Abstract
A high residential altitude impacts on the growth of children, and it has been suggested that linear growth (height) is more affected than body mass. The aim of the present study was to estimate the prevalence of obesity, overweight, underweight, and stunting in groups of native Tibetan children living at different residential altitudes (3700 vs 4300 m above sea level) and across ancestry (native Tibetan vs Han Chinese children living at the same altitude of 3700 m), as well as to examine the total effect of residential altitude and ancestry with stunting.
Two cross-sectional studies of 1207 school children aged 9 to 10 years were conducted in Lhasa in 2005 and Tingri in 2007. Conventional age- and sex-specific cutoff values were used for defining underweight, normal weight, overweight, or obesity, whereas stunting was defined from sex-specific height-for-age z-scores (≤−2.0).
The prevalence of underweight was high at 36.7% among Tingri Tibetan girls and 31.1% in Tingri Tibetan boys. The prevalence was statistically significant lower in Lhasa Tibetan girls (20.2%) than in both Tingri Tibetan girls and Han Chinese girls (33.7%), with a similar trend seen among boys. Severe and moderate stunting were found in 14.6% and 35.7%, respectively, of Tingri children, and near null among Han Chinese and native Tibetans in Lhasa. In logistic regression analyses, socioeconomic status and diet did not substantially change the observed crude association (total effect) (odds ratio [OR] = 3.3; 95% confidence interval [CI] 1.1–10.3) between ancestry and stunting. Similarly, adjustment for diet did not alter the crude association (direct effect) (OR = 101.3; 95% CI 37.1–276.4) between residential altitude and stunting.
The prevalence estimates of stunting and underweight were high, and clearly higher among native Tibetan children living at a higher residential altitude (Tingri) than the lower residential altitude (Lhasa), in addition to being higher among Han Chinese children than Tibetan children living at the same residential altitude (Lhasa). Thus, physical growth according to age, in terms of both height and weight, affected children living at an altitude of 4300 m above sea level.
INTRODUCTION
Growth serves as an overall indicator of child health at both sea level and high altitude,1 whereas a high residential altitude may lead to a delayed growth in children2 due to environmental stressors. High-altitude stressors include hypobaric hypoxia, low temperatures and relative humidity, high cosmic radiation, and in some instances, limited nutritional resources.3–5 Dang et al6 reported that Tibetan children <36 months and living >3500 m had a 2 to 6 time higher risk of becoming stunted in comparison to those living <3500 m when potential confounding factors (socioeconomic status [SES], child feeding patterns, morbidity, and maternal health care) were controlled for. They further reported a prevalence of stunting between 25.8% and 56.4% for altitudes between 3000 and 4500 m above sea level.6 Among Andean highlanders, the delay in growth is at its greatest during late childhood and adolescence.2 Haas et al5 reported that a high residential altitude affects linear growth (height) rather than body mass, and that this effect appears to start early in the postnatal period. Therefore, height-for-age (HAZ) z-score could serve as a measure for the long-term effects of high-altitude environmental stressors. Other indicators of growth, particularly weight-for-height z-score, measure more acute effects, for example responses to acute economic, environmental, and political factors.7
Delayed physical growth or stunting among highlanders has also been associated with low SES, including poor access to nutrients.8 Greksa et al9 reported that urban native children from La Paz, Bolivia (3600 m), were taller at all ages than rural children living at the same altitude, which could be due to differences in SES. In the same study,9 they further reported that the urban children had a similar height compared with urban high-altitude children from Peru living at an almost similar altitude (3800 m). These 2 populations have the same ancestry.9,10 However, Greksa et al11 and Stinson12 found a variation in growth between children with different ancestry living at the same altitude in the Andes. European children born and raised in the Andes were taller and heavier than the indigenous Aymara children, but the Europeans had a higher SES.11,12
Native Tibetans have the longest history of living at high altitude, approximately 22,000 years,13,14 and a comparison of the indicators of growth with Han Chinese immigrants (who have lived in Tibet for 1 to 3 generations) living at the same altitude may mainly indicate genetic differences, but could also be due to environmental and socioeconomic factors. Differences in indicators of physical growth among native Tibetan children living at different altitudes may mainly indicate effects of altitude, but could also be due to environmental and socioeconomic factors. In order to provide more information on the physical growth of children living in Tibet, the present study aimed at describing anthropometric measurements and estimating the prevalence of obesity, overweight, underweight, and stunting in native Tibetan and Han Chinese immigrant children living in Lhasa at an altitude of 3700 m and native Tibetan children living in Tingri at an altitude of 4300 m above sea level. We also aimed at examining the total effect of residential altitude and ancestry with stunting (HAZ z-score) by contrasting native Tibetans living at 3700 and 4300 m above sea level, and native Tibetan and Han Chinese children living at 3700 m, respectively.
We hypothesize that the physical growth indicators are less favorable in Tingri Tibetans and in Lhasa Han Chinese as compared with Lhasa Tibetan children, that is lower weight and height and a higher prevalence of stunting and underweight in Tingri Tibetan and Lhasa Han Chinese children than in Lhasa Tibetan children.
MATERIALS AND METHODS
Data were collected from a structured questionnaire and clinical examination, and we applied similar methods in the studies in Lhasa and Tingri, previously described by Bianba et al.15 The data collections were conducted indoors from August to November 2005 in Lhasa, and from September to October 2007 in Tingri.
Sample Size
In order to estimate sample size of our study population, we used expected mean and standard deviation from a similar study.16 Using the formula: n = 2kSD2/Δ2, where α = 0.05 and β = 0.20, and then k = 7.8, with SD = 5.8 and expected difference (Δ) between groups to be compared was 2 kg, we needed 131 in each group. We did separate analysis for boys and girls and compared native Tibetan and Han Chinese immigrant children at same altitude and native Tibetans at different altitudes. Therefore, we aimed at including 200 girls and 200 boys for all 3 study groups of children (ie, Tibetans in Lhasa, Tibetans in Tingri, and Han Chinese in Lhasa).
Data Collection and Variables
Nine primary schools out of 20 were randomly selected from Lhasa City, the capital of the Tibet Autonomous Region in China, whereas all 5 primary schools were selected from the rural Tingri district under the north face of Mt. Everest. One teacher from each of the selected schools prepared a list of all 9- to-10-year-old schoolchildren, which included 817 children from Lhasa and 490 children from Tingri. In Lhasa, 4 Tibetan children on the list were excluded due to respiratory health problems, hence yielding a total of 813 eligible children. One child refused to participate and 3 children lacked anthropometric measurements, thereby providing a participation rate of nearly 100%. Thus, 405 native Lhasa Tibetan children (207 boys and 198 girls) and 404 Lhasa Han Chinese children (235 boys and 169 girls) were included in the final analysis. In Tingri, 30 children on the list did not fulfill the criteria for age and were therefore excluded, yielding 460 eligible children. Moreover, 16 children did not go to school on the days of the data collection, 36 children lacked anthropometric measurements, and 10 children participated, but they were excluded because their parents did not agree to the use of their data for publication, producing a response rate of 87%. As a result, 203 girls and 195 boys were included in the final analysis in the rural area.
Study Group
“Study group” is a categorical variable which includes Lhasa Tibetans, Lhasa Han Chinese, and Tingri Tibetans. It is a measure of the effect of residential altitude when comparing Lhasa Tibetans and Tingri Tibetans, whereas the comparison of Lhasa Tibetans and Lhasa Han Chinese reflects the effect of ancestry. For selected analyses, Lhasa Han Chinese were further divided into lowland- and highland-born.
Anthropometric Measurements
An electronic scale (OMRON, HN-281, Shanghai, China) was applied to measure the body mass to the nearest 0.1 kg without shoes and wearing light clothes. A stadiometer (TZG, Shanghai, China) was used to measure the height to the nearest 0.5 cm. Chest and waist circumferences were measured before and after expiration with subjects standing and breathing normally, and the average of these 2 measurements was recorded. Body mass index (BMI) is defined as the body mass (in kg) divided by the square of the height (in meters).
Underweight, Overweight, Obesity, and Stunting
Deviations from normal physical growth were expressed as a prevalence of underweight, overweight, obesity, and stunting. International age- and sex-specific cutoff values were used for defining underweight, normal weight, overweight, or obesity according to Cole et al,17,18 and stunting was defined from sex-specific HAZ z-scores according to the WHO's 2007 growth reference.19,20 The raw measurements were converted to z-scores using the means and standard deviations of the data (z-score = [observed-median/SD]) according to the WHO's 2007 growth reference standards.20 As described by Dutta et al,21 children with a HAZ of −3 SD, −2 SD, and −1 SD were classified as having severe, moderate, and mild levels, respectively, of stunting; children with z-scores between −1 SD and +1 SD were considered to be normal, whereas children with z-scores of +1 SD, +2 SD, and +3 SD were considered to be overnourished, with the degree of overnourishment increasing with a higher SD.
SES
For estimating the association between ancestry and stunting in subanalyses, that is comparing Lhasa Tibetans and Lhasa Han Chinese, SES was measured from the question “How is your family financial condition (family economy) as compared to other families?”. The answer categories (poor, quite rich, rich) were operationalized into low SES (poor) and high SES (quite rich, rich). By a mistake, the same question was not included in Tingri. Thus, for estimating the association between residential altitude with stunting in subanalyses, that is comparing Lhasa Tibetans and Tingri Tibetans, adjustment for SES was not performed.
Diet Variation
In the present study, “Diet variety” is a variable that indicates the number of food types the child generally consumes at least once a week, which is among the 10 most healthy food types listed in the questionnaire: fish, fruits, raw vegetables, cooked vegetables, legumes, eggs, juice, nuts, cheese, and tsamba (Tibetan barley). “Diet variety” was further dichotomized into “healthy” (7–10 food types) and “unhealthy” (0–6 food types).
Statistical Methods
Data are reported as the mean ±1 SD, or as the mean with a 95% confidence interval (CI). The difference in mean values between groups was tested using a one-way ANOVA, and logistic regression models were applied to test the associations between the outcome variable (stunting) and study group (residential altitude and ancestry). The crude odds ratio (OR) indicates the unadjusted association (total effect) of altitude and ancestry with stunting (Model 1), and we adjusted for sex in Model 2. In order to investigate whether diet variety had any impact on the strength of association between residential altitude and ancestry with stunting, we adjusted for sex and diet variety in Model 3. Similar analyses (Models 1, 2, 3) were conducted with a change in the “study group” variable; the Lhasa Han Chinese group was subdivided into 2 groups: those born at high altitude (highland-born Han Chinese) versus those born at low altitude (lowland-born Han Chinese). In separate analyses of data restricted to Lhasa investigating the association between ancestry and stunting, we did adjustment for sex, diet variety, and SES (family economy). We used SPSS version 16.0 and Stata SE 9.0 for the data analyses, and the level of statistical significance was set at P < 0.05.
Ethical Considerations
The Tibet University Medical College (TUMC) was responsible for the data collection and ethical approval. Official permission for conducting the present studies was granted by the Health and Education Office and the TUMC. In addition, the permission for visiting the Tingri area was given by the Office of Frontier Defense in Lhasa. Information and consents forms were given to parents through the school leaders, and information about the study procedure was also given to the children. Participation was voluntary and the children could withdraw from the study for any reason at any time with no negative consequences. All information about the subjects was handled confidentially, and the names of the subjects were not registered. The list of identification number linking to names of participants was kept separately from the data file.
RESULTS
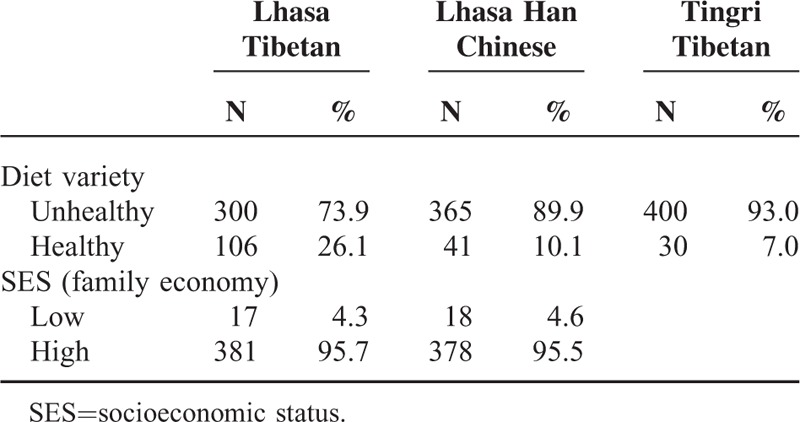
Sex-specific anthropometrical characteristics of Tingri Tibetan, Lhasa Tibetan, and Lhasa Han Chinese children are shown in Table 1. On average, the Lhasa Tibetan boys and girls were heavier (29.7 vs 22.5 kg; P < 0.001 for boys; 29.4 vs 22.4 kg; P < 0.001 for girls) and taller (134.8 vs 122.2 cm; P < 0.001 for boys; 135.9 vs 122.8 cm; P < 0.001 for girls), and had a higher BMI (16.3 vs 15.0 kg/cm2; P < 0.001 for boys; 15.8 vs 14.8 kg/cm2; P < 0.001 for girls) than the Tingri Tibetan; the Lhasa Tibetan boys and girls were statistically significant heavier, taller, and had a higher BMI than the Lhasa Han Chinese. Furthermore, the Lhasa Tibetan boys and girls also had larger chest and waist circumferences than the Tingri Tibetan (P < 0.001) and Lhasa Han Chinese (P < 0.001). The proportions of children with a healthy diet variation were 26.1%, 10.1%, and 7.0% among Lhasa Tibetan, Lhasa Han Chinese, and Tingri Tibetan children, respectively (Table 2). SES was only reported in Lhasa Tibetan and Lhasa Han Chinese children, and only 4.3% (Tibetan) and 4.6% regarded their family financial situation as poor (low SES), as compared with other families (Table 2).
TABLE 1.
Anthropometric Data of 9- to 10-Year-Old Lhasa Tibetan, Lhasa Han Chinese, and Tingri Tibetan Children
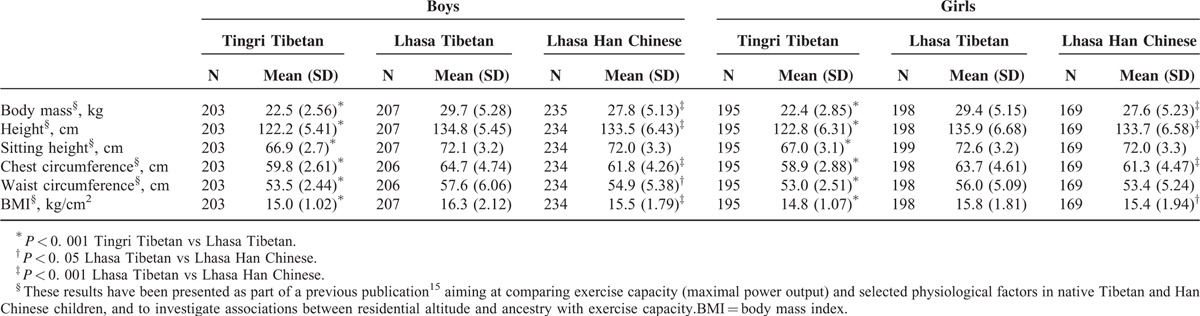
TABLE 2.
Prevalence of Healthy and Unhealthy Diet Variety Among 9- to 10-Year-Old Lhasa Tibetan, Lhasa Han Chinese, and Tingri Tibetan Children, and SES Among Lhasa Tibetan and Lhasa Han Chinese Children
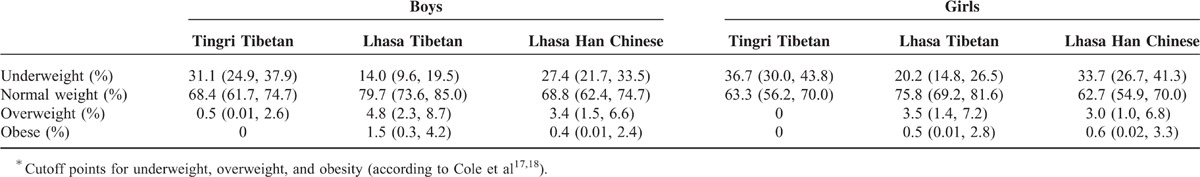
None of the Tingri Tibetans, and only 3.5% of Lhasa Tibetans and 3.0% of Han Chinese, were classified as overweight (Table 3). However, the prevalence of underweight was high at 36.7% (95% CI 30.0–43.8) among Tingri Tibetan girls and 31.1% (95% CI 24.9–37.9) in Tingri Tibetan boys. The prevalence was statistically significant lower in Lhasa Tibetan girls (20.2%; 95% CI 14.8–26.5) than in both Tingri Tibetan girls and Han Chinese girls (33.7%; 95% CI 26.7–41.3), with a similar trend seen among boys (Table 3).
TABLE 3.
Prevalence∗ of Underweight, Normal Weight, Overweight, and Obesity Among 9- to 10-Year-Old Children Living in Tibet (Percentage with 95% Confidence Intervals)
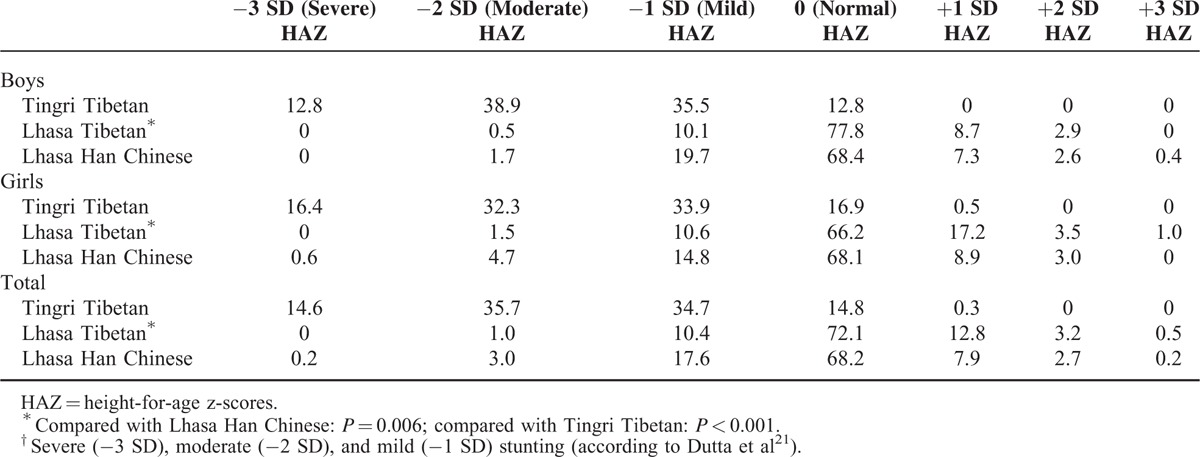
Severe stunting was found in 14.6% of Tingri children, whereas only 0.2% of Han Chinese and none of the Lhasa Tibetans were classified with severe stunting (Table 4). Among Tingri Tibetan children, 35.7% were moderately stunted, whereas this was the case for only 3.0% of Han Chinese and 1.0% of Lhasa Tibetan children.
TABLE 4.
Percentage Stunting† (HAZ) in 9- to 10-Year-Old Children Living in Tibet, by Sex and Ancestry
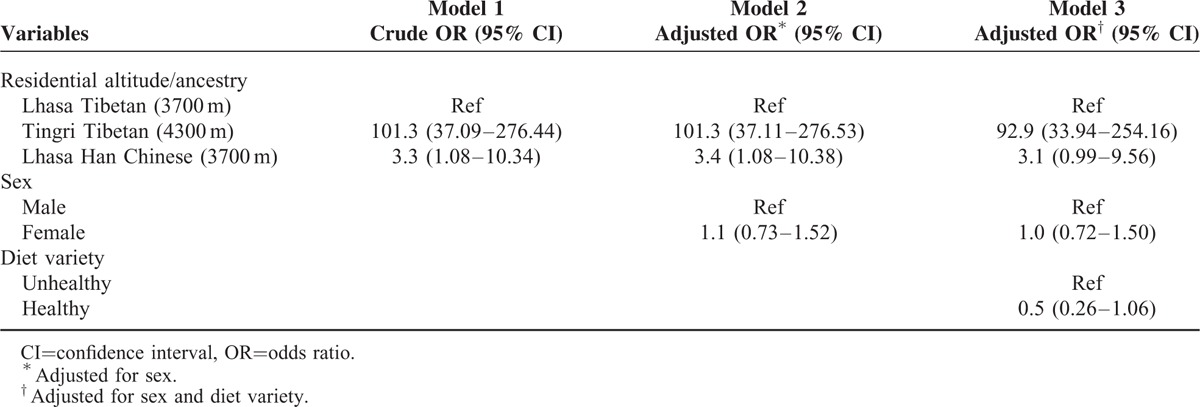
The associations between residential altitude and ancestry with stunting are shown in Table 5. As compared with Lhasa Tibetan children living at an residential altitude of 3700 m, Tingri Tibetan children at 4300 m had a 101.3 times higher odds for stunting, both in crude analyses (total effect) and after an adjustment for sex (Models 1 and 2). After an adjustment for diet variety, the OR did not change substantially (Model 3). Regarding ancestry, the odds for stunting among Lhasa Han Chinese was 3.3 times higher compared with Lhasa Tibetan children (Model 1), which did not change significantly after adjustments (Models 2 and 3). In separate analyses of data restricted to Lhasa, investigating the association between ancestry and stunting, an adjustment for SES did not affect the association, but lower SES was associated with stunting (OR = 8.8 [2.55–30.00]) (not shown in table). In separate analyses, where we split Lhasa Han Chinese into 2 groups, highland- and lowland-born, the OR for stunting became nonsignificant and slightly lower for the 2 groups, OR = 2.3 (0.54–9.68) for highland-born vs OR = 2.7 (0.90–8.15) for lowland-born (not shown in table).
TABLE 5.
Associations (OR) Between Residential Altitude and Ancestry With Stunting: Crude OR (Model 1); Adjustments for Sex (Model 2); Adjustments for Sex and Diet Variety (Model 3); Logistic Regression Analyses
DISCUSSION
The main findings of the present study were the high prevalence of stunting and underweight among children living in Tibet, with a higher prevalence among native Tibetans living at a higher residential altitude as compared with a lower residential altitude, and higher among Han Chinese compared with native Tibetan children living at the same residential altitude.
Several studies from high-altitude populations in Tibet6,16,22–25 and the Andes5,26,27 have reported a low height and weight as a measure of delayed growth, whereas few studies6,22 have reported prevalence estimates of underweight and stunting in Tibetan children. Our estimate of moderate and severe stunting indicates that every second child in Tingri had a low height for their age. This is higher than reported in a study of 8- to 14-year-old Tibetan children living in Shegar, Tingri, and Qi at 4000 to 4350 m, where a total prevalence of 28.3% was reported.28 When compared with the present study, parts of this discrepancy might be explained by methodological differences, including the presentation of average prevalence figures from rural and urban areas and sampling at a slightly lower mean altitude than Tingri (4300 m).
Previous studies have reported associations between (high) residential altitude and (low) birth weight,1,5,29,30 which may have subsequently resulted in a delayed growth.31
Some studies have shown that poor growth is related more to SES than to hypoxia,11,26,32 whereas others claim that residential altitude was more important.6,19,33 A community survey of Tibetan children living at altitudes between 3000 and 4000 m in Tibet demonstrated that there was a consistently better HAZ among children from urban than rural areas, although there was no direct association of stunting with residential altitude.32 In contrast to the present findings, Harris et al concluded that poor nutrition causes stunting in Tibetan children at high altitude. It has also been reported that the difference in growth between indigenous Aymara and migrant Europeans is related to different socioeconomic patterns.11,12 Moreover, a report by Pawson and Huicho34 based on 2 cross-sectional studies conducted in 1964 and 1999 in Peru suggested that a large part of the variation in physical growth was attributed to a variation in SES.
In a study of Tibetan children and adolescents aged 7 and 18 years living at 2261, 2780, 3407, 3658, and 4040 m, the children at the highest residential altitude (4040 m) presented a slower growth, lagging behind their counterparts in height at an altitude of 2261 by approximately 2 years. This indicates that hypoxia may be an important agent responsible for this delayed growth.35 Dang et al6 reported that residential altitude may result in a delay in height for younger children, independent of SES, child feeding patterns, morbidity, and maternal health care. The present study found no impact of diet variety on the associations between residential altitude and stunting and no impact of SES and diet variety on the association between ancestry and stunting. It is likely that the difference in stunting between Tibetan children living at 2 different altitudes may be due to altitude-induced hypoxia, which is considered to be the major factor of delayed growth at high altitude.3 From animal studies, it has been shown that the degree of hypoxia does not directly determine the degree of appetite and energy intake, and that protein restriction affects growth independently of whether the animals were hypoxic or not,36 whereas other animal studies indicate that hypoxia-like conditions decrease energy intake, hence reducing body mass.37,38
Differences in growth parameters, such as chest circumference, between different ancestral groups with a similar SES have been reported in high-altitude populations, thereby indicating a possible genetic effect.16 Recent genome-wide studies provide evidence of genetic adaptation to live at high altitude.39–46 In the present study, we found a significant difference in stunting between Lhasa Tibetan and Lhasa Han Chinese living at the same residential altitude, though with no changes in the strength of the association when including SES and diet variety in the analyses. For this reason, genetic differences in the ability of adaptation could have contributed to the observed variation in stunting variation between the 2 ancestries.
Methodological Discussion
An advantage of the present study is the high participation rates. However, participants from Tingri may be healthier than Lhasa children, which could lead to selection bias. The potential bias could be caused by different living conditions: children in Lhasa are living with their family and have a short travel distance to school, whereas children in rural need to travel a longer distance and stay in the school dormitory for a long time. Therefore, there may be healthier children in the schools in Tingri, and this may possibly have distorted the results toward a smaller difference between the 2 groups of children. The fieldworkers in 2 study sites (Lhasa and Tingri) were trained by the same person, which may reduce the systematic error in the measurements. In addition, we applied similar methods, internationally recognized, in both study sites. A weakness of the present study is that we have no common indicators of SES for all 3 groups of the population. It is difficult to identify a valid indicator of SES due to heterogeneity between Lhasa and Tingri in factors commonly used as indicators of the SES of a family. For example, in Tingri it is common that parents have low or no education and no income, but those who have several yaks and sheep will represent high SES in Tingri. In Lhasa, parents do not have animals, but most of the fathers have different levels of education and income.
We recommend conducting a specific study to identify the best measure of SES for high-altitude populations in Tibet. In new studies on altitude populations and stunting, we recommend the inclusion of measures of energy intake and expenditure and detailed measures of food quality and quantity, as well as including other factors that influence growth.
Other limitations of the study are the unavailability of data on parents’ height, which could have been included in analyses as a confounder when comparing native Tibetans with Han Chinese children. Finally, the comparison between native Tibetans with Han Chinese children would have been strengthened if we included data on Han Chinese living in Tingri. However, the population of Han Chinese in Tingri is small, and most of the few Chinese immigrant families send their children to schools in inland China or urban areas of Tibet due to the fact that all teaching is given in Tibetan language in rural schools. Thus, it is impossible to reach Han Chinese children in Tingri in a school setting, and it is hard to find 9- to 10-year-olds in Tingri at all.
CONCLUSIONS
Native Lhasa Tibetans are taller and heavier than those living at higher residential altitude, and also taller and heavier than Han Chinese living at the same altitude. None of the Tingri Tibetans, and only 3.5% of Lhasa Tibetans and 3.0% of Han Chinese, were classified as overweight. However, stunting and underweight were common among these populations, and an association was found between ancestry and stunting for children living at 3700 m in Lhasa. The higher prevalence of stunting among native Tibetan children living at a higher altitude (Tingri) compared with lower altitude (Lhasa) may represent delayed growth, and could be followed up in longitudinal studies.
An adjustment for diet variety did not substantially change the association between residential altitude and stunting, and there was no change in the association between ancestry and stunting after adjustment for SES and diet variety. This may indicate that there are some other factors related to living at higher altitude or having different ancestry that account for these associations, or that the SES and diet variables are too crude.
Acknowledgments
We are very thankful to the Network for University Cooperation Tibet-Norway for supporting this study. Thanks as well as to all the children who participated in this study and to our colleagues at the Tibet University Medical College, who gave great support in the data collection process.
Footnotes
Abbreviations: BMI = body mass index, HAZ = height-for-age, OR = odds ratio, SES = socioeconomic status.
This work was supported by the National 973 Program of China grants 2012CB518202 to T.W.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Niermeyer S, Yang P, Shanmina Drolkar, et al. Arterial oxygen saturation in Tibetan and Han infants born in Lhasa, Tibet. N Engl J Med 1995; 333:1248–1252. [DOI] [PubMed] [Google Scholar]
- 2.Greksa LP. Growth and development of Andean high altitude residents. High Alt Med Biol 2006; 7:116–124. [DOI] [PubMed] [Google Scholar]
- 3.Baker PT. Human adaptation to high altitude. Science 1969; 163:1149–1156. [DOI] [PubMed] [Google Scholar]
- 4.Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr 1981; 34:2540–2545. [DOI] [PubMed] [Google Scholar]
- 5.Haas JD, Moreno-Black G, Frongillo EA, Jr, et al. Altitude and infant growth in Bolivia: a longitudinal study. Am J Phys Anthropol 1982; 59:251–262. [DOI] [PubMed] [Google Scholar]
- 6.Dang S, Yan H, Yamamoto S. High altitude and early childhood growth retardation: new evidence from Tibet. Eur J Clin Nutr 2008; 62:342–348. [DOI] [PubMed] [Google Scholar]
- 7.Stevens GA, Finucane MM, Paciorek CJ, et al. Nutrition Impact Model Study G. Trends in mild, moderate, and severe stunting and underweight, and progress towards MDG 1 in 141 developing countries: a systematic analysis of population representative data. Lancet 2012; 380:824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niermeyer S, Andrade Mollinedo P, Huicho L. Child health and living at high altitude. Arch Dis Child 2009; 94:806–811. [DOI] [PubMed] [Google Scholar]
- 9.Greksa LP, Spielvogel H, Paredes-Fernandez L, et al. The physical growth of urban children at high altitude. Am J Phys Anthropol 1984; 65:315–322. [DOI] [PubMed] [Google Scholar]
- 10.Corella A, Bert F, Perez-Perez A, et al. Mitochondrial DNA diversity of the Amerindian populations living in the Andean Piedmont of Bolivia: Chimane, Moseten, Aymara and Quechua. Ann Hum Biol 2007; 34:34–55. [DOI] [PubMed] [Google Scholar]
- 11.Greksa LP, Spielvogel H, Caceres E. Effect of altitude on the physical growth of upper-class children of European ancestry. Ann Hum Biol 1985; 12:225–232. [DOI] [PubMed] [Google Scholar]
- 12.Stinson S. The effect of high altitude on the growth of children of high socioeconomic status in Bolivia. Am J Phys Anthropol 1982; 59:61–71. [DOI] [PubMed] [Google Scholar]
- 13.Aldenderfer MS. Moving up in the world: archaeologists seek to understand how and when people came to occupy the Andean and Tibetan plateaus. Am Sci 2003; 91:542–549. [Google Scholar]
- 14.Niermeyer S, Zamudio S, Moore LG. Hornbein TF, Schoene RB. The people. High Altitude: An Exploration of Human Adaptation. New York: Marcel Dekker; 2001. 43–99. [Google Scholar]
- 15.Bianba, Berntsen S, Andersen LB, et al. Exercise capacity and selected physiological factors by ancestry and residential altitude: cross-sectional studies of 9–10-year-old children in Tibet. High Alt Med Biol 2014; 15:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weitz CA, Garruto RM, Chin CT, et al. Morphological growth and thorax dimensions among Tibetan compared to Han children, adolescents and young adults born and raised at high altitude. Ann Hum Biol 2004; 31:292–310. [DOI] [PubMed] [Google Scholar]
- 17.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole TJ, Flegal KM, Nicholls D, et al. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 2007; 335:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panesar NS. Why are the high altitude inhabitants like the Tibetans shorter and lighter? Med Hypotheses 2008; 71:453–456. [DOI] [PubMed] [Google Scholar]
- 20.WHO growth reference data page. 2007. http://www.who.int/childgrowth/standards/height_for_age/en/. [Google Scholar]
- 21.Dutta A, Pant K, Puthia R, et al. Prevalence of undernutrition among children in the Garhwal Himalayas. Food Nutr Bull 2009; 30:77–81. [DOI] [PubMed] [Google Scholar]
- 22.Dang S, Yan H, Yamamoto S, et al. Poor nutritional status of younger Tibetan children living at high altitudes. Eur J Clin Nutr 2004; 58:938–946. [DOI] [PubMed] [Google Scholar]
- 23.Weitz CA, Garruto RM. Growth of Han migrants at high altitude in central Asia. Am J Hum Biol 2004; 16:405–419. [DOI] [PubMed] [Google Scholar]
- 24.Weitz CA, Garruto RM, Chin CT, et al. Growth of Qinghai Tibetans living at three different high altitudes. Am J Phys Anthropol 2000; 111:69–88. [DOI] [PubMed] [Google Scholar]
- 25.Weitz CA, Garruto RM, Chin CT, et al. Morphological growth of Han boys and girls born and raised near sea level and at high altitude in western China. Am J Hum Biol 2000; 12:665–681. [DOI] [PubMed] [Google Scholar]
- 26.Leonard WR. Nutritional determinants of high-altitude growth in Nunoa, Peru. Am J Phys Anthropol 1989; 80:341–352. [DOI] [PubMed] [Google Scholar]
- 27.Leonard WR, DeWalt KM, Stansbury JP, et al. Growth differences between children of highland and coastal Ecuador. Am J Phys Anthropol 1995; 98:47–57. [DOI] [PubMed] [Google Scholar]
- 28.Argnani L, Cogo A, Gualdi-Russo E. Growth and nutritional status of Tibetan children at high altitude. Coll Antropol 2008; 32:807–812. [PubMed] [Google Scholar]
- 29.Moore LG. Human genetic adaptation to high altitude. High Alt Med Biol 2001; 2:257–279. [DOI] [PubMed] [Google Scholar]
- 30.Yip R. Altitude and birth weight. J Pediatr 1987; 111:869–876. [DOI] [PubMed] [Google Scholar]
- 31.Yip R, Binkin NJ, Trowbridge FL. Altitude and childhood growth. J Pediatr 1988; 113:486–489. [DOI] [PubMed] [Google Scholar]
- 32.Harris NS, Crawford PB, Yangzom Y, et al. Nutritional and health status of Tibetan children living at high altitudes. N Engl J Med 2001; 344:341–347. [DOI] [PubMed] [Google Scholar]
- 33.Frisancho AR, Borkan GA, Klayman JE. Pattern of growth of lowland and highland Peruvian Quechua of similar genetic composition. Hum Biol 1975; 47:233–243. [PubMed] [Google Scholar]
- 34.Pawson IG, Huicho L. Persistence of growth stunting in a Peruvian high altitude community, 1964–1999. Am J Hum Biol 2010; 22:367–374. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YBWY, Wu TY. High Altitude Diseases. 1985; Xining: Qinghai People's Publishing House, 67–80. [Google Scholar]
- 36.Bozzini CE, Lezon CE, Norese MF, et al. Evidence from catch-up growth and hoarding behavior of rats that exposure to hypobaric air lowers the body-mass set point. Growth Dev Aging 2005; 69:81–88. [PubMed] [Google Scholar]
- 37.Monge C, Leon-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev 1991; 71:1135–1172. [DOI] [PubMed] [Google Scholar]
- 38.Mortola JP. Hypoxic metabolism in mammals. News Physiol Sci 1993; 8:79–82. [Google Scholar]
- 39.Beall CM, Cavalleri GL, Deng L, et al. Natural selection on EPAS1 (HIF2a) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci 2003; 107:11459–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bigham ABM, Pinto D, Mao X, et al. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet 2010; 6:e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng Y, Yang Z, Zhang H, et al. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol 2011; 28:1075–1081. [DOI] [PubMed] [Google Scholar]
- 42.Simonson TS, Yang Y, Huff CD, et al. Genetic evidence for high-altitude adaptation in Tibet. Science 2010; 329:72–75. [DOI] [PubMed] [Google Scholar]
- 43.Wang BZY-B, Zhang H, Lin F, et al. On the origin of Tibetans and their genetic basis in adapting high-altitude environments. PLoS One 2011; 6:e17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang K, Ouzhuluobu, Peng Y, et al. Identification of a Tibetan-specific mutation in the hypoxic gene EGLN1 and its contribution to high-altitude adaptation. Mol Biol Evol 2013; 30:1889–1898. [DOI] [PubMed] [Google Scholar]
- 45.Xu SLS, Yang Y, Tan J, et al. A genome-wide search for signals of high altitude adaptation in Tibetans. Mol Biol Evol 2011; 28:1003–1011. [DOI] [PubMed] [Google Scholar]
- 46.Yi X, Liang Y, Huerta-Sanchez E, et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 2010; 329:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


