Abstract
The aim of this study was to analyze the correlation between the first diagnosis of high-grade Vaginal Intraepithelial Neoplasia (HG-VaIN: VaIN 2–VaIN 3) and the cytological abnormalities on the referral pap smear.
All the women with histological diagnosis of HG-VaIN consecutively referred to the Gynecological Oncology Unit of the Aviano National Cancer Institute (Aviano, Italy) from January 1991 to April 2014 and with a pap smear performed in the 3 months before the diagnosis were considered, and an observational cohort study was performed.
A total of 87 women with diagnosis of HG-VaIN were identified. Major cytological abnormalities (HSIL and ASC-H) on the referral pap smear were significantly more frequent than lesser abnormalities (ASC-US and LSIL) in postmenopausal women (64.9% vs 36.7%, P = 0.02) and in women with a previous diagnosis of HPV-related cervical preinvasive or invasive lesions (70.5% vs 39.5%, P = 0.01). Diagnosis of VaIN 3 was preceded by major cytological abnormalities in most of the cases (72.7% vs 27.3%, P < 0.001).
The diagnosis of HG-VaIN can be preceded by different abnormalities on referral pap smear. Major abnormalities are usually reported in postmenopausal women and in women with previous cervical HPV-related disease. However, ASC-US or LSIL do not exclude HG-VaIN, especially VaIN2. An accurate examination of the whole vaginal walls (or vaginal vault) must be performed in all the women who underwent colposcopy for an abnormal pap smear, and a biopsy of all suspicious areas is mandatory.
INTRODUCTION
Vaginal intraepithelial neoplasia (VaIN) is a rare human papillomavirus (HPV)-related premalignant condition, histologically diagnosed, characterized by dysplastic changes in the vaginal epithelium, without stromal invasion.1 It accounts for only 0.4% of female lower genital tract intraepithelial lesions,2,3 with an incidence from 0.2 to 2 per 100,000 women/year.4 Therefore, its natural history is not well known.
The persistent high-risk HPV infection is considered the necessary condition for the development of VaIN.1,5 Multiple sexual partners and early stage at sexual debut,6 smoking,5,7 and immunosuppression,8 increasing the likelihood of HPV infection, are described as further risk factors involved in the development of vaginal dysplasia. Furthermore, the presence of previous or synchronous cervical dysplastic lesions,9 or a previous hysterectomy for HPV-related cervical invasive or preinvasive lesions,10 are believed to be associated to the development of VaIN.
Although the risk factors for VaIN are similar to those described for cervical intraepithelial neoplasia (CIN), its incidence appear to be 100-fold lower;10 this is believed to be due to the lack of a vulnerable squamocolumnar junction in the vagina.11 Additionally, it is speculated that HPV infection of the vagina occurs as frequently as in the cervix, but a lytic cell reaction in the vaginal epithelium enables the regression of lesions, in contrast to the characteristic latent infection in the cervix which causes persistent dysplasia.12,13
VaIN is typically diagnosed through a colposcopy-guided biopsy of suspicious areas after an abnormal referring pap smear, and the number of diagnosis has increased steadily over the recent years because of the widespread use of routine screening cytology tests.12
Most of the authors classified VaIN into low-grade lesions (mild dysplasia, VaIN 1) and high-grade lesions (high-grade vaginal intraepithelial neoplasia [HG-VaIN]: VAIN 2 and VaIN3, corresponding to moderate and severe dysplasia) according to the depth of tissue involved.7,9,11,13–15
To our knowledge, extensive data on cervical or vaginal cytology leading to the diagnosis of HG-VaIN are not available in the literature and only 1 study analyzed the abnormal referring cytology as a potential risk factor for the development of VaIN, though involving also low-grade VaIN.13 In this study, we restricted the analysis only on HG-VaIN, since these lesions can be assumed as the true precursor of vaginal cancer.13,16 The aim was to analyze the correlation between the first diagnosis of HG-VaIN and previous cytological abnormalities on the referral pap smear.
METHODS
All the women with histological diagnosis of VaIN 2 and VaIN 3 consecutively referred to the Gynecological Oncology Unit of the Aviano National Cancer Institute (Aviano, Italy) from January 1991 to April 2014 were considered. Only women with a cytological assessment on pap smear performed in the 3 months before the diagnosis were included in the analysis. Women with synchronous cervical intraepithelial lesions or cervical invasive cancer were excluded. Patients were identified by searching our clinical databases, and the medical records of women fulfilling the study inclusion criteria were retrospectively analyzed in an observational cohort study. Data obtained included information regarding pertinent medical and surgical history and sociodemographic characteristics of each woman.
Cytological abnormalities were classified accordingly to the Bethesda system terminology.17
High-grade squamous intraepithelial lesions (HSIL) and atypical squamous cells, cannot rule out high-grade SIL (ASC-H) were considered “major cytological abnormalities.” Atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesions (LSIL) were considered “lesser abnormalities.”
The diagnosis of HG-VaIN was made through biopsy of suspicious areas detected on colposcopic examination. Similarly, any cervical suspicious areas were biopsied to exclude the presence of synchronous preinvasive or invasive lesions of the uterine cervix. All the women considered underwent colposcopy because of an abnormal pap smear.
Colposcopic examinations were performed and recorded accordingly to the 2011 revised colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy.18 The colposcopies performed before the introduction of the 2011 International Federation for Cervical Pathology and Colposcopy terminology were revised accordingly.
Statistical analysis was performed using IBM SPSS version 22.0. The χ2 testing and Fisher exact test were used, as appropriate, to evaluate associations. Probability <0.05 was considered statistically significant.
Institutional Internal Review Board approval (CRO IRB n. 17/2013) was obtained.
RESULTS
From January 1991 to April 2014, 153 women referred to the Gynecological Oncology Unit of the Aviano National Cancer Institute (Aviano, Italy) were diagnosed with HG-VaIN for the first time. The mean age of these women was 45.2-years old (SD ± 13.9, range 18–78 years). Among them, 50 women showed synchronous cervical intraepithelial lesions histologically assessed or cervical invasive cancer and were excluded. Similarly 16 women were occasionally diagnosed with HG-VaIN, without a referral pap smear and were not included in the analysis. Thus, the study cohort included 87 women with diagnosis of HG-VaIN.
The mean age in the study cohort was 54.2 years (SD ± 12.8, range 31–78 years) and, in particular, 57 women (65.5%) were in postmenopausal status. Tobacco use was reported in 21 women (24.1%) and HIV infection was present in 2 cases (2.3%). Previous diagnosis of HPV-related cervical disease (CIN, carcinoma in situ or invasive cancer) was reported in 44 cases (50.6%). Forty-four women (50.6%) previously underwent hysterectomy; in particular, hysterectomy was performed because of CIN or invasive cervical cancer in 31 cases, endometrial cancer in 5 cases, and ovarian cancer in 1 case. Hysterectomy for benign conditions was performed in 7 cases. Ten women in the study cohort underwent radiation therapy after surgery for uterine malignancies.
Clinical characteristics of the study population regarding the referral pap smear results, the abnormal colposcopic findings and the grade of vaginal lesions detected, are reported in Table 1.
TABLE 1.
Clinical, Colposcopic, and Histological Characteristics of the Study Group (n = 87)
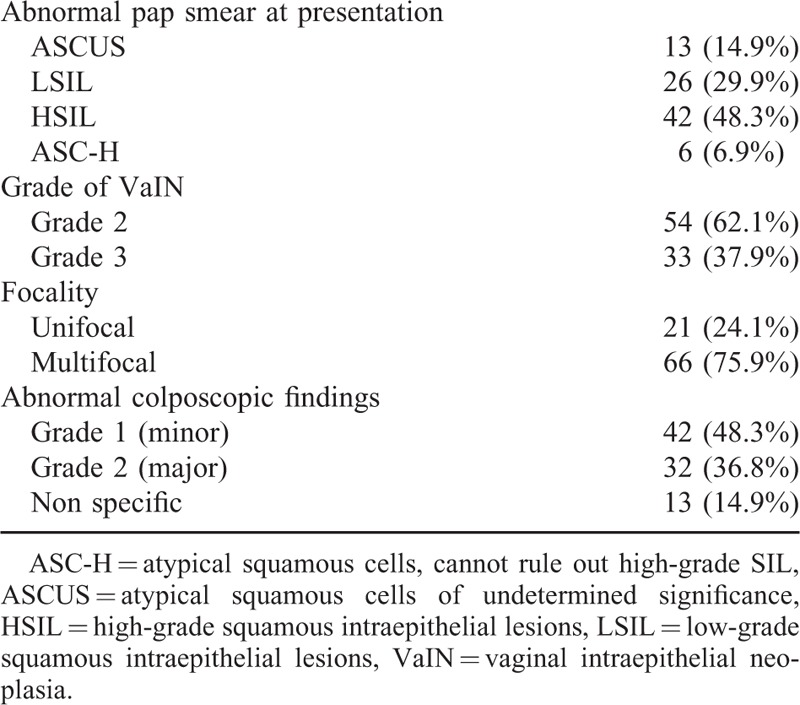
Major cytological abnormalities, as previously defined, were reported in 48 cases (55.2%); the remaining 39 women were diagnosed with lesser cytological abnormalities. No case of atypical glandular cells on pap smear was reported.
The referral pap smear results in correlation with the subsequent histological findings of VaIN are reported in Table 2.
TABLE 2.
Cytological Correlation to Histological Findings in the Study Population
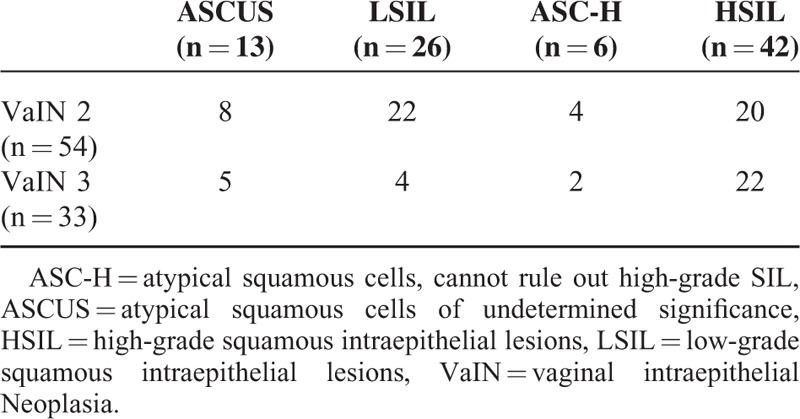
Among women diagnosed with HG-VaIN, the most frequent cytological abnormality on the previous pap smear was HSIL (42 cases, 48.3%). However, the proportion of previous major cytological abnormalities and lesser abnormalities on the referral pap smear was similar (55.2% vs 44.8%, P = 0.2).
In the whole cohort, 33 cases of VaIN 3 were found, among them, the proportion of women previously diagnosed with major cytological abnormalities was significantly higher than those previously diagnosed with lesser abnormalities (72.7% vs 27.3%, P < 0.001). Conversely, in the 54 cases of women diagnosed with VaIN 2, the proportion of women previously diagnosed with major cytological abnormalities and with lesser abnormalities was similar (44.4% vs 55.6%, P = 0.3).
The clinical characteristics of the study cohort in correlation with the referring cytological abnormalities are reported in Table 3.
TABLE 3.
Clinical Characteristics of the Study Cohort in Correlation With the Referring Cytological Abnormalities
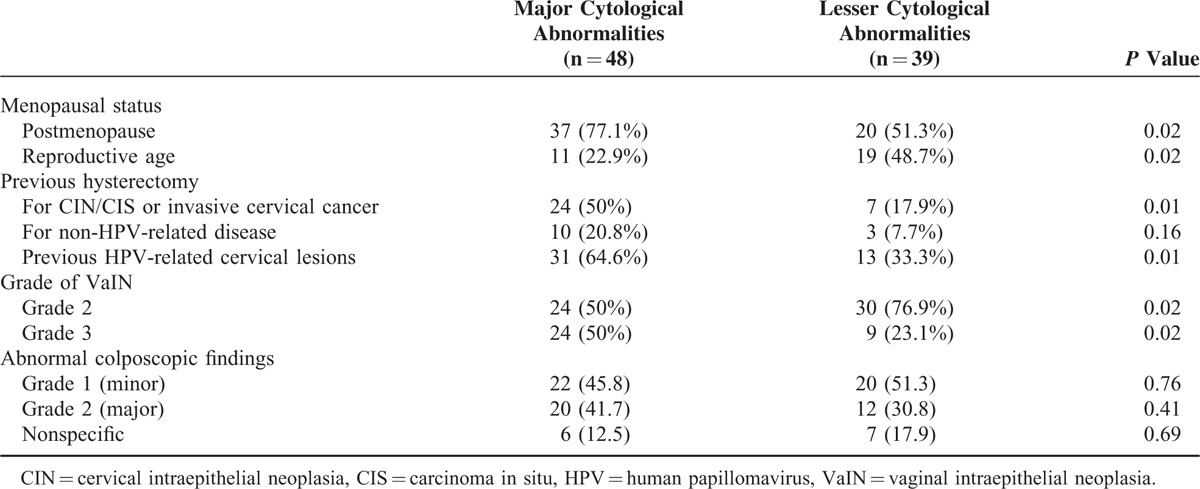
In postmenopausal women (n = 57), referral pap smear results of major abnormalities were detected in 37 cases (64.9%), with lesser abnormalities in the remaining 20 cases (35.1%). The rate of major abnormalities was significantly higher in these women, compared to premenopausal women (64.9% vs 36.7%, P = 0.02).
Similarly, referral pap smear results of major cytological abnormalities were significantly more frequent in women previously treated with hysterectomy (70.8% vs 32.5%, P < 0.001). In particular, this difference was confirmed in the 31 women treated with hysterectomy for premalignant or malignant HPV-related cervical lesions compared to the remaining women (77.4% vs 42.8%, P = 0.01).
Considering only women treated with hysterectomy for non-HPV-related malignancies (endometrial or ovarian cancer) or benign conditions (n = 13) the difference in referral pap smear results of major and lesser cytological abnormalities were not significant (20.8% vs 7.7%, P = 0.16).
In the 44 women with previous HPV-related cervical lesions (CIN or invasive cervical cancer), the rate of major abnormalities on referring pap smear was significantly higher (70.5% vs 39.5%, P = 0.01).
DISCUSSION
HG-VaIN is a very rare and asymptomatic premalignant condition, diagnosed through colposcopy-guided biopsy after an abnormal pap test result. The true prevalence of this condition is actually unknown but, in the last decades, its incidence is increased because of the widespread use of routine screening cytology tests.19
The correlation between abnormal pap smear and CIN has been investigated to an extent in the literature, but extensive data on cytological abnormalities leading to the diagnosis of HG-VaIN are lacking. To our knowledge, only 1 study analyzed the correlation between VaIN and the referring cytological abnormalities detected on pap smear, reporting a high-grade referral cytology in 89% of women diagnosed with VaIN 2-3.13
In our cohort, considering all the women diagnosed with HG-VaIN, the proportion of major abnormalities and lesser abnormalities on the referral pap smear was similar.
The most frequent cytological abnormality on the referral pap smear was HSIL, but in 44.8% of the cases, the diagnosis of HG-VaIN was preceded by lesser cytological abnormalities. This datum appear to be of particular interest, mainly when compared to data regarding the correlation between CIN and previous cytological abnormalities on referral pap smear: a high grade cervical dysplasia is preceded by major cytological abnormalities in most of the cases.20,21
Obviously, the detection of an abnormal pap smear requires a subsequent colposcopy with an accurate examination of the entire lower genital tract. An accurate examination of the whole vaginal walls and vault must be performed, and a biopsy of all suspicious areas is mandatory, even when colposcopy is preceded by lesser cytological abnormalities.
Postmenopausal status and previous HPV-related cervical invasive or preinvasive lesions are well known risk factors for the development of HG-VaIN.10,22 This correlation could be probably linked to the causal role of HPV infection. The persistent high-risk HPV infection is considered the necessary condition for the development of HG-VaIN.1,5 Therefore, women with a history of HPV-related disease of the lower genital tract are more likely to develop vaginal lesions. The potential progression of these lesions could determine severe dysplastic lesions of the vaginal epithelium leading to major cytological abnormalities on referral pap smear.
A previous hysterectomy for HPV-related cervical invasive or preinvasive lesions is believed to be another important risk factor for the development of VaIN.
Few studies, regarding occasional detection of VaIN during the usual cytological follow-up in women who underwent hysterectomy for cervical malignancies, were performed.23,24 Kalogirou et al23 conducted a study to determine the number of patients with CIN3 of the uterine cervix, treated with hysterectomy, who subsequently developed VaIN. The authors tried to define whether the following development of VaIN could justify intensive vaginal cytological and colposcopic follow-up. In the entire study population of 993 hysterectomized women, 41 (4.1%) developed VaIN, and the diagnosis was preceded by the detection of ASCUS pap smear in 42% of cases. However in this study, the authors considered both low grade and HG-VaIN together. Coughlan et al24 evaluated the effectiveness of vaginal cytology following hysterectomy for cervical cancer. They considered 123 women with cervical cancer treated with primary hysterectomy and, among them, 12 women (9.7%) developed cytological abnormalities on the routine follow pap smears. Mild dyskaryosis (corresponding to LSIL according to 2001 Bethesda system classification) was reported in 7 cases and severe dyskaryosis (corresponding to HSIL according to 2001 Bethesda system classification) in the remaining 5 cases. Only 6 women had positive colposcopic findings, with subsequent diagnosis of HG-VaIN in 4 cases and invasive vaginal carcinoma in the remaining 2 cases. However also in this case, the authors did not correlate the grade of vaginal dysplasia with the previous cytological abnormalities.
The role of routine follow-up with pap smear in women previously treated with hysterectomy for benign disease is currently on debate. Previous studies reported a 0 to 0.46% of positive vault cytology,25,26 thus some authors assert that there is insufficient evidence to recommend routine vaginal smear screening in women after total hysterectomy for benign disease.27 The American Society for Colposcopy and Cervical Pathology28 and the American College of Obstetricians and Gynecologists29 do not recommend routine vaginal smear screening in women who have had a hysterectomy and who do not have a history of CIN 2, CIN 3, or cervical cancer.
In our cohort, 13 women (14.9 %) were diagnosed with HG-VaIN after a hysterectomy performed for non-HPV-related malignancies (endometrial cancer or ovarian cancer) or for benign conditions. However, 12 of them had a history of HPV-related cervical lesions conservatively treated before the hysterectomy. The only 1-remaining case could have had an undiagnosed HPV-related disease at the time of hysterectomy or could have acquired an HPV infection after the hysterectomy. This data remark the importance of the cytological follow-up in women after hysterectomy for benign disease, when risk factors for VaIN are identified. Thus is the history of HPV-related disease of the lower genital tract, rather than hysterectomy itself, that has to be considered as a risk factor for development of HG-VaIN.
Among HG-VaIN, VaIN 3 is currently considered as the true precursor of vaginal cancer by some authors,14 since a significant rate of occult superficially invasive vaginal cancer is reported in histological specimens of women excised for this condition. For instance, Hoffman et al30 considering only women excised for VaIN 3, reported a 28% of occult invasive vaginal cancers, while the rate appear to be lower when VaIN 2 and VaIN3 are considered together (12% in a study by Indermaur et al31).
In our cohort, considering only women with histological diagnosis of VaIN 3, we detected major cytological abnormalities on the referral pap smear in most of the cases. However, ASCUS or LSIL on referral pap smear cannot exclude HG-VaIN, especially VaIN 2. This findings remark the importance of an accurate colposcopic examination, involving also the vaginal walls, in all the women with any abnormality on pap smear, mostly when other risk factors (such as postmenopausal status or history of HPV-related lesions of the lower genital tract) are detected.
In this study, we reported 87 cases of women with Hg-VaIN, that is, a relatively large number, considering the low rate of this condition in the general population.4
Moreover, all the colposcopies and vaginal biopsies and the subsequent histological evaluation were performed in a single institution, by the same gynecologists and by the group of pathologists of Aviano National Cancer Institute, with particular expertise in gynecologic–oncologic disease.
However, due to its retrospective nature, this study has a potential limitations, since the data collected are limited to those already reported in the medical charts.
The data of this study could be useful for clinicians to provide an appropriate counseling to women with abnormal pap test. These results stress the importance of an accurate colposcopic examination in women with abnormal pap smear, not only to identify and treat premalignant lesions of the uterine cervix, but also to identify and treat high-grade dysplastic lesions of vagina, preventing the development of invasive cancers. Colposcopic examination of vagina may be technically difficult, mainly in women after hysterectomy, since the lesions can develop deep in the vaginal cuff and could be not easily observed. Thus, colposcopy should be performed by qualified gynecologists with particular expertise in the lower genital tract premalignant diseases.
Footnotes
Abbreviations: ASCUS = atypical squamous cells of undetermined significance, CIN = cervical intraepithelial neoplasia, HG-VaIN = high-grade vaginal intraepithelial neoplasia, HPV = human papillomavirus, HSIL = high-grade squamous intraepithelial lesions, LSIL = low-grade squamous intraepithelial lesions, VaIN = vaginal intraepithelial neoplasia.
Study concept and design: FS, AC; acquisition of data: GDP, FM, MB; analysis and interpretation of data: FS, NC, GG, AC; drafting of the manuscript: FS, NC; critical revision of the manuscript for important intellectual content: FS, GG, AC; and statistical analysis: FS, NC.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Smith JS, Backes DM, Hoots BE, et al. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet Gynecol 2009; 113:917–924. [DOI] [PubMed] [Google Scholar]
- 2.Cardosi RJ, Bomalaski JJ, Hoffman MS. Diagnosis and management of vulvar and vaginal intraepithelial neoplasia. Obstet Gynecol Clin North Am 2001; 28:685–702. [DOI] [PubMed] [Google Scholar]
- 3.Gurumurthy M, Cruickshank ME. Management of vaginal intraepithelial neoplasia. J Low Genit Tract Dis 2012; 16:306–312. [DOI] [PubMed] [Google Scholar]
- 4.Duong TH, Flowers LC. Vulvo-vaginal cancers: risks, evaluation, prevention and early detection. Obstet Gynecol Clin North Am 2007; 34:783–802. [DOI] [PubMed] [Google Scholar]
- 5.Madsen BS, Jensen HL, van den Brule AJ, et al. Risk factors for invasive squamous cell carcinoma of the vulva and vagina–population-based case-control study in Denmark. Int J Cancer 2008; 122:2827–2834. [DOI] [PubMed] [Google Scholar]
- 6.Daling JR, Madeleine MM, Schwartz SM, et al. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol Oncol 2002; 84:263–270. [DOI] [PubMed] [Google Scholar]
- 7.Sherman JF, Mount SL, Evans MF, et al. Smoking increases the risk of high-grade vaginal intraepithelial neoplasia in women with oncogenic human papillomavirus. Gynecol Oncol 2008; 110:396–401. [DOI] [PubMed] [Google Scholar]
- 8.Hankins CA, Lamont JA, Handley MA. Cervicovaginal screening in women with HIV infection: a need for increased vigilance? CMAJ 1994; 150:681–686. [PMC free article] [PubMed] [Google Scholar]
- 9.Zeligs KP, Byrd K, Tarney CM, et al. A clinicopathologic study of vaginal intraepithelial neoplasia. Obstet Gynecol 2013; 122:1223–1230. [DOI] [PubMed] [Google Scholar]
- 10.Sillman FH, Fruchter RG, Chen YS, et al. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol 1997; 176:93–99. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Hu D, Xu S, et al. Clinical features, treatment and outcomes of vaginal intraepithelial neoplasia in a Chinese tertiary centre. Ir J Med Sci Nov 2014. [DOI] [PubMed] [Google Scholar]
- 12.Sugase M, Matsukura T. Distinct manifestations of human papillomaviruses in the vagina. Int J Cancer 1997; 72:412–415. [DOI] [PubMed] [Google Scholar]
- 13.Gunderson CC, Nugent EK, Elfrink SH, et al. A contemporary analysis of epidemiology and management of vaginal intraepithelial neoplasia. Am J Obstet Gynecol 2013; 208:410.e1–410.e6. [DOI] [PubMed] [Google Scholar]
- 14.Frega A, Sopracordevole F, Assorgi C, et al. Vaginal intraepithelial neoplasia: a therapeutical dilemma. Anticancer Res 2013; 33:29–38. [PubMed] [Google Scholar]
- 15.Ratnavelu N, Patel A, Fisher AD, et al. High-grade vaginal intraepithelial neoplasia: can we be selective about who we treat? BJOG 2013; 120:887–893. [DOI] [PubMed] [Google Scholar]
- 16.Massad LS. Outcomes after diagnosis of vaginal intraepithelial neoplasia. J Low Genit Tract Dis 2008; 12:16–19. [DOI] [PubMed] [Google Scholar]
- 17.Solomon D, Davey D, Kurman R, et al. Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119. [DOI] [PubMed] [Google Scholar]
- 18.Bornstein J, Bentley J, Bösze P, et al. 2011 Colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet Gynecol 2012; 120:166–172. [DOI] [PubMed] [Google Scholar]
- 19.Wee WW, Chia YN, Yam PK. Diagnosis and treatment of vaginal intraepithelial neoplasia. Int J Gynaecol Obstet 2012; 117:15–17. [DOI] [PubMed] [Google Scholar]
- 20.Ingkapairoj N, Luanratanakorn S, Chumworathayi B, et al. Incidences of cervical intraepithelial neoplasia 2-3 or cancer pathologic diagnoses in patients with a high grade squamous intraepithelial lesion pap smear attending a colposcopy clinic at srinagarind hospital. Asian Pac J Cancer Prev 2012; 13:6203–6206. [DOI] [PubMed] [Google Scholar]
- 21.Khan KA, Smith DA, Thrall MJ. Only a small fraction of high-grade cervical lesions are discovered after an interpretation of atypical squamous cells of undetermined significance when using imager-assisted, liquid-based papanicolaou tests and the Bethesda 2001 system. Arch Pathol Lab Med 2013; 137:936–941. [DOI] [PubMed] [Google Scholar]
- 22.Johnston GA, Jr, Klotz J, Boutselis JG. Primary invasive carcinoma of the vagina. Surg Gynecol Obstet 1983; 156:34–40. [PubMed] [Google Scholar]
- 23.Kalogirou D, Antoniou G, Karakitsos P, et al. Vaginal intraepithelial neoplasia (VAIN) following hysterectomy in patients treated for carcinoma in situ of the cervix. Eur J Gynaecol Oncol 1997; 18:188–191. [PubMed] [Google Scholar]
- 24.Coughlan C, McAuliffe F, Bermingham N, et al. Vaginal cytology following primary hysterectomy for cervical cancer: is it useful? Ir J Med Sci 2006; 175:45–49. [DOI] [PubMed] [Google Scholar]
- 25.Mouithys P, Papadopoulos C, Allier G, et al. Is it necessary to make screening pap smears after hysterectomy? Gynecol Obstet Fertil 2003; 31:620–623. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Sodhani P, Singh V, et al. Role of vault cytology in follow-up of hysterectomized women: results and inferences from a low resource setting. Diagn Cytopathol 2013; 41:762–766. [DOI] [PubMed] [Google Scholar]
- 27.Fetters MD, Fischer G, Reed BD. Effectiveness of vaginal Papanicolaou smear screening after total hysterectomy for benign disease. JAMA 1996; 275:940–947. [PubMed] [Google Scholar]
- 28.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis 2012; 16:175–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin Number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman MS, DeCesare SL, Roberts WS, et al. Upper vaginectomy for in situ and occult, superficially invasive carcinoma of the vagina. Am J Obstet Gynecol 1992; 166:30–33. [DOI] [PubMed] [Google Scholar]
- 31.Indermaur MD, Martino MA, Fiorica JV, et al. Upper vaginectomy for the treatment of vaginal intraepithelial neoplasia. Am J Obstet Gynecol 2005; 193:577–580. [DOI] [PubMed] [Google Scholar]


