Abstract
Although extremity chronic osteomyelitis is common in China, updated data were still limited regarding its characterizations. The present study aimed to review clinical features of extremity chronic osteomyelitis in Southern China.
A retrospective analysis was conducted in the patients who had sought medical attention from January 2010 to April 2015 for extremity chronic osteomyelitis in Nanfang Hospital in Southern China. Clinical data were collected and analyzed.
A total of 394 patients (307 males and 87 females) were included, giving a gender ratio of 3.53. The median age at first diagnosis was 42 years for all. The most frequent type was traumatic osteomyelitis (262 cases, 66.50%), which was mainly caused by open injury (166 cases, 63.36%) and during a road accident (91 cases, 34.73%). Single-site infection accounted for 81.98% (323 cases), with tibia (126 cases), femur (79 cases), calcaneus (37 cases), and toes (37 cases) as the top sites. The positive rate of intraoperative culture was 70.63% (214/303), 78.97% (169/214) of which was monomicrobial infection. Staphylococcus aureus (59 cases) was the most frequent bacteria for monomicrobial infection, followed by Pseudomonas aeruginosa (29 cases) and Escherichia coli (11 cases). The positive ratios of preoperative serum white blood cell (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin (PCT), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) were 21.63%, 64.92%, 53.27%, 42.25%, 72.82%, and 66.67%, respectively. The most frequently used intravenous antibiotic was cephalosporins. The overall cure rate was 77.74%, with a total amputation rate of 16.75%.
In this representative Chinese cohort, extremity chronic osteomyelitis was mostly caused by open injury and during a road accident, predominated in males and favored the tibia. S. aureus was the most frequent pathogenic organism. Preoperative elevated levels of serum IL-6, TNF-α, and ESR may be helpful diagnostic indicators of the disease. Most patients achieved a favorable clinical efficacy after appropriate treatment.
INTRODUCTION
Chronic osteomyelitis, defined as a long-term infection of the bone marrow, is characterized by low-grade inflammation caused by persistent pathogenic microorganisms with sequestrum and/or fistulous tract.1 Nowadays, it is still one of the most challenging disorders for clinicians due to its long disease course, complex treatment, and a high risk of recurrence.
In addition to the challenging aspects of the disorder, its clinical characteristics are also varied, which may be affected by geographical and time factors. From the perspective of geographical factors, its characterizations differ in different countries. In contrast to the developed countries, the disease is more frequent in developing ones.2–4 This is probably associated with factors such as economic foundation, lifestyle, and quality of medical services. From the time perspective, its clinical pictures have markedly changed in the past few decades.5 It used to be a sequelae of acute hematogenous osteomyelitis,6,7 but has now increasingly resulted from trauma,8–10 orthopedic implant,11,12 and diabetic foot.13,14 In addition, the spectrum of pathogenic organisms accounting for this disease also changed. Organisms besides bacteria can also cause bone infection, such as virus, protozoan, and fungus.15–17 Moreover, with the widespread use of orthopedic implants, biofilm bacteria are becoming frequent in chronic infections.11,18
In recent years, the incidence of extremity chronic osteomyelitis in China is rising at a rapid speed, mainly caused by a sharply growing number of road or industrial accidents.19 This long course disorder is exerting great pressures on its patients, both economically20 and psychologically.21 Additionally, the continuously increasing number of its victims renders it to be a considerable social problem. Therefore, how to solve this intractable health problem poses great challenges for doctors.
To solve this problem, clinicians are required to make early diagnosis and provide appropriate treatment in order to reduce its risk of recurrence, reconstruct limb function, and improve quality of life of the patients. It is also necessary to recognize the clinical characteristics and treatment strategies of the disorder. However, to the best of our knowledge, current studies are still limited characterizing extremity chronic osteomyelitis, especially in Chinese population. Therefore, this study was aimed to review the clinical features and management of extremity chronic osteomyelitis in Chinese patients, including gender, age at incidence, anatomical site, infecting organisms, levels of different serum inflammation markers for diagnosis, and treatment strategies.
MATERIALS AND METHODS
Study Design, Setting, and Data Source
The present study, designed as a retrospective characterization of extremity chronic osteomyelitis, was conducted in Nanfang Hospital, affiliated to Southern Medical University, a tertiary medical center in Guangzhou, Southern China. Patients’ data were collected using the electronic medical records of the hospital. Index term “osteomyelitis” and the time limit from January 1, 2010 to April 30, 2015 were set for search. The records retrieved initially were reviewed for eligibility assessment. Follow-up data were obtained by searching medical records or by telephone. Written consents from the participants were waived due to the retrospective design of the present study. However, their personal information was anonymized and de-identified before analysis. This study was approved by the ethical medical committee of the hospital.
Inclusion and Exclusion Criteria
Chronic osteomyelitis, defined as a persistent infection of bone marrow for more than 10 weeks,11 was diagnosed on the basis of intraoperative histopathological tests, or cultures from at least 2 infection sites with the same organism or a definite sinus tract connecting directly the bone. Eligible were the records of the patients with a diagnosis of extremity chronic osteomyelitis, which contained at least 1 of the following data: gender, age at first diagnosis, infected body side and anatomical site, intraoperative microorganism culture outcome, preoperative serum values of white blood cell (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin (PCT), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and treatment strategies with cure rates. Excluded were records of the patients diagnosed with acute osteomyelitis or chronic osteomyelitis in nonextremity bones (eg, clavicle, scapula, spine, and mandible). In addition, if a patient had multiple medical records (multiple hospitalizations), only his or her relevant records were kept for data collection and analysis.
Statistical Analysis
Statistical analysis was performed using the SPSS 17.0 software (SPSS Inc, Chicago, IL). Descriptive statistics were conducted for all variables. The distribution of data was assessed for normality using the Kolmogorov–Smirnov test. Continuous variables were expressed as the mean ± standard deviation or median with interquartile range (IQR) depending on data distribution. For normally distributed data, Student t test or one-way analysis of variance (ANOVA) was used to compare the differences between 2 independent or more than 2 groups. Otherwise, the Mann–Whitney U test or Kruskal–Wallis H test was used. Dichotomous variables were expressed as percentages and events. Chi-square test was used to compare differences of rates among different groups. Statistically significant difference was defined as P value of <0.05.
RESULTS
A total of 791 medical records were collected initially. Repeated medical records were removed to keep only 1 relevant record for patients who had multiple hospitalizations. After the records were further reviewed, a total of 394 consecutive patients were included finally.
Classification
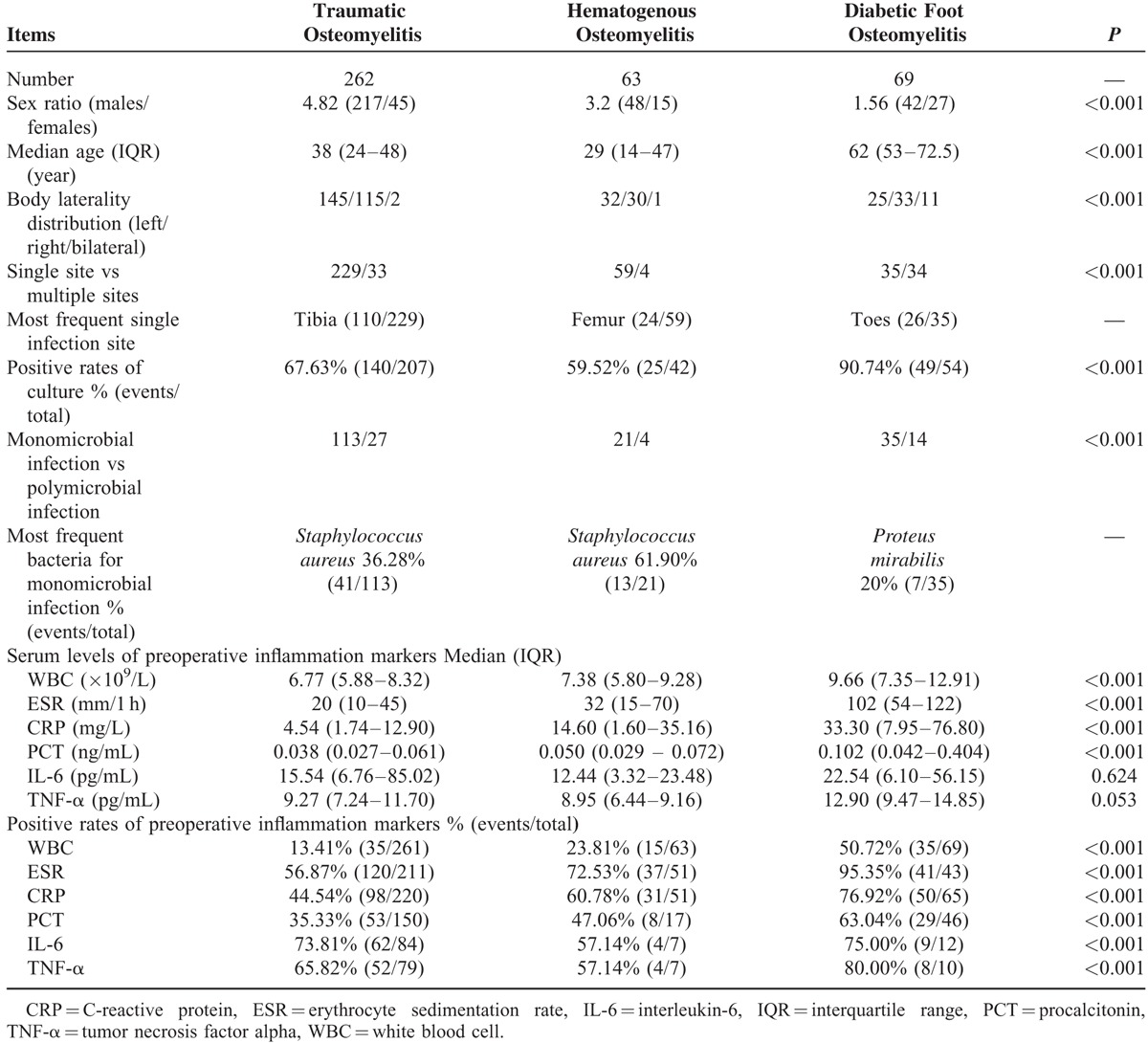
According to the mechanism of infection by classification by Waldvogel et al,22 traumatic osteomyelitis (including iatrogenic factors) was the most frequent type (66.50%, 262 cases). Chronic osteomyelitis secondary to hematogenous spread and diabetic foot accounted for 15.98% (63 cases) and 17.51% (69 cases), respectively (Table 1).
TABLE 1.
Comparisons Among Different Types of Chronic Osteomyelitis by Waldvogel Classification
Injury Features of Traumatic Osteomyelitis
Of the 262 cases of traumatic osteomyelitis, 166 were caused by open injury (63.36%) and 84 by closed injury (32.06%). Detailed injury information was unavailable in 12 cases (4.58%).
As for injury types, road accident lay on the top (91 cases, 34.73%), followed by blunt injury (73 cases, 27.86%), sharp injury (40 cases, 15.27%), and fall injury (23 cases, 8.78%). Such injury information was unavailable in 35 cases (13.36%).
Gender Ratio and Age at First Diagnosis
The present study included 307 males and 87 females, giving a gender ratio of 3.53 for a male predilection. As summarized in Table 1, gender ratios differed statistically among the 3 types of infection (P < 0.001), ranging from 1.56 (diabetic foot osteomyelitis), to 3.20 (hematogenous osteomyelitis) and to 4.82 (traumatic osteomyelitis).
The median age at first diagnosis for all was 42 years (IQR: 24–54 years), with 40 years (IQR: 24–51 years) and 49 years (IQR: 29–63 years) for males and females, respectively (P < 0.001). Approximately 80% of the patients suffered from the disease at 15 to 65 years of age (313 cases). The top 3 age periods were 45 to 50 years, 40 to 45 years, and 50 to 55 years, respectively. Stratified analysis also found a significant age difference among the 3 different types of osteomyelitis (traumatic vs hematogenous vs diabetic = 38 vs 29 vs 62 years, P < 0.001) (Table 1).
Infection Side and Site
The total numbers of infection on the left, right, and bilateral body sides were 202 (51.27%), 178 (45.18%), and 14 (3.55%) cases respectively. Altogether, 323 cases (81.98%) had a single infection site, and 71 (18.02%) had multiple sites that mainly occurred in diabetic foot osteomyelitis (49.28%).
Of the patients with a single-site infection, 292 (90.40%) had a lesion in a lower limb and 31 (9.60%) in an upper limb. The most frequent single infection site was tibia (126 cases, 39.00%), followed by femur (79 cases, 24.46%), calcaneus (37 cases, 11.46%), and toes (37 cases, 11.46%) (Figure 1). In addition, the tibia was the most common site in traumatic osteomyelitis, while the femur and toes were the most common sites in hematogenous and diabetic foot osteomyelitis, respectively.
FIGURE 1.
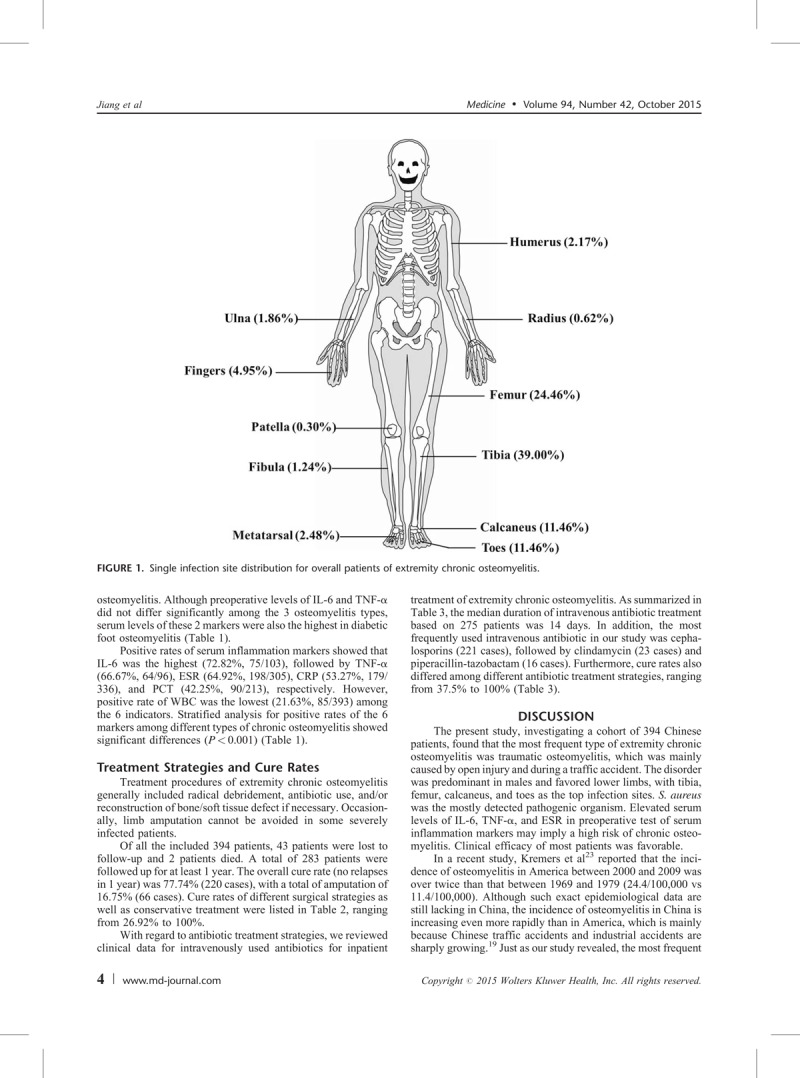
Single infection site distribution for overall patients of extremity chronic osteomyelitis.
Intraoperative Pathogenic Microorganism Culture
Altogether, 303 patients had records of organism cultures in our study. The positive rate of culture for all was 70.63% (214 cases), 78.97% (169 cases) of which were monomicrobial infection. Furthermore, a significant difference was identified regarding the positive rate of culture among the 3 types of osteomyelitis (P < 0.001). Diabetic foot osteomyelitis exhibited the highest rate of culture positivity, followed by traumatic and hematogenous osteomyelitis (Table 1).
The top 3 bacteria accounting for monomicrobial infection for all were Staphylococcus aureus (59 cases, 34.91%), Pseudomonas aeruginosa (29 cases, 17.16%), and Escherichia coli (11 cases, 6.51%). In addition, the most frequently detected bacteria were S. aureus in traumatic and hematogenous osteomyelitis, but Proteus mirabilis in diabetic foot osteomyelitis (Table 1).
Moreover, the bacteria that were detected in more than 5 patients also included Enterococcus faecalis (10 cases, 5.92%), P. mirabilis (9 cases, 5.32%), Acinetobacter baumannii (6 cases, 3.55%), Enterobacter cloacae (5 cases, 2.96%), and Proteus vulgaris (5 cases, 2.96%).
Preoperative Levels and Positive Rates of Serum Inflammation Markers
Cut-off values of serum inflammatory markers provided by Clinical Laboratory were WBC: 9.50 × 109/L, ESR for males: 15 mm/1 h, for females: 20 mm/h, CRP: 5 mg/L, PCT: 0.05 ng/mL, IL-6: 7.0 pg/mL, TNF-α: 8.1 pg/mL.
Comparisons of preoperative values of the 6 markers among the 3 types of osteomyelitis revealed significant differences in WBC, ESR, CRP, and PCT (P < 0.001), demonstrating that preoperative levels of the 4 markers were the highest in diabetic foot osteomyelitis and the lowest in traumatic osteomyelitis. Although preoperative levels of IL-6 and TNF-α did not differ significantly among the 3 osteomyelitis types, serum levels of these 2 markers were also the highest in diabetic foot osteomyelitis (Table 1).
Positive rates of serum inflammation markers showed that IL-6 was the highest (72.82%, 75/103), followed by TNF-α (66.67%, 64/96), ESR (64.92%, 198/305), CRP (53.27%, 179/336), and PCT (42.25%, 90/213), respectively. However, positive rate of WBC was the lowest (21.63%, 85/393) among the 6 indicators. Stratified analysis for positive rates of the 6 markers among different types of chronic osteomyelitis showed significant differences (P < 0.001) (Table 1).
Treatment Strategies and Cure Rates
Treatment procedures of extremity chronic osteomyelitis generally included radical debridement, antibiotic use, and/or reconstruction of bone/soft tissue defect if necessary. Occasionally, limb amputation cannot be avoided in some severely infected patients.
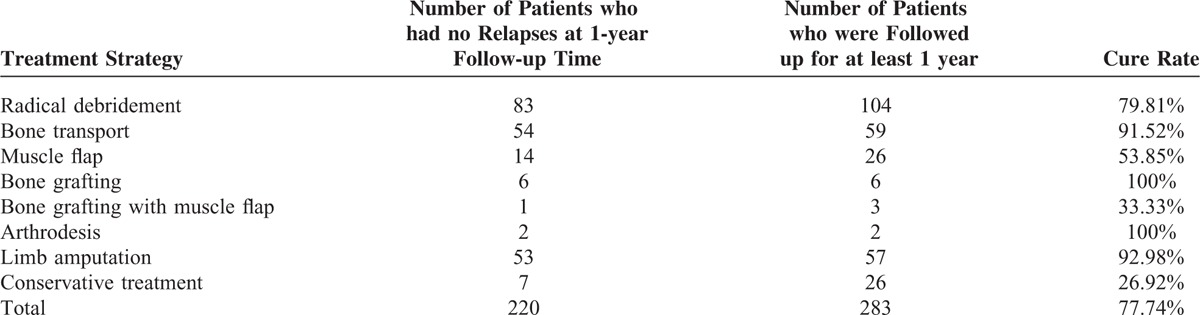
Of all the included 394 patients, 43 patients were lost to follow-up and 2 patients died. A total of 283 patients were followed up for at least 1 year. The overall cure rate (no relapses in 1 year) was 77.74% (220 cases), with a total of amputation of 16.75% (66 cases). Cure rates of different surgical strategies as well as conservative treatment were listed in Table 2, ranging from 26.92% to 100%.
TABLE 2.
Cure Rates of Different Surgical Strategies and Conservative Treatment for Extremity Chronic Osteomyelitis
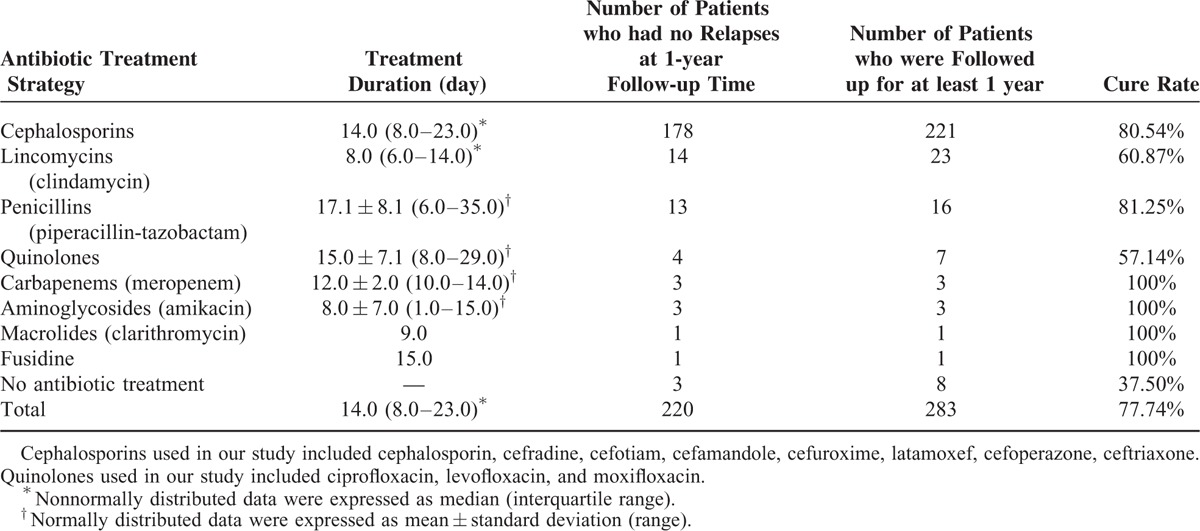
With regard to antibiotic treatment strategies, we reviewed clinical data for intravenously used antibiotics for inpatient treatment of extremity chronic osteomyelitis. As summarized in Table 3, the median duration of intravenous antibiotic treatment based on 275 patients was 14 days. In addition, the most frequently used intravenous antibiotic in our study was cephalosporins (221 cases), followed by clindamycin (23 cases) and piperacillin-tazobactam (16 cases). Furthermore, cure rates also differed among different antibiotic treatment strategies, ranging from 37.5% to 100% (Table 3).
TABLE 3.
Intravenous Antibiotics for Inpatient Treatment of Extremity Chronic Osteomyelitis
DISCUSSION
The present study, investigating a cohort of 394 Chinese patients, found that the most frequent type of extremity chronic osteomyelitis was traumatic osteomyelitis, which was mainly caused by open injury and during a traffic accident. The disorder was predominant in males and favored lower limbs, with tibia, femur, calcaneus, and toes as the top infection sites. S. aureus was the mostly detected pathogenic organism. Elevated serum levels of IL-6, TNF-α, and ESR in preoperative test of serum inflammation markers may imply a high risk of chronic osteomyelitis. Clinical efficacy of most patients was favorable.
In a recent study, Kremers et al23 reported that the incidence of osteomyelitis in America between 2000 and 2009 was over twice than that between 1969 and 1979 (24.4/100,000 vs 11.4/100,000). Although such exact epidemiological data are still lacking in China, the incidence of osteomyelitis in China is increasing even more rapidly than in America, which is mainly because Chinese traffic accidents and industrial accidents are sharply growing.19 Just as our study revealed, the most frequent type of this disease was traumatic osteomyelitis (76.85%) in China, while it was diabetes mellitus associated infection (27%) in America.23 This difference may originate from great variances between the 2 countries, such as lifestyle (eg, dietary habit), safety consciousness, traffic situation, economic status, and quality of medical services.
We found that extremity chronic osteomyelitis favored males, in accordance with the report by Kremers et al in America.23 However, our ratio (3.53) was significantly higher than theirs23 (1.38) (P < 0.001). In consistent with the study by Kremers et al,23 we also found that females tended to have a greater age than males at incidence. But our median ages reported were lower than those reported by Kremers et al,23 not only for all but also for both genders. We consider that the differences may be a consequence of different distributions of osteomyelitis types between the 2 studies.
In the present study, we found that the tibia was the site most susceptible to extremity chronic osteomyelitis as well as to traumatic osteomyelitis in Southern China. This may be associated with its unique anatomical location, bone structure, and limited blood supply. But for hematogenous osteomyelitis, the most affected site was the femur. Consequently, adequate blood supply to the femur may increase the risk of hematogenous osteomyelitis. We found that all the cases of diabetic mellitus associated osteomyelitis occurred at foot. This is probably because such a type of infection is usually a complication of diabetic foot. It is interesting that calcaneal osteomyelitis was also frequent in our study, which can be explained by the following reasons. It is known that calcaneal fracture mostly occurred in a fall, the great violence of which usually results in an open injury. In addition, scanty soft tissues surrounding the calcaneus also increase the risk of bone infection. Moreover, even puncture wound, medical injection, and insect sting are reported24–26 to lead to calcaneal infections.
Our positive rate of intraoperative organism culture was about 71%, close to 75% as reported by Kremers et al23 It is known that the positive rate of culture is affected by several factors, such as culture time and conditions, antibiotic use before culture, and biofilm bacteria.11 It is recommended that patients suspected of chronic osteomyelitis should stop antibiotic use for at least 2 weeks before culture, and at least 3 independent infection sites should be selected for culture. Moreover, the regular culture time is 1 week for all cases. If a negative culture outcome is obtained, the duration should be extended to 14 days and culture conditions may be changed for fungal and acid-fast bacillus. In accordance with previous studies, we found that the mostly detected bacterium was S. aureus. Polymicrobial infection was frequent, especially in diabetic foot osteomyelitis. Furthermore, P. mirabilis and P. aeruginosa were mostly identified in the patients diagnosed with diabetic foot osteomyelitis and calcaneal osteomyelitis, respectively. However, considering that the cases included were limited, we should take cautious attitude toward the above outcomes.
Preoperative levels of serum inflammatory markers may be helpful for diagnosis of extremity chronic osteomyelitis. In the present study, we found that the values and positive rates of inflammatory markers differed among different types of chronic osteomyelitis. In general, patients with diabetic foot osteomyelitis had greater values than those with another 2 types of osteomyelitis, implying that such a type of infection may be more severe. In addition, our positive rates differed among different markers and different groups as well (Table 1). It should be noted that the predictive values should be based on not only a single indicator but also a combination of these indicators. In a recent study, Stucken et al27 reported that the predicted probabilities of infection achieved 100% when associated with positive tests of WBC, ESR, and CRP. They also reported that approximately 20% of the patients still had infections even when all the above 3 indicators of theirs were in normal ranges. Due to the unspecific characteristic of the inflammatory markers, cautious attitude should be taken when using these serum indicators for diagnosis of chronic osteomyelitis or classifying different types of chronic osteomyelitis. In addition, although IL-6 and TNF-α achieved higher positive rates than other markers in patients with chronic osteomyelitis, currently, the 2 serum markers are still not widely used in the routine laboratory parameters for the diagnosis of chronic osteomyelitis.
Treatment of extremity chronic osteomyelitis should be based on comprehensive consideration of systematic and local conditions. The general procedures for management of infection in the long bones consist of radical debridement, systematic and local antibiotic use, and bone/soft tissue defect reconstruction. In our study, the overall cure rate was 77.74%, with a total amputation rate of 16.75%. Cure rates differed with regard to different treatment strategies, ranging from 26.92% to 100%. However, considering the very limited sample size for treatment methods of arthrodesis and bone grafting, we should take prudent attitude toward the outcomes. In our study, 37 cases were treated conservatively, firstly because the health status of some patients did not allow surgical treatment and secondly because some gave up surgical interventions for personal reasons such as financial difficulties. Taken the lowest cure rate of conservative treatment, it is not recommended to select this treatment method if the patients can tolerate surgery.
The selection of antibiotics should be based on culture outcomes and antimicrobial susceptibility results. In our study, the most frequently used intravenous antibiotic was cephalosporins, which is probably associated with broad antibacterial spectrum of these antibiotics. With respect to the duration of antibiotic therapy, several studies28–30 recommended that a total duration of 4 to 6 weeks may be sufficient. The median duration of intravenous antibiotics in the present study was 2 weeks. However, it never meant that all patients could stop antibiotic use after intravenous therapy for 14 days. It depends on systematic and local infection status, surgical strategy and immune status of the patients to decide whether intravenous antibiotic treatment can be stopped or not. For severely infected patients, we recommended an additional oral antibiotic therapy for 2 to 4 weeks. As for the route of antibiotic administration, an updated systematic review31 concluded that similar clinical efficacy can be achieved between oral and parenteral antibiotics for management of osteomyelitis if the bacteria are sensitive to the antibiotic used.
The present study had several limitations. As it was conducted in a single medical center in Southern China, it may not well characterize the extremity chronic osteomyelitis in Southern China. Therefore, multicenter studies should be performed to obtain more accurate information. In addition, although we reported the positive rates of different inflammation markers, the sample sizes for some markers (IL-6, TNF-α) were still far from enough. Further investigations should be conducted on the roles of local levels of the inflammation markers for diagnosis, potential influencing factors of the markers, and optimal combination models of the markers for assisted diagnosis. Moreover, we were unable to provide clinical data for oral antibiotic therapy because most patients received oral antibiotics out of hospital. Such information was unavailable in the electronic medical records in our hospital. The follow-up time of the current study was short and a longer follow-up is necessary to better evaluate the clinical efficacy of different treatment strategies.
In summary, our present study, based on a review of 394 patients in Southern China, suggested that extremity chronic osteomyelitis was mostly caused by open injury and during a road accident, predominated in males and favored lower limbs. S. aureus was the most frequent pathogenic organism. Preoperative elevated levels of serum IL-6, ESR, and TNF-α may be helpful diagnostic indicators of the disease. Despite of varied cure rates for different treatment strategies, the overall clinical efficacy was favorable.
ACKNOWLEDGMENTS
The authors thank Prof. Allen P. Liang for revising this manuscript. The authors are also grateful for funding support by Natural Science Foundation of China (Grant No. 81572165), Guangdong Provincial Natural Science Fund Doctor Startup Project (Grant No. S2012040006946), and China Postdoctoral Science Foundation Funded Project (Grant No. 2015M572340).
Footnotes
Abbreviations: ANOVA = analysis of variance, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, IL-6 = interleukin-6, IQR = Interquartile Range, PCT = procalcitonin, TNF-α = tumor necrosis factor alpha, WBC = white blood cell.
This study was supported by Natural Science Foundation of China (Grant No. 81572165), Guangdong Provincial Natural Science Fund Doctor Startup Project (Grant No. S2012040006946), and China Postdoctoral Science Foundation Funded Project (Grant No. 2015M572340).
Jiang N and Ma YF contributed equally to this study.
The authors certify that they, or any members of their immediate families, have no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, and so on) that might pose a conflict of interest in connection with the submitted article.
REFERENCES
- 1.Prieto-Perez L, Perez-Tanoira R, Petkova-Saiz E, et al. Osteomyelitis: a descriptive study. Clin Orthop Surg 2014; 6:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Museru LM, McHaro CN. Chronic osteomyelitis: a continuing orthopaedic challenge in developing countries. Int Orthop 2001; 25:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am 2004; 86-A:2305–2318. [DOI] [PubMed] [Google Scholar]
- 4.Wirbel R, Hermans K. Surgical treatment of chronic osteomyelitis in children admitted from developing countries. Afr J Paediatr Surg 2014; 11:297–303. [DOI] [PubMed] [Google Scholar]
- 5.Walter G, Kemmerer M, Kappler C, et al. Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int 2012; 109:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islam GTJ, Darton T, Townsend R. Bone and joint infections. Surgery (Oxford) 2013; 31:187–192. [Google Scholar]
- 7.Sheehy SH, Atkins BA, Bejon P, et al. The microbiology of chronic osteomyelitis: prevalence of resistance to common empirical anti-microbial regimens. J Infect 2010; 60:338–343. [DOI] [PubMed] [Google Scholar]
- 8.Hogan A, Heppert VG, Suda AJ. Osteomyelitis. Arch Orthop Trauma Surg 2013; 133:1183–1196. [DOI] [PubMed] [Google Scholar]
- 9.Schenker MLYS, Baldwin KD, Ahn J, et al. Does timing to operative debridement affect infectious complications in open long-bone fractures? A systematic review. J Bone Joint Surg Am 2012; 94:1057–1064. [DOI] [PubMed] [Google Scholar]
- 10.Penn-Barwell JG, Bennett PM, Fries CA, et al. Severe open tibial fractures in combat trauma: management and preliminary outcomes. Bone Joint J 2013; 95-B:101–105. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med 2014; 276:111–119. [DOI] [PubMed] [Google Scholar]
- 12.Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep 2008; 10:394–403. [DOI] [PubMed] [Google Scholar]
- 13.Sagray BA, Malhotra S, Steinberg JS. Current therapies for diabetic foot infections and osteomyelitis. Clin Podiatr Med Surg 2014; 31:57–70. [DOI] [PubMed] [Google Scholar]
- 14.Lesens O, Desbiez F, Vidal M, et al. Culture of per-wound bone specimens: a simplified approach for the medical management of diabetic foot osteomyelitis. Clin Microbiol Infect 2011; 17:285–291. [DOI] [PubMed] [Google Scholar]
- 15.McMahon A. Bone and joint infections. Paediatr Child Health 2011; 21:546–551. [Google Scholar]
- 16.Gabrielli E, Fothergill AW, Brescini L, et al. Osteomyelitis caused by Aspergillus species: a review of 310 reported cases. Clin Microbiol Infect 2014; 20:559–565. [DOI] [PubMed] [Google Scholar]
- 17.Gamaletsou MN, Rammaert B, Bueno MA, et al. Aspergillus osteomyelitis: epidemiology, clinical manifestations, management, and outcome. J Infect 2014; 68:478–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkins M, Hall-Stoodley L, Allan RN, et al. New approaches to the treatment of biofilm-related infections. J Infect 2014; 69 Suppl 1:S47–S52. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Li Y, Wang QK, et al. Status of road safety and injury burden: China. J Orthop Trauma 2014; 28 Suppl 1:S41–S42. [DOI] [PubMed] [Google Scholar]
- 20.Hackett DJ, Rothenberg AC, Chen AF, et al. The economic significance of orthopaedic infections. J Am Acad Orthop Surg 2015; 23 Suppl:S1–S7. [DOI] [PubMed] [Google Scholar]
- 21.Tseng CH, Huang WS, Muo CH, et al. Increased depression risk among patients with chronic osteomyelitis. J Psychosom Res 2014; 77:535–540. [DOI] [PubMed] [Google Scholar]
- 22.Waldvogel F, Medoff G, Swartz M. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med 1970; 282:198–206. [DOI] [PubMed] [Google Scholar]
- 23.Kremers HM, Nwojo ME, Ransom JE, et al. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am 2015; 97:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suresh SS, Zaki H, Shalamzari JE, et al. Osteomyelitis calcaneum due to a scorpion sting. J Foot Ankle Surg 2014; 53:340–343. [DOI] [PubMed] [Google Scholar]
- 25.Wronka KS, Sinha A. Calcaneal osteomyelitis following steroid injection for plantar fasciitis: a case report. Foot Ankle Spec 2012; 5:253–255. [DOI] [PubMed] [Google Scholar]
- 26.Laughlin RT, Reeve F, Wright DG, et al. Calcaneal osteomyelitis caused by nail puncture wounds. Foot Ankle Int 1997; 18:575–577. [DOI] [PubMed] [Google Scholar]
- 27.Stucken C, Olszewski DC, Creevy WR, et al. Preoperative diagnosis of infection in patients with nonunions. J Bone Joint Surg Am 2013; 95:1409–1412. [DOI] [PubMed] [Google Scholar]
- 28.Senneville E, Nguyen S. Current pharmacotherapy options for osteomyelitis: convergences, divergences and lessons to be drawn. Expert Opin Pharmaco 2013; 14:723–734. [DOI] [PubMed] [Google Scholar]
- 29.Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012; 54:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tone A, Nguyen S, Devemy F, et al. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: a multicenter open-label controlled randomized study. Diabetes Care 2015; 38:302–307. [DOI] [PubMed] [Google Scholar]
- 31.Conterno LO, Turchi MD. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev 2013; 9:Cd004439. [DOI] [PMC free article] [PubMed] [Google Scholar]


