Supplemental Digital Content is available in the text
Abstract
Celiac disease (CD) is common in Caucasians, but thought to be rare in Asians. Our aim was to determine the prevalence of CD in Chinese patients with chronic diarrhea predominant irritable bowel syndrome (IBS-D).
From July 2010 to August 2012, 395 adult patients with IBS-D and 363 age and sex-matched healthy controls were recruited in Zhongnan Hospital of Wuhan University and Xiaogan Central Hospital in Hubei province, central China. Patients with IBS-D were diagnosed according to the Rome III criteria. Serum Immunoglobulin (IgA/IgG) anti-human tissue transglutaminase (anti-htTG)-deamidated gliadin peptide (DGP) antibodies were measured in a single ELISA (QUANTA Lite h-tTG/DGP Screen). Upper endoscopy with duodenal biopsies and HLA-DQA1 and HLA-DQB1 genotyping were performed in seropositive subjects and a gluten-free diet was prescribed.
Seven IBS-D patients (7/395, 1.77%) and 2 healthy controls (2/363, 0.55%), were positive for anti-htTG/DGP antibodies. Of these 9 cases, 1 was lost to follow-up, 3 were suspected to have CD and 5 were eventually diagnosed as CD with intestinal histological lesions classified as Marsh Type II in 2 and Type III in 3. Of these 5 diagnosed CD patients, 4 (4/395, 1.01%) were from the IBS-D group and 1 (1/363, 0.28%) from the healthy control had asymptomatic CD. Two Type III CD patients with relatively high titers in the serologic assay were homozygous and heterozygous for haplotype HLA-DQA1∗03-DQB1∗03:03 (HLA-DQ9.3), respectively.
In the present study, CD was present in 1.01% of patients with IBS-D and in 0.28% of the control group. We like to suggest that the haplotype HLA-DQA1∗03-DQB1∗03:03 (HLA-DQ9.3), which is common in Chinese, is a new susceptibility factor for CD in China. Larger screening and genetic studies are needed in the Chinese population of different regions.
INTRODUCTION
Celiac disease (CD) is one of the gluten-related disorders including also nonceliac gluten sensitivity (NCGS) and wheat allergy. CD is characterized by chronic mucosal inflammation with infiltration of intraepithelial lymphocytes (IELs) in the epithelium and plasma cells in the lamina propria, with crypt hyperplasia and villous atrophy primarily in the upper small intestine. The classical form of CD presents with symptoms and signs of malabsorption such as chronic diarrhea, bloating, weight loss, and abdominal pain but more recent clinically silent nonclassical presentations in adults include iron-deficiency anemia, osteoporosis, gastroesophageal reflux, constipation, weight loss, neurologic symptoms, dermatitis herpetiformis, hypoproteinemia, hypocalcemia, and elevated liver enzyme levels.1 CD occurs in genetically susceptible individuals exposed to dietary gluten. Approximately 90% of Caucasian patients with CD are carriers of the human leukocyte antigen (HLA)-DQ2.5 (DQA1∗05-DQB1∗02) heterodimers encoded in cis (DQ2.5cis) or trans (DQ2.5trans) configuration and the remainder carry either DQ8 (DQA1∗03-DQB1∗03:02), HLA-DQ2.2 (DQA1∗02-DQB1∗02) heterodimers, or HLA-DQA1∗05 only. These HLA-DQ gene variants at the major histocompatibility complex on chromosome 6p21.3 are present in 30% to 43% of the general European population and explain ∼40% of the disease heritability, whereas large genome-wide association studies have brought the number of non-HLA loci each contributing only a small risk to 39 explaining ∼14%, at present leaving 46% as “missing heritability”.2 After a strict gluten-free diet (GFD), the clinical symptoms and histological features in CD patients improve in the great majority of patients.3,4
CD is an inflammatory autoimmune disease that affects genetically predisposed individuals. It is triggered by the ingestion of gluten and other related proteins in barley, rye, and possibly oats. The interaction of genetic and environmental factors leads to loss of gluten tolerance and the development of intestinal lesions characterized by increased number of lymphocytes in the epithelium and lamina propria, destruction of the villi, alteration of epithelial cells, and mucosal remodeling with the presence of auto-antibodies to the enzyme tissue transglutaminase-2 (tTG2). The lesion and inflammatory bowel changes resolve when gluten is removed from the diet.5
In Caucasians the average prevalence is estimated to be about 1% to 2% according to several epidemiological studies using specific serological tests, evaluated by different methods and markers.1 The prevalence in the countries of Northern Europe is slightly higher than the prevalence in the Mediterranean. The Scandinavian countries, the United Kingdom, and Ireland have shown a prevalence ranging from 1% to 2.5%.6 The average prevalence of CD in the United States is very similar to the one observed in Europe.7 In the last years, a greater awareness of CD and a more active search through information campaigns and the dissemination of knowledge by patients associations and internet active groups have contributed to a higher prevalence. Despite the lack of data, the prevalence in Asian populations including Chinese was predicted to be low, due to the rice-based staple diets.8 The knowledge on CD in China has started in recent years, though no formal epidemiological studies have been performed yet.9,10 These reports are of great importance since they confirmed the occurrence of CD in China.
Irritable bowel syndrome (IBS) is a chronic condition characterized by abdominal discomfort or pain and altered bowel habit. The prevalence of IBS in Europe and North America is estimated to be 10% to 15% based on the Rome II and III criteria, and in the Asia–Pacific region the prevalence is somewhat lower (2.9% to 15.6%) but is increasing,11 particularly in countries with developing economies.12 A small meta-analysis revealed that the prevalence of biopsy-proven CD in cases meeting diagnostic criteria for IBS is more than 4-fold higher than in controls without IBS.13
The availability of highly sensitive and specific serologic assays for CD has led to increased recognition that the disease is more common than assumed. Sugai et al14,15 and Hogen Esch et al16 reported that the IgA/IgG human-tTG/DGP enzyme-linked immunosorbent assay (ELISA) is one of the most sensitive screening tests for CD. Using an h-tTG/DGP combined ELISA, we carried out a study of serological screening for CD in adult Han Chinese patients with chronic diarrhea predominant IBS (IBS-D) that is the most frequent form of IBS and represents a high risk group to suffer from CD and healthy individuals. In addition, in serologic positive subjects we performed endoscopic and biopsy examinations, HLA-DQ genotyping, to estimate the prevalence of CD in central China.
METHODS
Recruitment of Patients With Chronic Diarrhea and Healthy Controls
Between July 2010 and August 2012, 417 consecutive patients diagnosed with IBS-D in Zhongnan Hospital of Wuhan University in Wuhan city and Xiaogan Central Hospital near Wuhan, both in Hubei Province, central China were recruited. All the patients were Han Chinese referred by doctors from the departments of Gastroenterology of these hospitals. All patients completed a questionnaire, which comprised sex, date of birth, height, weight, education status, dietary habit, staple food, current clinical manifestations, especially gastrointestinal symptoms and signs including detailed information of diarrhea, previous and current medications, personal and family medical history, etc. Twenty-two subjects were excluded in accordance with the exclusion criteria given below, and no significant differences exist in age, sex, body mass index (BMI), and indications for referral between those included in and those excluded from the study. All patients were on a normal gluten containing diet consisting of wheat, and oat at the time of inclusion. A total of 183 men and 212 women, mean age 48.37 ± 11.6, and BMI 21.6 ± 4.1 (kg/m2, mean ± SD) were included. The inclusion criteria were age greater than 18 but less than 80 years old; diagnosis of IBS-D according to the Rome III criteria; and a signed consent form. Patients were excluded if they took NSAIDs, suffered from intestinal tuberculosis or antibiotic-associated diarrhea 4 weeks prior to the study; were diagnosed with villous adenomas, microscopic colitis, or inflammatory bowel disease; had a history of gastro-intestinal cancer; were positive for antibody to human immunodeficiency virus, hepatitis B antigen, or hepatitis C virus; or were syringe drug users or cocaine inhalers.
In addition, 363 age and sex-matched healthy controls taking the annual routine health examinations in Zhongnan Hospital of Wuhan University and Xiaogan Central Hospital were recruited in this study. Healthy individuals did not fill out the above-mentioned questionnaire but they were confirmed to be normal in the examination during the period of the study and were excluded when chronic diarrhea or the same exclusion items used for IBS-D patients were apparent. A total of 173 men and 190 women, mean age 47.47 ± 15.18 (years), and BMI 24.13 ± 3.95 (kg/m2, mean ± SD) were included. BMI of healthy controls was significantly higher than of IBS-D patients (P < 0.001). The study protocol was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University. All included subjects signed the consent form for the project.
Diagnostic Criteria for Celiac Disease
CD was diagnosed when the anti-htTG/DGP antibodies test was positive, a Marsh II or III abnormality was found in the small intestinal biopsy specimen, and an objective response to a GFD was obtained. The Marsh classification was used for histological grading of the duodenal biopsy specimens.17
H-tTG/DGP Enzyme-Linked Immunosorbent Assay
Approximate 3 mL venous blood was obtained from each individual, and then centrifuged at 3000 rpm for 10 minutes. The serum samples were stored at −80°C until use. All serum samples were subjected to IgA/IgG anti-htTG/DGP ELISA (QUANTA Lite, h-tTG/DGP ELISA; number 704575, Inova Diagnostics Inc., San Diego, CA), which is a solid phase enzyme immunoassay for the quantitative detection of IgA/IgG antibodies to synthetic DGP and the native h-tTG. In accordance with recommendations by the manufacturer, serum samples were defined to be positive for anti-htTG/DGP ELISA antibodies when the measured values were 20 Units or above.
HLA-DQ Typing
Genomic DNA of seven (No. 1, No. 2, No. 3, No. 4, No. 5, No. 8, No. 9) serologic positive subjects was extracted from 250 μL serum using the EasyMAG NucliSens extraction system (BioMérieux Benelux BV, Zaltbommel, The Netherlands). 2 mL NucliSens lysis buffer was added to the serum and incubated for 10 minutes at room temperature. The mixture was then added to the EasyMAG vessel and 50 μL of magnetic silica was subsequently added. The DNA was extracted on the EasyMAG machine (BioMérieux Benelux BV, Zaltbommel, The Netherlands) using the Generic 2.0.1 program. Elution was performed in 25 μL NucliSens Extraction buffer 3.18 EDTA-anticoagulated peripheral blood nucleated cells of the other 2 serologic positive subjects (No. 6 and No. 7) were available for extraction of genomic DNA. For HLA-DQA1 and HLA-DQB1 genotyping, polymerase chain reaction-amplified exon 2 amplicons were generated for low- to medium resolution typing in a combined, single-stranded conformation polymorphism–heteroduplex assay by a semiautomated electrophoresis and gel-staining method on the PhastSystem (Amersham Pharmacia Biotech, Uppsala, Sweden). This method has been validated by using a panel of reference DNA against the Dynall Allset sequence-specific primers high-resolution typing kits (Dynal A.S., Oslo, Norway).19,20
Endoscopic and Histological Assessments and Intraepithelial Lymphocyte Counting in Patients Positive for Anti-htTG/DGP ELISA Antibodies
Patients positive for IgA/IgG anti-htTG/DGP antibodies were asked to accept endoscopic examination of the duodenum and biopsies. At the endoscopy, 4 to 6 biopsies were obtained from the descendent duodenum at different levels distal to the papilla. The biopsies were fixed with 10% buffered formalin, embedded in paraffin blocks, and stained with hematoxylin and eosin. Two experienced pathologists blindly evaluated the histologic pattern under the microscope and assigned a Marsh classification to the biopsy findings. Type 0 indicates a normal small intestinal architecture sufficient to exclude celiac disease, type I (infiltrative) is characterized by increased IELs, type II (hyperplastic) indicates also crypt hyperplasia, and an additional partial, subtotal, or total villous atrophy characterizes the type III (destructive) lesion.21 Moreover, all histological sections were examined by light microscopy (×400 magnification), and the numbers of epithelial cells and IELs in a randomly chosen, uninterrupted length of the villous epithelium (>500 cells) were counted. The average number of IELs within 100 intestinal epithelial cells was calculated, and 40 IELs/100 epithelium cells was defined as the upper limit of the normal.22–24
Treatment of a Gluten-Free Diet for Patients Positive for Anti-htTG/DGP IgA/IgG
Patients positive for anti-htTG/DGP IgA/IgG were asked to receive GFD treatment. During a GFD, it was advised to avoid all gluten-containing food. Patients were monitored by periodic hospital visits for assessment of symptoms, physical examinations, and adherence to the GFD (Figure 1). Positive response to GFD treatment was defined when strictly compliant patients had improvement of clinical symptoms including chronic diarrhea, abdominal pain, and decreased serum antibody level compared with pre-GFD.
FIGURE 1.
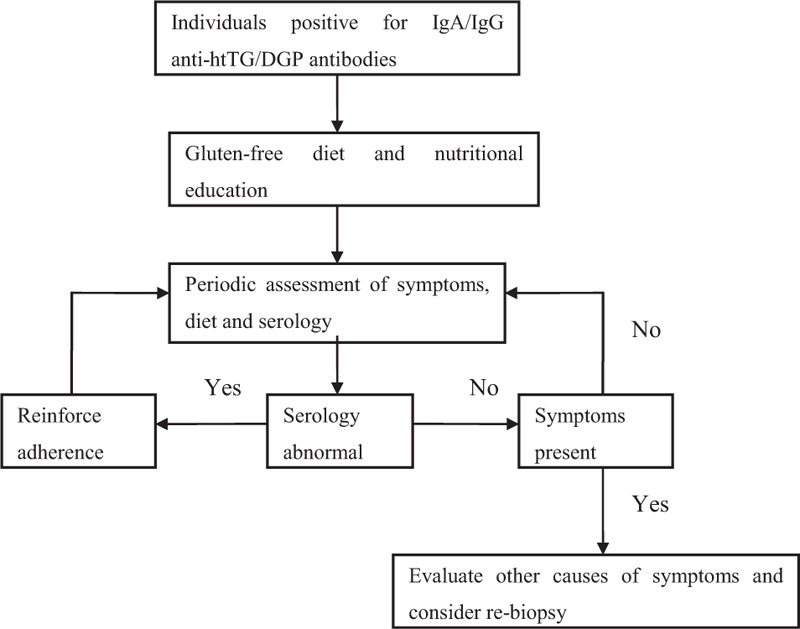
Treatment and monitoring of patients positive for IgA/IgG anti-htTG/DGP antibodies.
Evaluation of Clinical Symptoms
The criteria for evaluating clinical manifestations was based on 4 symptoms: abdominal pain; abdominal discomfort (eg, bloating, abnormal stress sense); change in bowel habits; change in bowel traits. The severity score for each symptom was evaluated as: 0, asymptomatic; 1, mild (no influence on daily life or sleep); 2, moderate (obvious symptoms with mild limitation of daily life); 3, severe (symptoms significantly affect the daily life). The clinical symptoms were assessed before and after GFD therapy and the total score (0–12) was calculated according to the above rating standard.
Statistical Analysis
The overall prevalence was reported using relative frequencies and percentages with the corresponding 95% confidence interval (95% CI) on the basis of the binomial distribution. Mean, standard deviations (SD), or median and range were used as continuous statistics while evaluating using Student t test. Frequency differences were compared using Fisher exact test. Statistical significance was established at the P value <0.05. All calculations were performed using SPSS for Windows, version 17.0 (SPSS, Inc, Chicago, IL). Figures were drawn using GRAPHPAD Prism 5.0 (GraphPad Software, Inc, San Diego, CA).
RESULTS
Serum IgA/IgG Anti-htTG/DGP Antibodies in IBS-D Patients and Healthy Controls
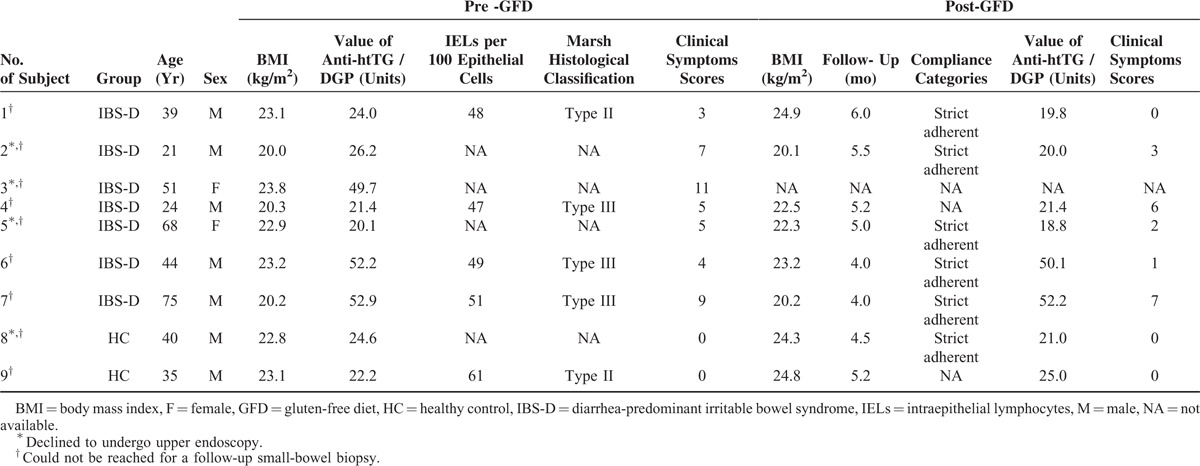
The values of cases positive for the IgA/IgG anti-htTG/DGP antibodies are shown in Table 1 and Supplemental Digital Content, http://links.lww.com/MD/A463 (see Figure, Supplemental Digital Content, http://links.lww.com/MD/A463 which illustrates the serum IgA/IgG anti-htTG/DGP antibodies as determined by ELISA in patients with IBS-D and healthy controls, in relation to sex). There was no significant difference between the values in males and females in both IBS-D patients and healthy controls. Seven (1.77%) IBS-D patients and 2 (0.55%) healthy controls were positive for IgA/IgG anti-htTG/DGP antibodies. However, this difference was not significant (1.77% vs. 0.55%, P = 0.41). Two IBS-D patients (No. 6 and No. 7) had relatively high titers (≥50 Units) compared with the other serologic screening test positive subjects.
TABLE 1.
The Demographic and Clinical Features of IgA/IgG Anti-htTG/DGP Positive Patients and Healthy Controls and Post-GFD Serum Antibody Level and Clinical Assessments
Duodenal Biopsy and Histological Assessment
Table 1 shows features of the 9 IgA/IgG anti-htTG/DGP positive subjects. Of these, 5 subjects (No. 1, No. 4, No. 6, No. 7, and No.9) underwent upper endoscopy and duodenal biopsies for histological assessment and showed increased numbers (range: 47–61) of IELs per 100 epithelial cells. In addition, subject No. 1 had enlargement of the crypts (Marsh II; Figure 2A); subject No. 4 villous atrophy and cryptic hyperplasia (Marsh III; Figure 2B); subjects No. 6 and No. 7 villous atrophy and cryptic hyperplasia (Marsh III; Figures 2C and D), respectively; subject No. 9 enlargement of crypts (Marsh II; Figure 2E).
FIGURE 2.
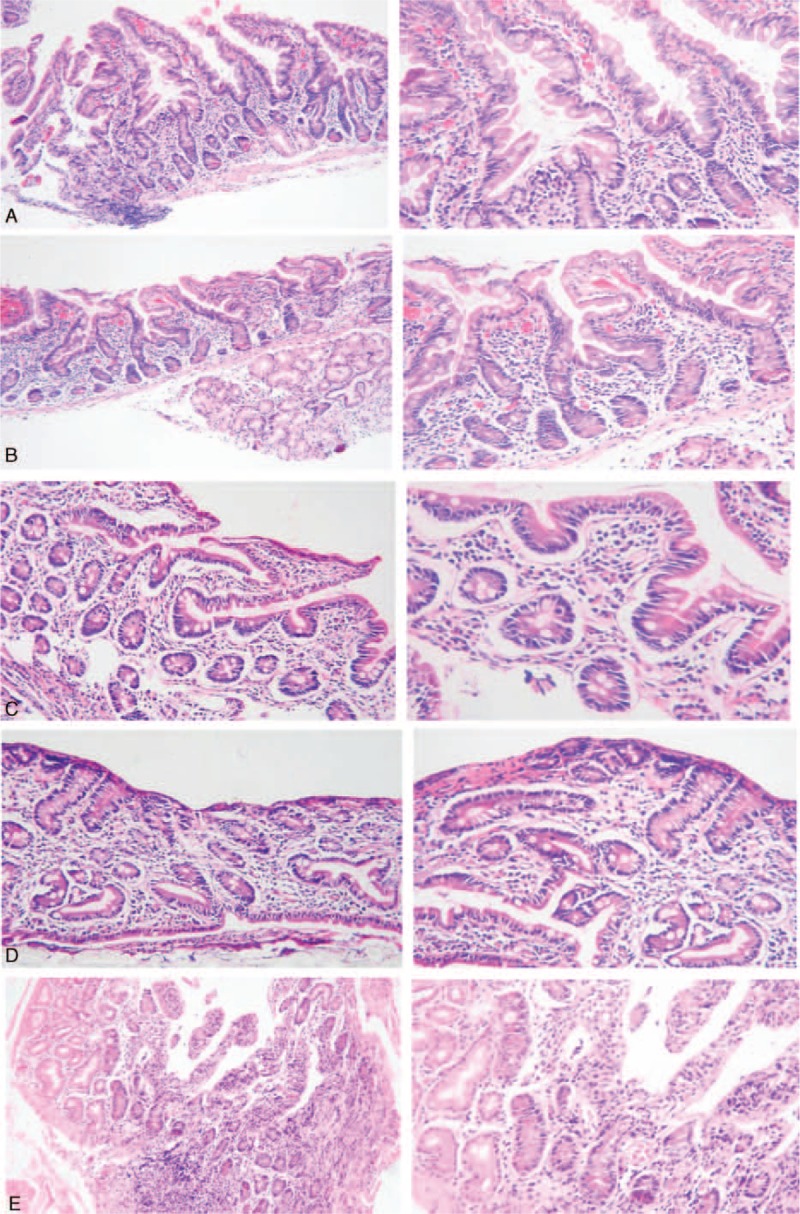
Histological assessment of duodenal biopsies of subjects No. 1 (A), No. 4 (B), No. 6 (C), No.7 (D) low (×40) and high (×100 magnification), and No. 9 (E) low (×100) and high (×200 magnification) are on the left and right respectively.
Change in the IgA/IgG Anti-htTG/DGP Antibody Levels and Clinical Responses in Positive Subjects Following Treatment With a Gluten-Free Diet
Among the 9 subjects positive for IgA/IgG anti-htTG/DGP antibodies, all except 1 (No. 3) who was lost to follow-up were advised to receive a GFD treatment. Of the 8 subjects, patients No. 1, No. 2, No. 5, No. 6, No. 7, and No. 8 strictly followed the GFD requirements for a median duration of 4.9 months, whereas patients No. 4 and No. 9 refused a GFD. The changes of serum IgA/IgG anti-h-tTG/DGP antibodies during follow-up in months in these 6 subjects with GFD and the other 2 without GFD are shown in Figure 3. The post-GFD clinical responses, as well as the categories of the patients’ compliance with the GFD, are demonstrated in Table 1. All the subjects refused to re-undergo upper endoscopy.
FIGURE 3.
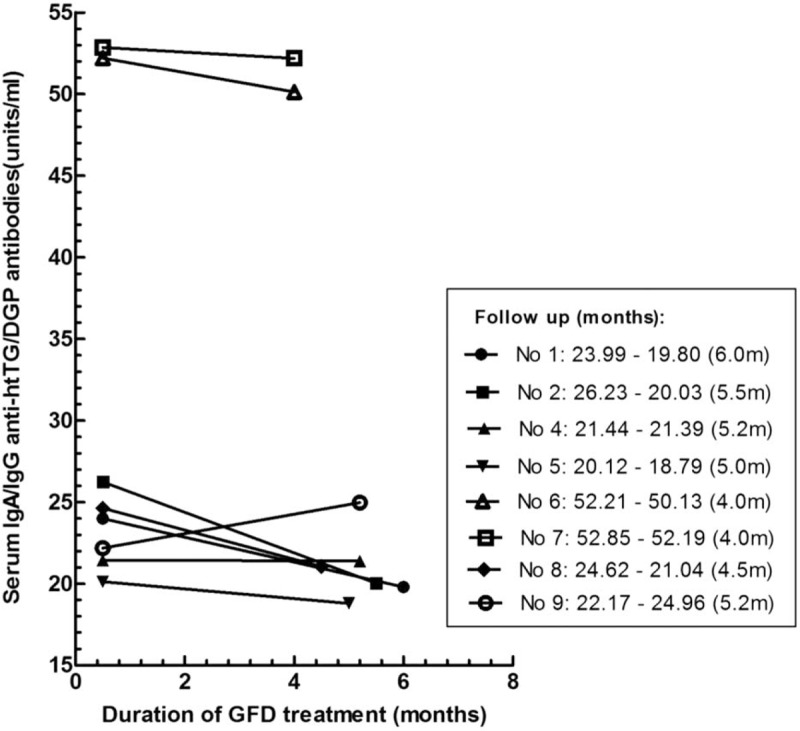
Changes of serum IgA/IgG anti-htTG/DGP antibodies during follow-up in subjects with gluten-free diet (GFD) and without GFD. Subjects No. 1, No. 2, No. 5, No. 6, No. 7, and No. 8 with initially positive for IgA/IgG anti-htTG/DGP antibodies, following treatment of GFD for a median duration of 4.9 months (range 4.0–6.0 mo). No. 4 and No. 9 had no gluten restrictions.
HLA-DQ Genotyping
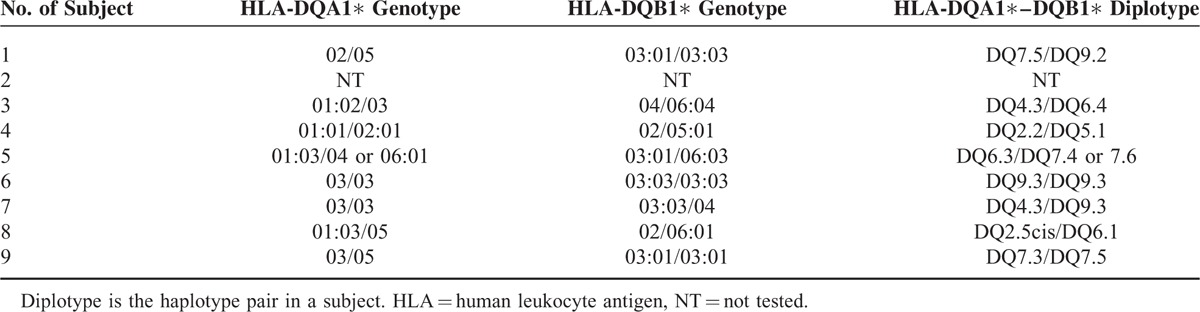
HLA-DQA1 and DQB1 genotypes of all subjects except No. 2 were established (Table 2). Subject No. 8 was heterozygous for the CD-associated haplotype HLA-DQA1∗05-DQB1∗02 (DQ2.5cis). Subject No. 6 was homozygous and subject No. 7 was heterozygous for the haplotype HLA-DQA1∗03-DQB1∗03:03 (DQ9.3), and both were negative for the CD-associated DQ2.5 and DQ8 (HLA-DQA1∗03-DQB1∗03:02). Subject No. 4 was a heterozygous carrier of HLA-DQA1∗02-DQB1∗02 (DQ2.2). Subjects No. 1 and No. 9 were heterozygous for the haplotype HLA-DQA1∗05-DQB1∗03:01 (DQ7.5) containing only half of DQ2.5 (ie, HLA-DQA1∗05) with subject No. 1 also containing half of DQ9.3 (ie, heterozygous for HLA-DQB1∗0303). Subjects No. 3 and No. 5 did not carry any of these allelic combinations mentioned in either the cis or trans configuration.
TABLE 2.
HLA-DQA1 and HLA-DQB1 Genotypes As Well As Inferred HLA-DQA1-DQB1 Diplotypes in Seropositive Subjects
Diagnosis of Patients With IBS-D and Healthy Controls
Of 9 serological positive subjects, 5 (No. 1, No. 4, No. 6, No. 7, and No. 9) presented with type II or type III intestinal histological lesions were considered to suffer from celiac disease. One subject (No. 8) from the healthy control group was considered to be a potential CD candidate, unfortunately the control did not accept the possibility of taking a biopsy of the small intestine. Two other subjects (No. 2, No. 5) were only suspected to have CD in the absence of histologic evidence. The last subject (No. 3) was lost to follow-up. Of note, 3 individuals in IBS-D group who were negative for anti-htTG/DGP IgA/IgG, complained of abdominal pain, diarrhea, bloating, or tiredness after the ingestion of gluten-containing food in the questionnaire and the medical record, but they did undergo neither gluten challenge nor GFD, neither skin prick tests/specific immunoglobulin E (SPTs/sIgE) nor histological examination; therefore, they were suspected to have gluten intolerance, either nonceliac gluten sensitivity or wheat allergy. No other gluten-related findings were observed in the control group due to lack of further investigation.
DISCUSSION
The present study confirms that celiac disease exists in patients with IBS and healthy subjects in the Han Chinese population. With the traditional rice staple food gradually being replaced by Western-style food containing a high content of wheat8 CD appears to inevitably become a health problem in China. Performance of the serological test in a clinical setting depends on patient characteristics (eg, age, genetic predisposition, and IgA deficiency), pretest probability, stage of disease, and ingested amounts of gluten. IgA deficiency in Chinese is much lower than in Western populations.25,26 We used the IgA/IgG human-tTG/DGP ELISA with a high sensitivity of 96.8% and a specificity of 100.0% with a cut-off level of 20 Units established in citizens from the United States.27 Although CD appears to be much less common than in Western countries, the serum IgA/IgG anti-htTG/DGP antibody values showed statistical differences between the IBS-D group and the healthy control group (8.19 ± 2.36 vs. 6.75 ± 3.11, P = 0.0001). Of note, using the same composite antigen ELISA, Sugai et al detected much higher antibody titers in Caucasian CD patients.14 The total amount of wheat in the Chinese diet is probably much less than in other countries. Unfortunately, we have not quantified and registered the age of introduction of gluten intake in our subjects. However, we think that these values do not affect the diagnosis of CD, particularly when the other diagnostic criteria such as duodenal biopsy specimen's histological assessment, clinical symptoms, and the response to GFD were observed.28 Among the 9 individuals suspected of having CD on the ground of histological biopsy abnormalities, 2 of Table 1 (No. 4 and No. 9) were confirmed to have CD by histology, 3 (No. 1, No. 6, and No. 7) were confirmed to have CD by both histology, 1 (No. 3) was lost to follow-up, and 3 (No. 2, No. 5, and No. 8) were suspected to have CD by response to GFD but without histological evidence.
Duodenum and jejunal biopsy assessments have been the gold standard diagnostic test for CD during the last 30 years. An increase in the number of IELs is the first and most sensitive index of the effects of CD.29 In the present study, 5 seropositive subjects agreed to undergo upper endoscopy and to have duodenal mucosa biopsies taken. Histological lesions (Type II or Type III) marked the diagnosis of CD in all 5 cases.
We noted that in 1 healthy subject (No. 9) positive for anti-htTG/DGP antibodies, the diagnostic duodenal biopsy histological assessment was Type II and this individual probably has CD but is asymptomatic. Indeed, it has been reported that although a broad spectrum of symptoms may be associated with untreated CD, many patients, especially those presenting in adulthood, have Marsh I histological abnormalities but often anemia and osteoporosis.30–32 Also, long-term observation indicates that the majority of these patients will eventually develop typical histological lesions of CD at some time.33 Thus, we suppose that the actual prevalence of CD in China may be possibly even higher than our finding and that silent cases may underestimate it as those individuals will not be screened.
The 2 healthy individuals with abnormal antibody levels were asymptomatic, whereas from the 5 patients with IBS-D 1 had type 1 diabetes mellitus and 4 had associated signs and symptoms, such as malnutrition in 1 and anemia in 4. In line with studies in Western countries, our data further demonstrate that CD is more common in patients with IBS-D diagnosed with strict application of Rome III criteria, compared with the general population. Our results and others in the literature would suggest that routine screening for CD in patients with symptoms of IBS is necessary, at least in patients who present with gastrointestinal symptoms, including chronic diarrhea, malabsorption, weight loss, and abdominal distention.
DQ2.5, DQ8, and DQ9.3 in the General Population and the Study Population
The frequency of HLA-DRB1∗03, uniquely carrying the haplotype DQ2.5cis (HLA-DQA1∗05-DQB1∗02), is 4.1% in the Han population of Wuhan, Hubei province in accordance with a range between 3% and 5% on mainland China.34,35 Since the frequency of HLA-DQB1∗02 in Han Chinese from Hubei is 10.7%, the frequency of DQ2.5trans (HLA-DQA1∗05/DQB1∗02) amounts 6.6%. Therefore, with a frequency between 4.1% and 10.7% DQ2.5 is common in the general population under study.35 The frequency of HLA-DQB1∗0302, mainly present in China on haplotypes with HLA-DRB1∗04-DQA1∗03, is 6.2% in the Han population of Wuhan in accordance with a range between 4.5% and 5.6% in other regions of mainland China, HLA-DQA1∗03:02/DQB1∗03:03:02 is strongly associated with susceptibility to childhood-onset ocular myasthenia gravis in Southern Han Chinese.36,37
In our study, 1 out of 9 subjects (No. 8) was heterozygous for the CD-associated HLA-DQ2.5cis. No subject was positive for HLA-DQ8. Subject No. 6 is homozygous for HLA-DQ9.3 (HLA-DQA1∗03-DQB1∗0303) and No.7 is heterozygous for HLA-DQ9.3. HLA-DQB1∗0303 has a frequency of 18.4% in Hubei and is present on both DQ9.2 (HLA-DQA1∗02-DQB1∗0303) and DQ9.3 haplotypes. The frequency of HLA-DQ9.3 is high in mainland China, ranging from 13.8% to 21.9%.37 Islet autoantibodies are associated with HLA-DQ genotypes in Han Chinese patients with type 1 diabetes and their relatives.38 HLA-DQ9.3 carries aspartate at DQβ57 but is otherwise identical to HLA-DQ8 in the peptide binding cleft. Recently, a dominant DQ9-restricted gluten epitope (DQ8-glut-1) previously identified in DQ8-positive CD patients revealed a strong T-cell response and sustained strong binding to DQ9.3 demonstrating DQ9 is a susceptibility factor for CD.39 We also noticed that both subjects No. 6 and No. 7 had relatively high titers (52.2 and 52.9 Units, respectively) in the sensitive serologic screening test when compared with the other CD patients with levels just above the cut-off value of 20 Units. Thus, we hypothesize that in addition to a lower gluten intake variations in genetic background across different populations, such as the haplotype HLA-DQA1∗03-DQB1∗03:03 (DQ9.3) (with a frequency less than 1% in American whites) determines a lower humoral response. HLA-DQ9.3 might very well be a new susceptibility factor for CD in China.
In our study, 5 subjects were finally diagnosed with CD and 3 were diagnosed as suspected CD. As for the HLA-DQ2.5/DQ8 status, subject No. 4 is associated with DQ2.2, subject's No. 1 and No. 9 with DQX.5, and both No. 3 and No. 5 patients have HLA status of DQX.x, all of which obviously indicate to observe probably big differences in CD-associated genotype background between Central Han Chinese and white Caucasian patients. We noticed that in our research subject No. 8, who belongs to the healthy control group, is HLA-DQ2.5 positive, was without appearance of clinical symptoms, and had high antibody level that decreased after GFD. Thus very like he has CD in an asymptomatic form and a close follow-up is indicated. There are some limitations in our study, such as the relatively small number of patients and controls and the selection covering 2 large referral centers in the Hubei Province. Moreover, 4 out of 9 seropositive individuals declined to undergo upper endoscopy and diagnostic biopsies taken and could therefore, at best, be diagnosed as suspected CD.
Interestingly, in response to the questionnaire of our study on CD, 3 of IBS-D patients who were negative for CD in the serological marker test reported intestinal and/or extraintestinal symptoms after ingestion of gluten containing food. This suggests that these patients were suffering from other gluten-related disorders, nonceliac gluten sensitivity,40,41 or wheat allergy.42 However, in our study neither double-blind, placebo-controlled gluten challenge nor SPTs/sIgE was performed, nor has the influence of “Fermentable Oligo-Di-Monosaccharides and Polyols” (FODMAPs) in their diets been studied. These aspects are certainly worth investigating in future studies with our patients.40,41,43
Recently while this manuscript was in preparation Lu et al described a series of Asian, mainly Chinese, patients with IBS who were tested positive for IgA DGP, and improved on a gluten exclusion diet but without celiac disease.44 A recent study performed in Iran has demonstrated that many IBS patients are gluten-sensitive, and their symptoms could be adequately controlled with a gluten-free diet only. These authors suggest that gluten sensitivity should be investigated first, and then elimination of FODMAP might be considered in nonresponders for whom a gluten-free diet proves to be ineffective in controlling the symptoms. This approach is interesting to follow in future studies.45
In conclusion, the prevalence of celiac disease in patients with IBS-D is 1.01% (4/395) and 0.28% (1/363) in the control group. The haplotype HLA-DQA1∗03-DQB1∗03:03 (HLA-DQ9.3), which is common in Chinese but has a low frequency in Caucasians, is probably a susceptibility factor for CD in China. Larger screening and genetic studies in different regions are needed to confirm this finding.
Footnotes
Abbreviations: anti-htTG = anti-human tissue transglutaminase, BMI = body mass index, CD = celiac disease, DGP = deamidated gliadin peptide, DNA = deoxyribonucleic acid, ELISA = enzyme-linked immunosorbent assay, GFD = gluten-free diet, HLA = human leukocyte antigen, IBS = irritable bowel syndrome, IBS-D = diarrhea-predominant irritable bowel syndrome, IEL = intraepithelial lymphocyte, Ig = immunoglobulin, NCGS = nonceliac gluten sensitivity, NSAID = nonsteroidal anti-inflammatory drug, SD = standard deviation, SPTs/sIgE = skin prick tests/specific immunoglobulin E, tTG2 = tissue transglutaminase-2.
All the authors have reviewed and approved the final manuscript submitted. Guarantor of the article: BX and since 2015 GZ.
HW, GZ, and LL contributed equally.
Specific author contributions: HW contributed to conception, funding, acquisition of data and revised the manuscript critically for important intellectual content.
GZ contributed to design, acquisition, analysis, and interpretation of data and revised the manuscript critically for important intellectual content.
LL contributed to acquisition, analysis, and interpretation of data and wrote the major part of the manuscript.
JBAC contributed to acquisition of data and revised the manuscript critically for important intellectual content.
AY contributed to acquisition of data.
JK contributed to acquisition of data.
GY contributed to analysis of data.
MW contributed to analysis of data.
JW, BMEvB, and SAM revised the manuscript critically for important intellectual content.
ASP contributed to conception and design and revised the manuscript critically for important intellectual content.
BX contributed to funding, conception, and design, revised the manuscript critically for important intellectual content and study supervision.
The authors have no conflicts of interest to disclose.
Study Highlights: What Is Current Knowledge: patients with symptoms, signs, or laboratory evidence suggestive of malabsorption should be tested for celiac disease.
Celiac disease is common in Caucasians with HLA-DQ2.5 in ∼95% and HLA-DQ8 in most other patients.
HLA-DQ9.3, rare in Caucasians, can also confer risk by the presence of DQ9-restricted gluten-specific T cells.
Celiac disease is considered uncommon in Chinese and their HLA-DQ distribution unknown.
What Is New Here
Serological screening for celiac disease revealed that celiac disease is present in adult patients with diarrhea-predominant irritable bowel syndrome from central China.
HLA-DQ9.3, common in China, but not HLA-DQ2.5/DQ8 was found in 2 patients with histologic abnormalities on duodenal biopsies classified as a Marsh Type III lesion and supported by positive serology.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med 2007; 357:1731–1743. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Wijmenga C, Withoff S. From genome-wide association studies to disease mechanisms: celiac disease as a model for autoimmune diseases. Semin Immunopathol 2012; 34:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mooney PD, Hadjivassiliou M, Sanders DS. Coeliac disease. BMJ 2014; 348:g1561. [DOI] [PubMed] [Google Scholar]
- 4.Mooney PD, Hadjivassiliou M, Sanders DS. Emerging drugs for coeliac disease. Expert Opin Emerg Drugs 2014; 19:533–544. [DOI] [PubMed] [Google Scholar]
- 5.Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat Rev Immunol 2013; 13:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catassi C, Yachha SK. Fasano A, Troncone R, Branski D. The global village of celiac disease. Basel, Switzerland, Frontiers in Celiac Disease. vols. 23–31.. Karger:2008. [Google Scholar]
- 7.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012; 107:1538–1544. [DOI] [PubMed] [Google Scholar]
- 8.Cummins AG, Roberts-Thomson IC. Prevalence of celiac disease in the Asia-Pacific region. J Gastroenterol Hepatol 2009; 24:1347–1351. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Xia B, von Blomberg BME, et al. Coeliac disease in China, a field waiting for exploration. Rev Esp Enferm Dig 2010; 102:472–477. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Xia B, von Blomberg BME, et al. Coeliac disease: emerging in China? Gut 2010; 59:418–419. [DOI] [PubMed] [Google Scholar]
- 11.Gwee KA, Bak YT, Ghoshal UC, et al. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol 2010; 25:1189–1205. [DOI] [PubMed] [Google Scholar]
- 12.Quigley EM, Abdel-Hamid H, Barbara G, et al. A global perspective on irritable bowel syndrome: a consensus statement of the World Gastroenterology Organisation Summit Task Force on irritable bowel syndrome. J Clin Gastroenterol 2012; 46:356–366. [DOI] [PubMed] [Google Scholar]
- 13.Ford AC, Chey WD, Talley NJ, et al. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med 2009; 169:651–658. [DOI] [PubMed] [Google Scholar]
- 14.Sugai E, Hwang HJ, Vazquez H, et al. New serology assays can detect gluten sensitivity among enteropathy patients seronegative for anti-tissue transglutaminase. Clin Chem 2010; 56:661–665. [DOI] [PubMed] [Google Scholar]
- 15.Sugai E, Vazquez H, Nachman F, et al. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol 2006; 4:1112–1117. [DOI] [PubMed] [Google Scholar]
- 16.Hogen Esch CE, Csizmadia GD, van Hoogstraten IM, et al. Childhood coeliac disease: towards an improved serological mass screening strategy. Aliment Pharmacol Ther 2010; 31:760–766. [DOI] [PubMed] [Google Scholar]
- 17.Marsh MN. The immunopathology of the small intestinal reaction in gluten-sensitivity. Immunol Invest 1989; 18:509–531. [DOI] [PubMed] [Google Scholar]
- 18.Huijsmans CJ, Poodt J, Damen J, et al. Single nucleotide polymorphism (SNP)-based loss of heterozygosity (LOH) testing by real time PCR in patients suspect of myeloproliferative disease. PLoS One 2012; 7:e38362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crusius JBA. The Immunogenetics of Chronic Inflammatory and Autoimmune Disease. Amsterdam, The Netherlands: Vrije Universiteit; 2002. [Google Scholar]
- 20.Hadithi M, von Blomberg B, Crusius J, et al. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann Intern Med 2007; 147:294–302. [DOI] [PubMed] [Google Scholar]
- 21.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999; 11:1185–1194. [DOI] [PubMed] [Google Scholar]
- 22.Kakar S, Nehra V, Murray JA, et al. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am J Gastroenterol 2003; 98:2027–2033. [DOI] [PubMed] [Google Scholar]
- 23.Kulshrestha MK, Honan WP, Aluwihare N, et al. Coeliac disease and disseminated lymphomatosis. J R Soc Med 1998; 91:594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mino M, Lauwers GY. Role of lymphocytic immunophenotyping in the diagnosis of gluten-sensitive enteropathy with preserved villous architecture. Am J Surg Pathol 2003; 27:1237–1242. [DOI] [PubMed] [Google Scholar]
- 25.Agardh D. Antibodies against synthetic deamidated gliadin peptides and tissue transglutaminase for the identification of childhood celiac disease. Clin Gastroenterol Hepatol 2007; 5:1276–1281. [DOI] [PubMed] [Google Scholar]
- 26.Agardh D, Bjorck S, Agardh CD, et al. Coeliac disease-specific tissue transglutaminase autoantibodies are associated with osteoporosis and related fractures in middle-aged women. Scand J Gastroenterol 2009; 44:571–578. [DOI] [PubMed] [Google Scholar]
- 27.Sugai E, Moreno ML, Hwang HJ, et al. Celiac disease serology in patients with different pretest probabilities: is biopsy avoidable? World J Gastroenterol 2010; 16:3144–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med 2010; 123:691–693. [DOI] [PubMed] [Google Scholar]
- 29.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992; 102:330–354. [PubMed] [Google Scholar]
- 30.Monzon H, Forne M, Gonzalez C, et al. Mild enteropathy as a cause of iron-deficiency anaemia of previously unknown origin. Dig Liver Dis 2011; 43:448–453. [DOI] [PubMed] [Google Scholar]
- 31.Esteve M, Carrasco A, Fernandez-Banares F. Is a gluten-free diet necessary in Marsh I intestinal lesions in patients with HLADQ2, DQ8 genotype and without gastrointestinal symptoms? Curr Opin Clin Nutr Metab Care 2012; 15:505–510. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Banares F, Alsina M, Modolell I, et al. Are positive serum-IgA-tissue-transglutaminase antibodies enough to diagnose coeliac disease without a small bowel biopsy? Post-test probability of coeliac disease. J Crohns Colitis 2012; 6:861–866. [DOI] [PubMed] [Google Scholar]
- 33.Dickey W, Hughes DF, McMillan SA. Patients with serum IgA endomysial antibodies and intact duodenal villi: clinical characteristics and management options. Scand J Gastroenterol 2005; 40:1240–1243. [DOI] [PubMed] [Google Scholar]
- 34.Yuan J, Gao J, Li X, et al. The tip of the “celiac iceberg” in China: a systematic review and meta-analysis. PLoS One 2013; 8:e81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong F, Xiong P, Yang Z, et al. [An investigation of the polymorphism of HLA class II alleles in the Han population in Hubei Province of China]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 1999; 16:216–219. [PubMed] [Google Scholar]
- 36.Zhu WH, Lu JH, Lin J, et al. HLA-DQA1∗03:02/DQB1∗03:03:02 is strongly associated with susceptibility to childhood-onset ocular myasthenia gravis in Southern Han Chinese. J Neuroimmunol 2012; 247:81–85. [DOI] [PubMed] [Google Scholar]
- 37.Yu RB, Hong X, Ding WL, et al. Polymorphism of the HLA-DQA1 and -DQB1 genes of Han population in Jiangsu Province. China Chin Med J (Engl) 2006; 119:1930–1933. [PubMed] [Google Scholar]
- 38.Wang J, Miao D, Babu S, et al. Prevalence of autoantibody-negative diabetes is not rare at all ages and increases with older age and obesity. J Clin Endocrinol Metab 2007; 92:88–92. [DOI] [PubMed] [Google Scholar]
- 39.Bodd M, Tollefsen S, Bergseng E, et al. Evidence that HLA-DQ9 confers risk to celiac disease by presence of DQ9-restricted gluten-specific T cells. Hum Immunol 2012; 73:376–381. [DOI] [PubMed] [Google Scholar]
- 40.Volta U, Caio G, De Giorgio R, et al. Non-celiac gluten sensitivity: a work-in-progress entity in the spectrum of wheat-related disorders. Best Pract Res Clin Gastroenterol 2015; 29:477–491. [DOI] [PubMed] [Google Scholar]
- 41.Catassi C, Elli L, Bonaz B, et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): the Salerno Experts’ Criteria. Nutrients 2015; 2015:4966–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut 2013; 62:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuppan D, Pickert G, Ashfaq-Khan M, et al. Non-celiac wheat sensitivity: differential diagnosis, triggers and implications. Best Pract Res Clin Gastroenterol 2015; 29:469–476. [DOI] [PubMed] [Google Scholar]
- 44.Lu W, Gwee KA, Siah KT, et al. Prevalence of Anti-deamidated Gliadin Peptide Antibodies in Asian Patients With Irritable Bowel Syndrome. J Neurogastroenterol Motil 2014; 20:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shahbazkhani B, Sadeghipour A, Malekzadeh R, et al. Non-Celiac Gluten Sensitivity Has Narrowed the Spectrum of Irritable Bowel Syndrome: A Double-Blind Randomized Placebo-Controlled Trial. Nutrients 2015; 7:4542–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]


