Abstract
Epidermolysis bullosa (EB) is a rare disorder characterized by inherited skin adhesion defects with abnormal disruption of the epidermal–dermal junction in response to mechanical trauma. Our aim was to investigate a set of cytokine levels in serum samples from patients suffering from epidermolysis bullosa simplex (EBS), dystrophic epidermolysis bullosa (DEB), and healthy controls (HCs), exploring their potential correlations with antiskin autoantibody titers and disease activity. Forty patients afferent to the Dermatological Ward of Bari City Hospital and 9 HCs were enrolled and subdivided according to the dystrophic (DEB) and simplex forms (EBS). We found a significant increase in interleukin (IL)-1β plasmatic levels of DEB (P = 0.0224) and EBS (P = 0.0465) patients compared to HCs; IL-6 levels were significantly higher in DEB than in EBS patients (P = 0.0004) or HCs (P = 0.0474); IL-2 levels were significantly increased in DEB compared with EBS (P = 0.0428). Plasmatic tumor necrosis factor-β and interferon-γ were higher in DEB patients than in HCs (P = 0.0448 and 0.0229). Conversely, tumor necrosis factor-α was significantly decreased in DEB (P = 0.0034). IL-5 correlated with anti-BP180 (r = −0.5018, P = 0.0338), anti-BP230 (r = −0.6097, P = 0.0122), and anticollagen VII (r = −0.5166, P = 0.0405) autoantibodies; interferon-γ correlated with anti-BP180 (r = 0.9633, P < 0.0001), anti-BP230 (r = 0.9071, P < 0.0001), and anticollagen VII (r = 0.8619, P = 0.0045) autoantibodies. Score of disease severity was significantly correlated with IL-6 (r = 0.6941, P = 0.029) and IL-12 (r = 0.5503, P = 0.0272). The present study supports that EB might be considered a systemic inflammatory disease rather than a skin-limited disorder; clinical disease activity scores could be also integrated by laboratory data such as IL-6 and IL-12 dosage; biotherapies targeting specific cytokine networks probably represent a way to go in the future.
INTRODUCTION
Epidermolysis bullosa (EB) represents a rare group of severe inherited defects known as genodermatoses, and is characterized by abnormalities in skin adhesion. In particular, clinical phenotype is distinguished by an abnormal skin and mucosa fragility due to the disruption of epidermal–dermal junction in response to mechanical trauma, resulting in mutilating scars, local and systemic infections, syndactyly, and esophageal strictures.1 According to a recently introduced “onion skinning” approach to classification, EB can be distinguished taking into consideration the account type, the mode of inheritance, phenotype, immunofluorescence antigen-mapping findings, and mutations.2 Nevertheless, the authors did not change the previously used names for the major EB types to maintain continuity with past scientific literature and to prevent any confusion. In particular, EB types are identified according to the level in which blisters develop and also include the simplex form (EBS), with mechanical fragility and blistering confined to the epidermis; the junctional EB (JEB) consisting of all subtypes with blisters arising within the lamina lucida of the dermal–epidermal basement membrane; and the dystrophic form (DEB), including subtypes with blisters occurring within the uppermost dermis.
The simplex form derives mainly from mutations of K5 and K14 genes encoding for type I and II keratin intermediate filaments,3 whereas DEB derives from mutations in the COL7A1 gene encoding for type VII collagen—the major component of anchoring fibrils at the dermal–epidermal junction.4 However, genotype–phenotype correlations are not always found and several different phenotypes are reported for DEB regardless of the genetic background. In this context, the Birmingham Epidermolysis Bullosa Severity (BEBS) score is currently employed to assess disease activity.5
Antitype VII collagen and other antiskin autoantibodies (anti-BP180, anti-BP230) can be detected. Although their pathogenic role is unclear, their levels have proved to be significantly correlated with the BEBS score, thus suggesting that the phenotype severity may rely on autoimmune mechanisms.6,7 The potential occurrence of AA-amyloidosis and organ involvement,8–10 along with previous reported data on cytokine serum level imbalances,11–13 suggests that moderate to severe forms of EB should be regarded as systemic inflammatory disorders rather than local skin diseases.
Therefore, we investigated the levels of a core set of cytokines in serum samples from EBS and DEB patients, and healthy controls (HCs), exploring their potential correlations with antiskin autoantibody titers and disease activity.
MATERIALS AND METHODS
Patients
We collected serum samples from a cohort of 40 EB patients (15 men, 25 women) consecutively seen at the Dermatological Ward of Bari City Hospital and diagnosed by clinical assessment and immunofluorescence antigen mapping (IFM) and/or transmission electron microscopy. According to the cross-sectional design of the study, serum samples were collected once during routine follow-up visits. Patients were divided into 2 groups according to skin cleavage: intraepidermal blistering diseases (EBS) (15 patients, 7 men and 8 women, mean age 16 ± 7.3 years), and subepidermal blistering diseases (DEB) (25 patients, 8 men and 17 women, mean age 33 ± 6.3 years). Samples were also obtained from 9 HCs (1 man, 8 women, mean age 46.77 ± 5.23 years).
The primary aim of the study was to compare cytokine profiles of EBS patients, DEB patients, and HCs; the secondary aim was to investigate any potential correlation among cytokines levels, autoantibody titers, and clinical features. The BEBS score5 was used to perform clinical assessment of disease severity. Written informed consent was obtained both from patients and HCs. The study protocol was reviewed and approved by the Ethical Committee of the Medical University of Bari.
Cytokine Measurement
Serum cytokine levels of interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, tumor necrosis factor (TNF)-α, TNF-β, and interferon (IFN)-γ were measured using a human instant ELISA assay (e BIOSCIENCE, San Diego, CA), according to the manufacturer's instructions. Patients and HCs sera were added to each well of a microwell plate. Sera were incubated for 3 hours at room temperature (RT). After washing to remove any unbound proteins, tetramethylbenzidine (TMB) substrate was added and incubated for 10 minutes at RT. The acid solution was then added to each well to terminate the enzyme reaction and stabilize the color development. The value in each sample was obtained by comparing the optical density (OD) of the sample with the OD of the calibrator.
The detection of circulating antiskin autoantibodies was performed in all samples with an indirect immunofluorescence (IIF) method (Euroimmun, Lubeck, Germany) and with the ELISA methods (MBL, Nagoya, Japan). Samples were scored positive if a fluorescence reaction was observed at 1:10 sample dilution and autoantibody titers were expressed in units/mL.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 software. Continuous variables are expressed as mean ± standard deviation for normally distributed data. Since data were not homoscedastic, the Kruskal–Wallis test followed by post-hoc Dunn multiple comparison test was used to compare the titers of each cytokine assayed by ELISA methods in patients and in healthy individuals. Correlation analysis was carried out by using the Spearman rank test. P values <0.05 were considered statistically significant.
RESULTS
Serum Interleukin Levels in EB Patients
Cytokine levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, along with TNF-α, TNF-β, and IFN-γ, were analyzed in serum samples from EB patients and 9 HCs. In both EBS and DEB patients, IL-1β serum levels were found significantly increased when compared to HCs (P = 0.0465 and 0.0224, respectively), whereas IL-6 levels were significantly higher in DEB than in EBS patients or HCs (P = 0.0004 and 0.0474, respectively). In addition, IL-2 levels were also significantly increased in DEB compared to EBS patients (P = 0.0428), whereas serum concentration of TNF-β (P = 0.0448) and IFN-γ (P = 0.0229) was significantly higher in DEB patients than in HCs. Conversely, TNF-α was significantly decreased in DEB patients (Figure 2C, P = 0.0034) than in HCs. For other cytokines, no difference was detected. Figure 1 graphically shows significant differences in cytokine serum levels between EBS and DEB patients, and HCs.
FIGURE 2.

Main significant correlations identified between cytokine serum levels, autoantibody (anti-BP180, anti-BP230, anticollagen VII) titers, and Birmingham Epidermolysis Bullosa Severity (BEBS) score in overall EB patients enrolled. Data (pg/mL) are expressed as means; r and P values were obtained from Spearman test.
FIGURE 1.
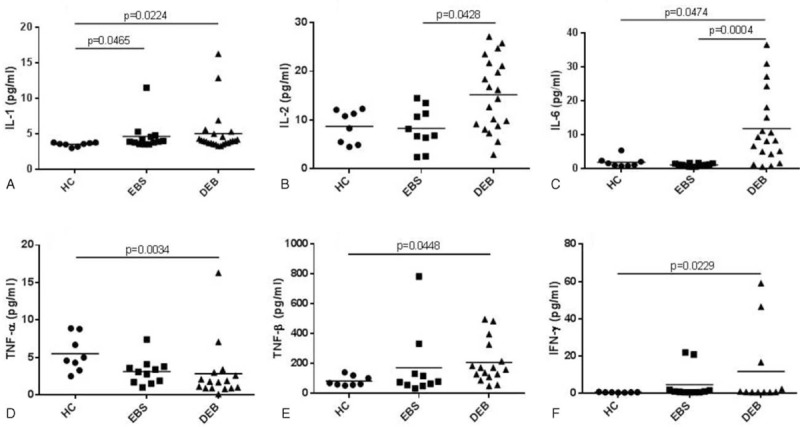
Significant differences in cytokine serum levels between patients with epidermolysis bullosa simplex (EBS), dystrophic epidermolysis bullosa (DEB), and healthy controls (HCs). Data (pg/mL) are expressed as means; since data were not homoscedastic, P values were obtained from Dunn multiple comparison test after Kruskal–Wallis test.
Clinical and Laboratory Correlations
The BEBS score, used to perform clinical assessment of disease severity, showed a positive correlation with IL-6 (r = 0.6941, P = 0.029) and IL-12 (r = 0.5503, P = 0.0272) serum levels. In addition, IL-5 serum levels inversely correlated with anti-BP180 (r = −0.5018, P = 0.0338), anti-BP230 (r = −0.6097, P = 0.0122), and anticollagen VII (r = −0.5166, P = 0.0405) autoantibody titers. Furthermore, IFN-γ levels were directly correlated with anti-BP180 (r = 0.9633, P < 0.0001), anti-BP230 (r = 0.9071, P < 0.0001), and anticollagen VII (r = 0.8619, P = 0.0045) autoantibody titers.
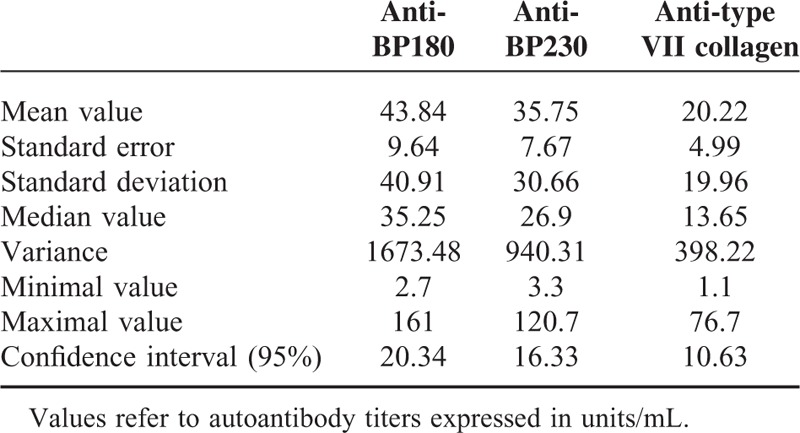
Finally, serum levels of IL-10 were found to be correlated with anticollagen VII (r = 0.48, P = 0.037) and with anti-BP230 titer (r = 0.45, P = 0.05). Table 1 provides more information about autoantibodies detected in the present study. Figure 2 shows the main correlations among cytokine serum levels, autoantibody titers, and BEBS score.
TABLE 1.
Detailed Information on BP180, BP230, and Type II Collagen Autoantibodies
DISCUSSION
Our cross-sectional study was aimed at searching for a peculiar cytokine pattern in EB patients and exploring the potential associations among cytokine profiles, autoantibody serum levels, and disease activity, evaluated by the BEBS score. In particular, we found increased IL-1β serum levels in EBS and DEB patients, whereas IL-2 was higher in DEB than in EBS patients, and IL-6 was higher in DEB patients than in both EBS patients and HCs. In addition, TNF-β and INF-γ levels were higher in patients affected by DEB, whereas TNF-α was significantly lower in such patients. Furthermore, IL-6 and IL-12 were positively correlated with the BEBS activity score, whereas IFN-γ and IL-5 were correlated with skin autoantibodies in a positive and negative manner, respectively.
These results strengthen that systemic involvement is hidden below mucocutaneous manifestations in EB patients. Indeed, extracutaneous features previously reported in patients with a more severe phenotype suggested a potential multisystem involvement in such patients. In particular, IL-1 has been referred to be responsible for abnormal fat metabolism, cardiovascular complications, renal sclerosis, weight loss, and systemic amyloidosis in most severe EB cases.8–10,14–16 In addition, IL-1β signaling was found constitutively activated in keratinocytes from EBS patients, resulting in activation of the Jun Amino-Terminal Kinases stress pathway and overexpression of IL-1β in a positive feedback loop.11 In line with these findings, our results support the role of IL-1β in inducing systemic involvement in EB patients.
According to previously reported data,12 we found high IL-6 serum levels in DEB patients. This cytokine, mainly produced by activated macrophages, monocytes, antigen-presenting cells, and lymphocytes, is capable of promoting Th17 cell and monocyte differentiation, and also inducing the production of acute-phase proteins, thus playing a key role in the switch from innate to acquired immunity.17 Noteworthy, IL-6 upregulation has been previously identified in DEB patients complaining of a more severe phenotype,18 thus supporting our findings showing a correlation of IL-6 (and IL-12) with the BEBS score. Noteworthy, since BEBS score is not adjusted for patients’ age, IL-6 and IL-12 dosage could provide more details on disease severity, especially among children.13 However, since IL-6 levels were found significantly higher in DEB than in EBS patients, the role of IL-6 on disease activity evaluation could be more important in the former group. In addition, IL-6 has previously proved to be significantly upregulated at the mRNA level in skin obtained from K5−/− mouse models.19 Consequently, both the role of IL-6 on pathogenesis and the possible relation with disease activity in EBS patients should be investigated on a larger number of patients.
To date, in vitro production of IL-2 from in vitro peripheral blood mononuclear cells (PBMCs) has been previously reported to be very low in DEB patients,20 and a diminished number of IL-2 receptors were also found in patients with severe forms of EB.21 Noteworthy, in the present study, IL-2 was found to be significantly increased in DEB than in EBS patients, thus making necessary further studies to better understand the role of IL-2 in such patients. In any case, the different IL-2 expression in DEB and EBS patients, and the higher IL-6 levels found in DEB than in EBS patients probably reflect diverse pathogenetic mechanisms supported by the different genetic defects.
Interestingly, we found decreased TNF-α serum levels in DEB patients compared to HCs. According to previous published data, mesenchymal stem cells preconditioning with TNF-α showed to improve transplantation functional utility in recessive DEB.22 In addition, collagen VII gene expression was reported to be increased by TNF-α through TGF-β signaling in HaCaT keratinocytes.23 In this regard, reduced TNF-α levels in EB patients could have a role in inducing a decreased or aberrant collagen VII production.
A significant increase in IFN-γ levels was also found in our DEB patients. In this context, IFN-γ proved to mightily activate the keratin K15 promoter in human epidermal keratinocytes,24 whereas IFN-γ blockers have been successfully proposed as possible pharmacological approach for DEB patients.25 Notably, in our DEB patients, IFN-γ positively correlated with anti-BP180, anti-BP230, and anticollagen VII autoantibody titers, pointing out its role in immunomodulation and regulation of adaptive immune response. Furthermore, collagen VII and BP230 autoantibody titers negatively correlated with IL-5, indicated as promoter of CD4+CD25+ T-regulatory cells that are involved in autoimmunity suppression.26
The altered cytokine levels highlighted in the present study could be the consequence of genetic defects and skin blistering rather than the keystone of etiopathogenetic mechanisms. Nevertheless, although further studies on larger populations are mandatory to better establish the real role for each cytokine, in the present study, IFN-γ and IL-5 were found related to antibody titers, thus suggesting their pathogenetic role in inducing deregulation of adaptive immune system in such patients. However, looking further into the future, our findings encourage the employment of biologic agents targeting specific cytokine networks. This alternative therapeutic approach could be useful to counteract both skin manifestations and systemic long-term complications such as AA-amyloidosis, thus avoiding life-threatening consequences and improving quality of life. Nevertheless, nonhealing blisters may ease infections in EB patients, and patients treated with biologic therapy are at an increased risk of developing infections. For these reasons, anti-IL-1 agents could represent an optimal opportunity because of their lower infectious risk than other biologics.27 Furthermore, wound healing might close potential opened doors to infections.
In conclusion, the imbalance of several cytokines highlighted in the present study confirms that EB is a systemic inflammatory disease rather than a skin-limited disorder. In addition, IL-6 and IL-12 dosage may represent a useful and not invasively laboratory tool, in addition to the currently used severity score aimed at establishing disease activity. Whether or not some of these cytokines may be a potential target of new biotherapies needs further investigations. This might open new avenues in the treatment of a complex disease often leading to life-threatening complications.
Footnotes
Abbreviations: BEBS = Birmingham Epidermolysis Bullosa Severity, DEB = dystrophic epidermolysis bullosa, EB = epidermolysis bullosa, EBS = epidermolysis bullosa simplex, HCs = healthy controls, IFM = immunofluorescence antigen mapping, IFN = interferon, IIF = immunofluorescence, IL = interleukin, JEB = junctional epidermolysis bullosa, OD = optical density, RT = room temperature, TMB = tetramethylbenzidine, TNF = tumor necrosis factor.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Tabolli S, Sampogna F, Di Pietro C, et al. Quality of life in patients with epidermolysis bullosa. Br J Dermatol 2009; 161:869–877. [DOI] [PubMed] [Google Scholar]
- 2.Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol 2008; 58:931–950. [DOI] [PubMed] [Google Scholar]
- 3.Bolling MC, Lemmink HH, Jansen GH, et al. Mutations in KRT5 and KRT14 cause epidermolysis bullosa simplex in 75% of the patients. Br J Dermatol 2011; 164:637–644. [DOI] [PubMed] [Google Scholar]
- 4.Christiano AM, Ryynanen M, Uitto J. Dominant dystrophic epidermolysis bullosa: identification of a Gly- > Ser substitution in the triple-helical domain of type VII collagen. Proc Natl Acad Sci USA 1994; 91:3549–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss C, Wong A, Davies P. The Birmingham Epidermolysis Bullosa Severity score: development and validation. Br J Dermatol 2009; 160:1057–1065. [DOI] [PubMed] [Google Scholar]
- 6.Tampoia M, Bonamonte D, Filoni A, et al. Prevalence of specific anti-skin autoantibodies in a cohort of patients with inherited epidermolysis bullosa. Orphanet J Rare Dis 2013; 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito S, Guez S, Manzoni F, et al. Epidermolysis bullosa and the partnership with autoimmunity: what should we assimilate? Immunol Res 2015; 61:63–69. [DOI] [PubMed] [Google Scholar]
- 8.Yamanaka K, Nakanishi T, Saito H, et al. Persistent release of IL-1 s from skin is associated with systemic cardio-vascular disease, emaciation and systemic amyloidosis: the potential of anti-IL-1 therapy for systemic inflammatory diseases. PLoS One 2014; 9:e104479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CC, Isomoto H, Hayashi T. Gastrointestinal amyloidosis secondary to inherited skin disorder. Gastroenterology 2012; 142:e9–e10. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko K, Kakuta M, Ohtomo Y, et al. Renal amyloidosis in recessive dystrophic epidermolysis bullosa. Dermatology 2000; 200:209–212. [DOI] [PubMed] [Google Scholar]
- 11.Wally V, Lettner T, Peking P, et al. The pathogenetic role of IL-1beta in severe epidermolysis bullosa simplex. J Invest Dermatol 2013; 133:1901–1903. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami Y, Oyama N, Ohtsuka M, et al. Increased serum levels of interleukin-6, immunoglobulin and acute phase protein in patients with the severe clinical form of inherited epidermolysis bullosa. J Dermatol 2005; 32:503–505. [DOI] [PubMed] [Google Scholar]
- 13.Annicchiarico G, Morgese MG, Fiore T, et al. HLA typing in epidermolysis bullosa patients: relevancy to gluten sensitivity. J Genet Syndr Gene Ther 2013; 4:182. [Google Scholar]
- 14.Fine JD, Hall M, Weiner M, et al. The risk of cardiomyopathy in inherited epidermolysis bullosa. Br J Dermatol 2008; 159:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine JD, Johnson LB, Weiner M, et al. Inherited epidermolysis bullosa and the risk of death from renal disease: experience of the National Epidermolysis Bullosa Registry. Am J Kidney Dis 2004; 44:651–660. [PubMed] [Google Scholar]
- 16.Annicchiarico G, Morgese MG, Brunetti L, et al. Improvement of renal function in epidermolysis bullosa patients after gluten free diet: two cases. Eur Rev Med Pharmacol Sci 2012; 6:138–141. [PubMed] [Google Scholar]
- 17.Samavedam UK, Kalies K, Scheller J, et al. Recombinant IL-6 treatment protects mice from organ specific autoimmune disease by IL-6 classical signalling-dependent IL-1ra induction. J Autoimmun 2013; 40:74–85. [DOI] [PubMed] [Google Scholar]
- 18.Odorisio T, Di Salvio M, Orecchia A, et al. Monozygotic twins discordant for recessive dystrophic epidermolysis bullosa phenotype highlight the role of TGF-beta signalling in modifying disease severity. Hum Mol Genet 2014; 23:3907–3922. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Chen J, Planko L, et al. Induction of inflammatory cytokines by a keratin mutation and their repression by a small molecule in a mouse model for EBS. J Invest Dermatol 2007; 127:2781–2789. [DOI] [PubMed] [Google Scholar]
- 20.Chopra V, Tyring SK, Johnson L, et al. Patients with severe forms of inherited epidermolysis bullosa exhibit decreased lymphokine and monokine production. J Clin Immunol 1990; 10:321–329. [DOI] [PubMed] [Google Scholar]
- 21.Chopra V, Tyring SK, Johnson L, et al. Peripheral blood mononuclear cell subsets in patients with severe inherited forms of epidermolysis bullosa. Arch Dermatol 1992; 128:201–209. [PubMed] [Google Scholar]
- 22.Perdoni C, McGrath JA, Tolar J. Preconditioning of mesenchymal stem cells for improved transplantation efficacy in recessive dystrophic epidermolysis bullosa. Stem Cell Res Ther 2014; 5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda H, Kon A, Ito N, et al. Keratinocyte-specific modulation of type VII collagen gene expression by pro-inflammatory cytokines (tumor necrosis factor-alpha and interleukin-1beta). Exp Dermatol 2005; 14:289–294. [DOI] [PubMed] [Google Scholar]
- 24.Radoja N, Stojadinovic O, Waseem A, et al. Thyroid hormones and gamma interferon specifically increase K15 keratin gene transcription. Mol Cell Biol 2004; 24:3168–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skurkovich B, Skurkovich S. Autoimmune diseases are connected with disturbances in cytokine synthesis, and therapy with IFN-gamma blockers is their main pathogenetic treatment. Ann N Y Acad Sci 2007; 1109:167–177. [DOI] [PubMed] [Google Scholar]
- 26.Tran GT, Hodgkinson SJ, Carter NM, et al. IL-5 promotes induction of antigen-specific CD4+CD25+ T regulatory cells that suppress autoimmunity. Blood 2012; 119:4441–4450. [DOI] [PubMed] [Google Scholar]
- 27.Cantarini L, Lopalco G, Caso F, et al. Effectiveness and tuberculosis-related safety profile of interleukin-1 blocking agents in the management of Behçet's disease. Autoimmun Rev 2015; 14:1–9. [DOI] [PubMed] [Google Scholar]


