evaluated during this study (6470 in group 1; 6210 in group 2). The reduction in the total value of costly administered drugs was 56% after guideline implementation. Such reduction in inappropriate prescribing accounts for the saving of 85,625 United States dollars (USD) monthly and estimated 1,027,500 USD annually.
Guideline implementation could improve the adherence of evidence-based drug utilization and resulted in significant cost savings in a major teaching medical center via a decrease in inappropriate prescribing of costly medications.
INTRODUCTION
Due to increasing concern about unjustifiable variations in clinical practice for the same condition, clinical practice guidelines have attracted worldwide attention with the aim of improving people's health, and a desire to make the best use of available health resources.1
Carefully constructed practice guidelines can decrease the unnecessary use of costly medications. This concept has generated unprecedented interest across all medical specialties in the past decade.2,3
A major concern is implementations of these guidelines to ensure that they are incorporated in clinical practice and are actually used in clinical decision making.4 Implementation of guidelines prevent medication errors significantly, improve appropriateness of prescription, and lead to significant cost saving.5 The application of evidence-based practices will maximize benefit to the patient by achieving optimum treatment outcomes while reducing cost and decreasing possible risks due to side effects and drug interactions.6
Since there are a limited number of studies in assessing the implementation of clinical practice guidelines for decreasing drug expenditure,7 we designed this study to examine the influence of guideline implementation on decreasing inappropriate use and consequently direct drug cost in a tertiary referral hospital. Decrease in patients’ medical expenditure and rationalizing the administration of medicine were 2 dedicated goals of this project.
MATERIALS AND METHODS
In May 2014, a consensus was developed in Shahid Faghihi Hospital regarding the need to control costly medications’ use. This hospital is a teaching referral hospital with 350 beds, affiliated to Shiraz University of Medical Sciences. The approval to this study was obtained from the institutional review board and HSR committee, office of vice chancellor for development of management and resources of Shiraz university of medical sciences (93-01-26-8297). Commenced in June 2014, an automated hospital information system (HIS) was used to identify the top costly medications in the mentioned hospital.
Calculating Costs
At first it seemed necessary to compute the costs of drugs according to the prices at which they were obtained from the Department of Pharmacy. The only thing that was taken into consideration was the hospital's acquisition expenditures, while the nursing or pharmacy labor costs associated with costly drugs use were not included.
Practice Guideline Implementation
The drugs which were targeted for practice guidelines implementation were those with largest budget (>10,000 USD/month) and wide use in the whole wards of hospital including albumin, pantoprazole, and enoxaparin.
An expert panel of different specialists (including internist, gastroenterologist, cardiologist, surgeon, neurologist, and clinical pharmacist) was formed to choose updated international consensus guidelines in literature that best matched local condition for the mentioned drugs.
Having selected international consensus guidelines for albumin,8 enoxaparine,9–11 and pantoprazole,12 preprinted order forms were developed accordingly for these drugs.
The forms were presented to all faculty members, resident physicians and nurses who were involved in the administration of the target drugs, and their final comments were invited. To maximize physicians’ adherence to the guidelines and evaluate their fidelity, several strategies13 were employed including:
the application of computer decision support program according to order forms;
periodic audit and feedback based on one-on-one consulting and telephone calls; and
interactive educational meetings.
In our study pool, demographic data, including age, sex, and admission wards, were obtained for each patient. It should be noted that the total number of albumin, enoxaparin, and pantoprazole that were administered for each patient during their hospital stay were recorded.
A control survey was performed in group 1 (pre-guideline implementation group) during the first 4 months to determine the appropriateness of target drug use in different wards based on selected guidelines.
At the start of guideline implementation, during the second 4 months in group 2, the hospital pharmacy began distributing target drugs according to the practice guidelines by confirmation of clinical pharmacist except in emergencies.
Outcome Analysis
The preliminary purpose of this study was to decrease inappropriate utilization of costly medications after the implementation of the clinical-based guidelines. Secondary purpose included the differences in making significant impact on cost between 2 groups in pre and postguideline implementation phases.
Specific outcome criteria such as venous thromboembolic events (VTE), gastrointestinal (GI) bleeding, length of hospital stay (LOS), and mortality rate were evaluated at the end.
Statistical Analysis
Chi-squared test and t test were applied for continuous and nominal data, where appropriate. Independent sample test was employed when appropriate, to compare the differences between pre and post-intervention. The results were compiled with SPSS version 20.0 (Systat Software, Inc., Chicago, IL), and descriptive statistics were analyzed. Pv < 0.05 were considered significant.
RESULTS
Patients’ Size and Characteristics
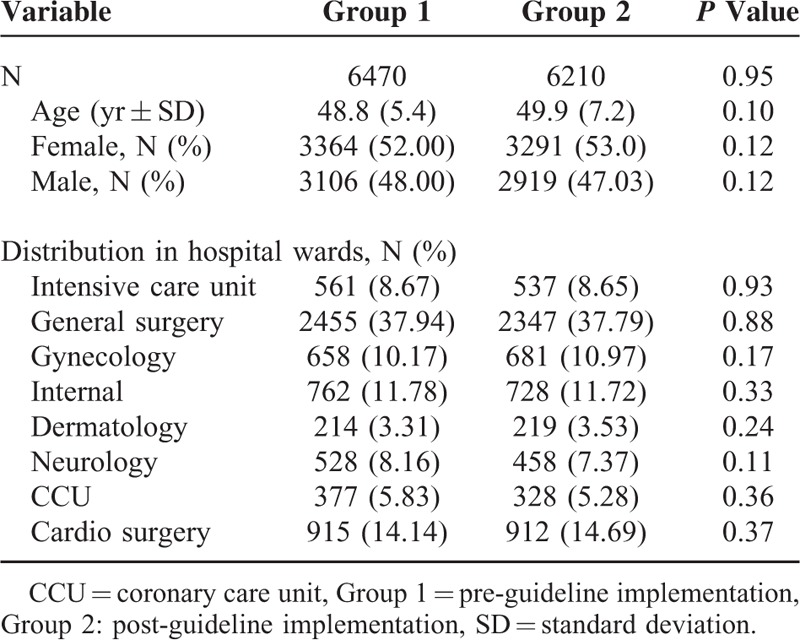
A total of 12,680 patients were included in the 8-month study period, with 6470 and 6210 subjects representing the group 1 (pre-guideline implementation group) and group 2 (post-guideline implementation group), respectively. The mean (standard deviation, SD) age of the patients was 49.35 (6.30) years and 52.48% were female. No significant differences in age and sex distribution were found between the 2 groups (Table 1).
TABLE 1.
Patient's Characteristics
Drug Utilization Review
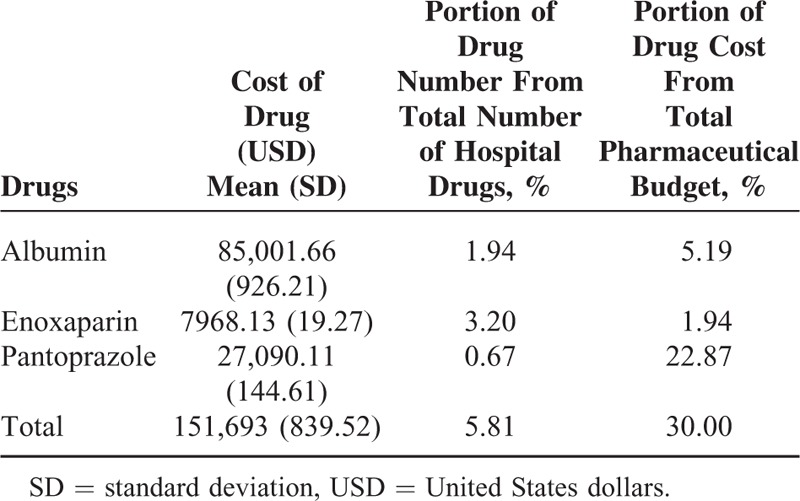
The drugs that we targeted consisted of albumin, enoxaparin, and pantoprazole which represented only 5.81% of total number of hospital drugs; however, the sum of their cost accounted for 30.0% of the total pharmaceutical budget with an average of 152,000 USD/month (Table 2).
TABLE 2.
Costly Drugs (Average Monthly Data) in Group 1 (Pre-guideline Implementation Group)
In order to determine the appropriateness of the target drugs use in different wards, we surveyed the prescriptions during 4 months in group 1. The results showed that 51.2% of albumin, 26% of enoxaparin, and 67% of pantoprazole administrations were inappropriate.
Pharmaceutical Savings as a Result of Practice Guidelines
In group 2, from October 2014 to January 2015, 2732 requests were made for these drugs. From which, 59.7% (1630/2732) were in accordance with guideline, whereas the other 40.3% (1102/2732) were the subject of intervention so the administration continuance were ceased and a feedback was given to the related physician in each case. The reduction in total number of drugs were 36%, 33%, and 40% in albumin, enoxaparin, and pantoprazole, respectively (Table 3, Pv < 0.0001).
TABLE 3.
Comparison of Monthly Pharmaceutical Expenditure in Both Groups
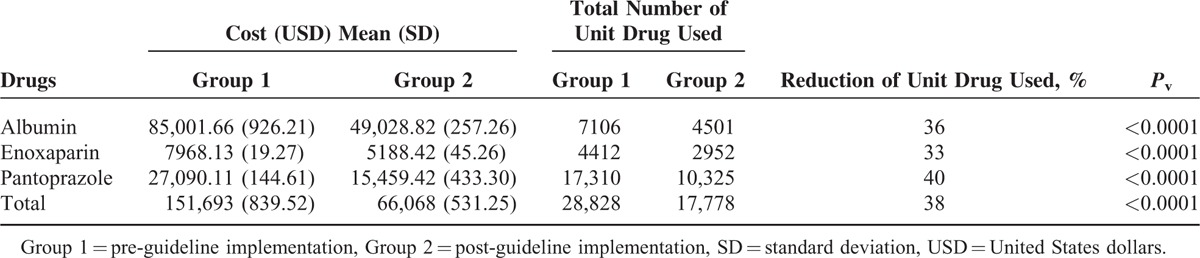
The effects of practice guidelines on the monthly costs of the target drugs are shown in Table 3. (Costs are calculated in USD and they are not adjusted to inflation.)
Mean baseline pharmaceutical costs of the 3 target drugs were calculated to be 151,693 ± 839.52 USD/month, from June to September 2014 in group 1. After the implementation of practice guidelines, the pharmaceutical costs per month have been reduced to 66,068 ± 531.25 USD from October 2014 to January 2015 in group 2 (Pv < 0.0001; Table 3).
The estimated total annual costs of group 1 and group 2 were 1,820,316 USD and 792,816 USD, respectively, which translates into an estimated annual cost saving of 1,027,500 USD (85,625 USD/month).
Overall, in group 1 we had 1156 orders of the target drugs per month of which approximately 53.2% were inappropriate according to the guidelines. In group 2, after guideline implementation, the inappropriate administration was reduced to 34.2 % (683 orders monthly) that makes a significant difference (Pv < 0/0001). So the finding suggests that by implementation of guidelines the number of inappropriate drug orders can be significantly reduced and led to 65.8% physician's adherence to guidelines (Fig. 1).
FIGURE 1.
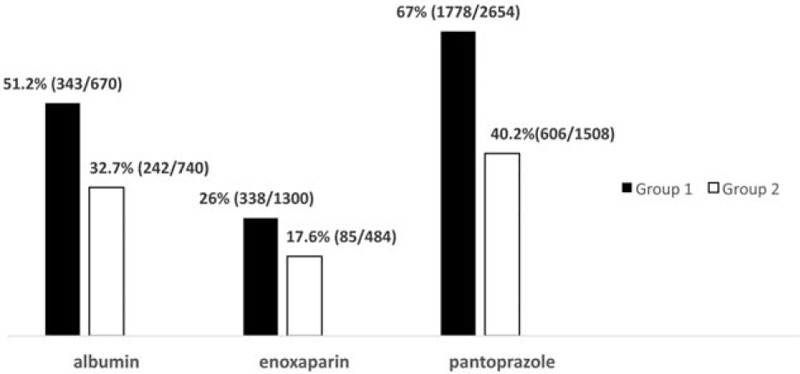
Trend of inappropriate orders for target drugs (%) before and after the employment of an evidence-based drug utilization management program. Group 1 = preguideline implementation, Group 2 = postguideline implementation.
Outcome Analysis
The reason for the overuse of the mentioned drugs is the physicians’ attempt to decrease the incidence of GI bleeding, VTE, and post-surgery recovery time by correction of patients’ hypo-albuminemia and consequently shortening LOS. Hence, in order to determine the consequence of reducing the target drugs overuse, we also proceeded to survey VTE, GI bleeding, VTE, raw, and pure death rate in groups 1 and 2.
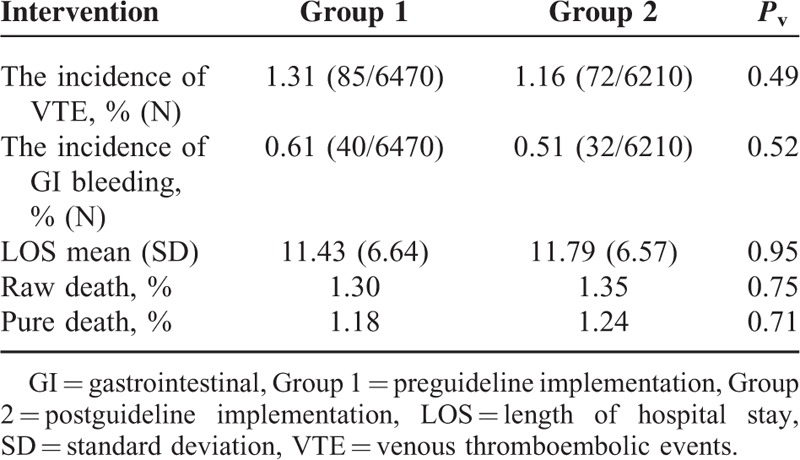
The incidence of VTE was 1.31% (85/6470) in group 1 and 1.16% (72/6210) in group 2, while the difference was not statistically significant (Pv = 0.49). GI bleeding incidence was measured to be 0.61 % (40/6470) and 0.51% (32/6210) in groups 1 and 2, respectively. The results demonstrated a slight decrease in the incidence GI bleeding in group 2; however, it was not statistically significant as well (Pv = 0.52).
LOS (mean ± SD) was 11.43 ± 6.64 days in group 1 versus 11.79 ± 6.57 days in group 2 (Pv = 0.95).
In total number of patients surveyed, there was no statistically significant relationship between either LOS of the 2 groups (Pv > 0.05) or the raw and pure death rates (Pv > 0.05) (Table 4).
TABLE 4.
Comparison of Outcomes Before and After Practice Guidelines Implementation
DISCUSSION
This is the first study from Iran which evaluates the budget saving effect of a guideline implementation for selected costly medications. It should be noted that in healthcare systems, drug expenditure is one of the largest components of the entire hospital operating budget, and increases continuously at a faster pace than other healthcare expenditures.14 Hence, drug costs remain as an area of focus for cost containment initiatives and clinical practice guidelines that attract significant attention of hospital managers.15
Inappropriate use of drugs is reported with varying degrees depending on the drug type and institution. According to a report from Food and Drug Organization of Health Ministry in 2008 in Iran, the highest cost paid for a single drug which was used in hospitals was devoted to albumin.16 Results from our study showed that based on guideline, 51.2% of albumin prescriptions had not been appropriately administered.
In the past few years, some studies in human albumin usage have been carried out in a number of countries. The studies revealed that 50% to 70% of prescriptions were inappropriate, imposing a substantial burden of costs to their healthcare system.17–19
Enoxaparin is a low-molecular weight heparin, which is widely used as curative or preventive treatment of the thromboembolic disorders.20 The present study showed that 26% of enoxaparin administration had been inappropriate. Similar result has been obtained from another comparable study that was performed on enoxaparin prescription for elderly patients, showing that a total of 28.7% of which were inappropriate.21
In the group 1 of our study, 67% of pantoprazole administrations were inappropriate according to the guidelines. The improper administration of proton pump inhibitors (PPIs) including pantoprazole has been shown in other studies as well. Batuwitage et al22 reported inappropriate PPI use in 54% of patients treated with PPIs in a primary care situation. Another study showed that up to 55% of patients for whom PPIs were administered were over-prescribed.23
Over the past few years inappropriate PPI usage has contributed to an increase in healthcare costs. In some reports similar to our results, pantoprazole ranked among the “Top-10” costly drugs which consumed the largest part of total medication costs.22,23
Due to limited healthcare resources, the appropriate use of existing fund seems to be crucial. Introducing guideline restrictions to prescribers at the point of order entry may reduce the number of inappropriate administrations. It is generally believed that implementing a restrictive policy on prescription of costly medications can avoid unnecessary administrations and complications and more people can be served. Since monitoring all pharmaceuticals that are used in hospital is next to impossible and also unnecessary, it is important to determine most costly medications with high impact on the budget.
It seems necessary to find proper strategies to propagate and implement evidence-based guidelines in order to improve physicians’ performance and increase their adherence to these guidelines.24–27
Hence, we used a combination of different methods such as preprinted order forms, local experts’ opinion as well as audit and feedback to optimize the compliance of the healthcare workers.26
In our setting, such interventions led to 65.8% physicians’ adherence to guidelines and consequently inappropriate drug use showed significant decrease in group 2 (Fig. 1).
The findings also suggest a declining trend of the target drug orders that in turn reflects the physicians’ education after the establishment of the institutional guidelines (Fig. 1).
Different modalities with the aim of increasing adherence to institutional guidelines have previously demonstrated favorable outcomes.28,29 Fraser et al30 showed that suggestions from a team of experts (an infectious disease fellow and a clinical pharmacist) including changes in antibiotic choice, dosing, or administration route reduced antibiotic charges per patient for nearly 400 USD in adult in-patients receiving parenteral antibiotics.
In another study by Gentry et al,31 implementation of antibiotic control program yielded a total cost saving of 291,885 USD (30.8% reduction) in a teaching hospital in the United States.
At the Hartford Hospital, the investigators reported a total cost avoidance of 777,462 USD over 4 years by a pharmacy-managed anemia protocol implementation in an outpatient dialysis setting.32
In the present study, by implementing the practice guidelines, major cost savings (56%) was produced via limiting the inappropriate use of costly drugs (Table 3) without any adverse impact on the clinical outcomes (Table 4).
In our tertiary hospital, we saved 85,625 USD/month by simply implementing guidelines for only 3 medications. If we assume that the same profile of drug usage continues, the magnitude of annually saving would be 1 million USD for only 1 hospital.
One of the major causes that the physicians stated for overprescribing the target drugs was that they were extremely cautious about their patients. In other words, there was a presumption that if guidelines criteria were implemented, it would put the patients at the risk of VTE, GI bleeding, or also lengthy surgery recovery and consequently lead to increasing LOS. However, our results showed that when administration is fully in accordance with guideline criteria, it does not increase the risk of aforesaid incidents, since there was no statistically relation between the incidences of VTE, GI bleeding, LOS, and death rates of the 2 groups (P value > 0.05).
Contrary to the physicians’ belief; LOS did not increase by declining drug administrations, besides a significant cost saving was achieved via the reduction of inappropriate and overuse of these drugs (Table 3).
In fact, the results implied that drugs overuse not only did not provide any excessive benefit, but also can potentially put the patients at risk of side effects and imposed a burden of unnecessary costs on them.
Interestingly in spite of the fact that our intervention mostly included declining drug administrations, LOS (P = 0.95) and mortality rate (P = 0.75) was not different when comparing the 2 groups before and after guideline implementation.
The main limitation of this study was failing to account the nursing or pharmacy labor costs associated with costly drugs use.
CONCLUSIONS
In conclusion, institutionalizing practice guidelines can lead to a significant decrease in the cost and proper use of drugs. Based on the results, implementing a drug-utilization management program, could improve the adherence of evidence-based drug utilization which would in turn result in 1 million USD savings annually only for 3 drugs. Therefore, it is recommended that institutions consider implementing such management programs in order to reduce inappropriate drug usage and decrease their costs.
ACKNOWLEDGMENTS
The authors express their gratitude to Dr Paria Mahmoudi and the Research Consulting Center (RCC) of Shiraz University of Medical Sciences for their assistant in English editing.
Footnotes
Abbreviations: GI = gastrointestinal, HISh = ospital information system, LOS = length of hospital stay, PPI = proton pump inhibitor, USD = United States dollars, VTEv = enous thromboembolic events.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Sackett DL, Rosenberg WMC, Gray JAM, et al. Evidence based medicine: what it is and what it isn’t. BMJ 1996; 312:71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marwick C. Pharmacoeconomics: is a drug worth its cost? JAMA 1994; 272:1395. [PubMed] [Google Scholar]
- 3.Kantor GSA, Chung F. Anesthesia drug cost, control and utilization in Canada. Can J Anaesth 1996; 43:9–16. [DOI] [PubMed] [Google Scholar]
- 4.Grimshaw JM, Russell IT. Achieving health gain through clinical guidelines II: ensuring guidelines change medical practice. Qual Health Care 1994; 3:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg W, Donald A. Evidence based medicine; an approach to clinical problem-solving. BMJ 1995; 310:1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schifalacqua MM, Shepard A, Kelley W. Evidence based practice: cost benefit of large sys implementation. Qual Manag Health Care 2012; 21:74–80. [DOI] [PubMed] [Google Scholar]
- 7.Buckley MS, Kane-Gill LS, Patel SA. Clinical and economic evaluation of an evidence-based institutional epoetin-utilization management program. Clin Ther 2013; 35:294–302. [DOI] [PubMed] [Google Scholar]
- 8.Liumbruno GM, Bennardello F, Lattanzio A, et al. Recommendations for the use of albumin and immunoglobulins. Blood Transfus 2009; 7:216–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133:381–453. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients undergoing surgery. NICE Clinical Guideline No. 46, pp. 1–160. Available at: http://www.nice.org.uk/CG046 Accessed March 31, 2008. [Google Scholar]
- 11.Kearon C. Natural history of venous thromboembolism. Circulation 2003; 107:22–30. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong TA, Coursin DB, Devlin J, et al. ASHP therapeutic guidelines on stress ulcer prophylaxis. Am J Health Syst Pharm 1999; 56:347–379. [DOI] [PubMed] [Google Scholar]
- 13.Glasgow R, McKay G, Piette J, et al. The RE-AIM framework for evaluating interventions: what can it tell us about approaches to chronic illness management? Patient Educ Couns 2001; 44:119–127. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman JM, Shah ND, Vermeulen LC, et al. Projecting future drug expenditures. Am J Health Syst Pharm 2006; 63:123–138. [DOI] [PubMed] [Google Scholar]
- 15.Heffler S, Smith S, Keehan S, et al. U.S health spending projections for 2004–2014. Health Aff 2005; 5:74–85. [DOI] [PubMed] [Google Scholar]
- 16.Jahangard-Rafsanjani Z, Javadi MR, Torkamandi H, et al. The evaluation of albumin utilization in a teaching university hospital in Iran. Iran J Pharm Res 2011; 10:385–390. [PMC free article] [PubMed] [Google Scholar]
- 17.Tarín-Remohi MJ, Sánchez-Arcos A, Santos-Ramos B, et al. Costs related to inappropriate use of albumin in Spain. Ann Pharmacother 2000; 34:1198–1205. [DOI] [PubMed] [Google Scholar]
- 18.Yim JM, Vermeulen LC, Erstad BL, et al. Albumin and nonprotein colloid solution use in US academic health centers. Arch Intern Med 1995; 155:2450–2455. [PubMed] [Google Scholar]
- 19.Tanzi M, Gardner M, Megellas M, et al. Evaluation of the appropriate use of albumin in adult and pediatric patients. Am J Health Syst Pharm 2003; 60:1330–1335. [DOI] [PubMed] [Google Scholar]
- 20.Deitcher SR, Kessler CM, Merli G, et al. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost 2006; 12:389–396. [DOI] [PubMed] [Google Scholar]
- 21.Pugh MJV, Fincke BG, Bierman AB, et al. Potentially inappropriate prescribing in elderly veterans: are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc 2005; 53:1282–1289. [DOI] [PubMed] [Google Scholar]
- 22.Batuwitage BT, Kingham JG, Morgan NE, et al. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J 2007; 83:66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Vliet EP, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther 2009; 29:213–221. [DOI] [PubMed] [Google Scholar]
- 24.Freemantle N, Harvey EL, Wolf F, et al. Printed educational materials: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2000; 2:172. [DOI] [PubMed] [Google Scholar]
- 25.Thomson MA, Oxman AD, Davis DA, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2000; 2:409. [DOI] [PubMed] [Google Scholar]
- 26.Thomson MA, Oxman AD, Haynes RB, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2000; 2:125. [DOI] [PubMed] [Google Scholar]
- 27.Jamtvedt G, Young JM, Kristoffersen DT, et al. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2006; 2:259. [DOI] [PubMed] [Google Scholar]
- 28.Devlin JW, Holbrook AM, Fuller HD. The effect of ICU sedation guidelines and pharmacist interventions on clinical outcomes and drug cost. Ann Pharmacother 1997; 31:689–695. [DOI] [PubMed] [Google Scholar]
- 29.Marshall J, Finn CA, Theodore AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med 2008; 36:427–433. [DOI] [PubMed] [Google Scholar]
- 30.Fraser GL, Stogsdill P, Dickens JD, Jr, et al. Antibiotic optimization. An evaluation of patient safety and economic outcomes. Arch Intern Med 1999; 157:1689–1694. [DOI] [PubMed] [Google Scholar]
- 31.Gentry CA, Greenfield RA, Slater LN, et al. Outcomes of an antimicrobial control program in a teaching hospital. Am J Health Syst Pharm 2000; 57:268–274. [DOI] [PubMed] [Google Scholar]
- 32.Quercia RA, Abrahams R, White CM, et al. Cost avoidance and clinical benefits derived from a pharmacy managed anemia program. Hosp Pharm 2000; 36:169–175. [Google Scholar]


