Abstract
To investigate whether the width of gastric serosal lesions in advanced gastric cancer patients have a predictive value for peritoneal recurrence and the 5-year survival rate.
A total of 1109 patients with advanced noncardia primary gastric adenocarcinoma, who underwent curative gastrectomy between January 1997 and December 2007, were included. Data about tumor size, longitudinal tumor location, resection type, serum albumin concentration, lymphatic/venous invasion, lymph node metastasis status, lesion size, histological and Borrmann type of tumor, as well as the recurrence rate and width of the gastric serosal lesions were collected and analyzed.
The peritoneal recurrence rate in patients with gastric serosal lesions ≤3 cm was lower than in patients with gastric serosal lesions >3 cm. Multivariate analyses of the 5-year survival rate variables for all patients revealed significant correlations with serum albumin concentrations (HR 1.382, P = 0.002, 95% CI 1.123–1.701), width of serosa changes (HR 1.377, P = 0.020, 95% CI 1.053–1.802), depth of invasion (HR 1.529, P < 0.001, 95% CI 1.288–1.814), and lymph node metastasis (HR 1.551, P < 0.001, 95% CI 1.420–1.694), whereas for recurrent patients only serum albumin concentrations (HR 2.000, P < 0.001, 95% CI 1.425–2.805), width of serosa changes (HR 1.867, P = 0.002, 95% CI 1.248–2.793), and lymph node metastasis (HR 1.521, P < 0.001, 95% CI 1.249–1.852) correlated with the 5-year survival rate.
Gastric serosal lesions >3 cm may indicate a high risk for peritoneal recurrence and serve as additional indicators for preventive postoperative adjuvant chemotherapies in patients with advanced gastric cancer.
INTRODUCTION
Gastric cancer is the third most common cause of cancer-related death, with the highest mortality rates in East Asia, including China. Approximately 60% of gastric cancers are diagnosed in these regions.1 The main treatment for gastric cancer is surgery but peritoneal recurrence is the most frequent pattern of metastasis even after curative resection.2,3 Since the prognosis of patients with peritoneal recurrence is dismal and the median survival time is only about 6 months, the identification of patients at high-risk of peritoneal recurrence is crucial to allow proper treatment strategies to be developed.
Over the last 20 years, peritoneal lavage cytology (PLC) has been the standard method used to detect peritoneal micrometastasis. However, the sensitivity of PLC for metastasis prognosis has been reported to be only 18%–50% and not all patients with positive peritoneal cytology will eventually develop a peritoneal recurrence. However, even patients with negative peritoneal cytology have developed peritoneal recurrence after curative resection.4,5 Other authors have noted that test execution variations might be the reason for the poor diagnostic accuracy of PLC.6 Therefore, additional approaches to identify definitively peritoneal metastasis are needed.
As up to 50% of patients with serosal invasion are known to develop peritoneal recurrence, even after curative resections,7 the present retrospective study was carried out to investigate the correlation between the magnitude of serosal changes and the incidence of peritoneal recurrence in order to determine the high-risk factors for peritoneal metastasis.
Patients and Methods
A cohort of 1109 patients with advanced stage, primary noncardia gastric adenocarcinoma8 were operated on (gastrectomy) between January 1997 and December 2007 in the Department of General Surgery of the Second Affiliated Hospital of Harbin Medical University. Patient pathological and operation reports together with subsequent follow-up clinical evaluation examination data were stored on our outpatient clinical computer database and available to us from remote terminals as required. The majority of the patients underwent total or partial gastrectomy together with a D2 lymphadenectomy performed by experienced surgeons, which closely followed the operation guidelines of the Japanese Research Society for Gastric Cancer.9 The exclusion criteria for analysis were gastric stump cancer or synchronous malignancy, tumors of the esophagogastric junction, any form of chronic inflammatory disease, the number of lymph nodes retrieved was <15, a definitive M1 classification, and peritoneal recurrence records that were erroneous. It is an indisputable fact that micrometastases are found in the peritoneal cavity of all PT4b stage cancers, and therefore these patients were excluded from the present study. All patients provided written informed consent for the study, which was approved by the Research Ethics Committee of Harbin Medical University.
Extent of Serosal Invasion
The Japanese Classification of Gastric Carcinoma27 was used as a guide to measure the width of the serosal invasion. Briefly, the entire serous membrane was visualized by opening the resected stomach along the lesser or greater curvature. The largest macroscopical change in the serosal surface was determined as the measurement (serosal invasion width) used in subsequent analysis. The changes were characterized thus: S0, no change (n = 770); Sn, maximum diameter of change ≤3 cm (n = 113); and Sw, maximum diameter of changes >3 cm (n = 226).
Follow-Up
All patients underwent periodic follow-up examinations and were assessed every 3 months during the first 2 years after surgery, then every 6 months for 3 years and afterwards yearly. During follow-up, patients were evaluated by physical examination, serum tumor markers (carcinoembryonic antigen, CA19-9 and CA125), endoscopy, chest radiography, abdominopelvic ultrasonography, and computed tomography. Survival duration was calculated from the time of surgery to death or the last follow-up date (December 31, 2011). The median follow-up duration was 52.2 months (range 3.8–119.4).
Peritoneal recurrence was determined by positive cytologic findings of ascitic fluid or by reoperative biopsy. Abdominal ultrasonography and computed tomography were performed for suspected clinical recurrence or because of an increase in tumor markers above pathological levels. The proof of peritoneal recurrence usually required sequential imaging that demonstrated progression of metastatic lesions. In rare situations, peritoneal recurrence was detected by the appearance of clinical signs such as intraabdominal mass or intractable intestinal obstruction. Tumors involving the ovaries (Kruchenberg tumors) and Douglas metastases detected clinically or radiologically were counted as peritoneal recurrences. Although some patients had multiple recurrence episodes, only the initial site of peritoneal recurrence was taken into consideration for statistical evaluations.
Statistical Analyses
SPSS (version 19.0; SPSS, Inc., Chicago, IL) was used for all statistical analyses. Data presented as percentages were compared using a Chi-square test; comparisons of 5-year survival rates with different variables among subgroups were analyzed using a log-rank Chi-square test; multivariate analysis was carried out to establish the hazard ratio with different variables. The Cox proportional hazard regression model was used to determine the optimal cutoff threshold for gastric lesion size. P-values <0.05 were considered to be statistically significant.
RESULTS
Threshold Size of Gastric Lesion
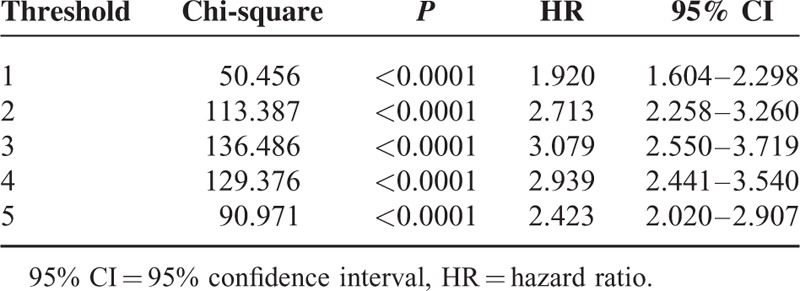
To determine the survival rates, changes in the serosal width threshold were calculated at 1 cm interval. The Cox proportional hazard regression model indicates that the optimal cutoff point is given by the largest Chi-square score determined. The most significant difference in survival rates was at the threshold value of 3 cm (Chi-square 136.486, P < 0.0001, HR 3.079, 95% CI 2.550–3.719) (Table 1).
TABLE 1.
Chi-Square Score and Hazard Ratio According to the Extent of Serosal Changes Calculated Using the Cox Proportional Hazard Regression Model
Correlations Between Clinical Parameters and Peritoneal Recurrence
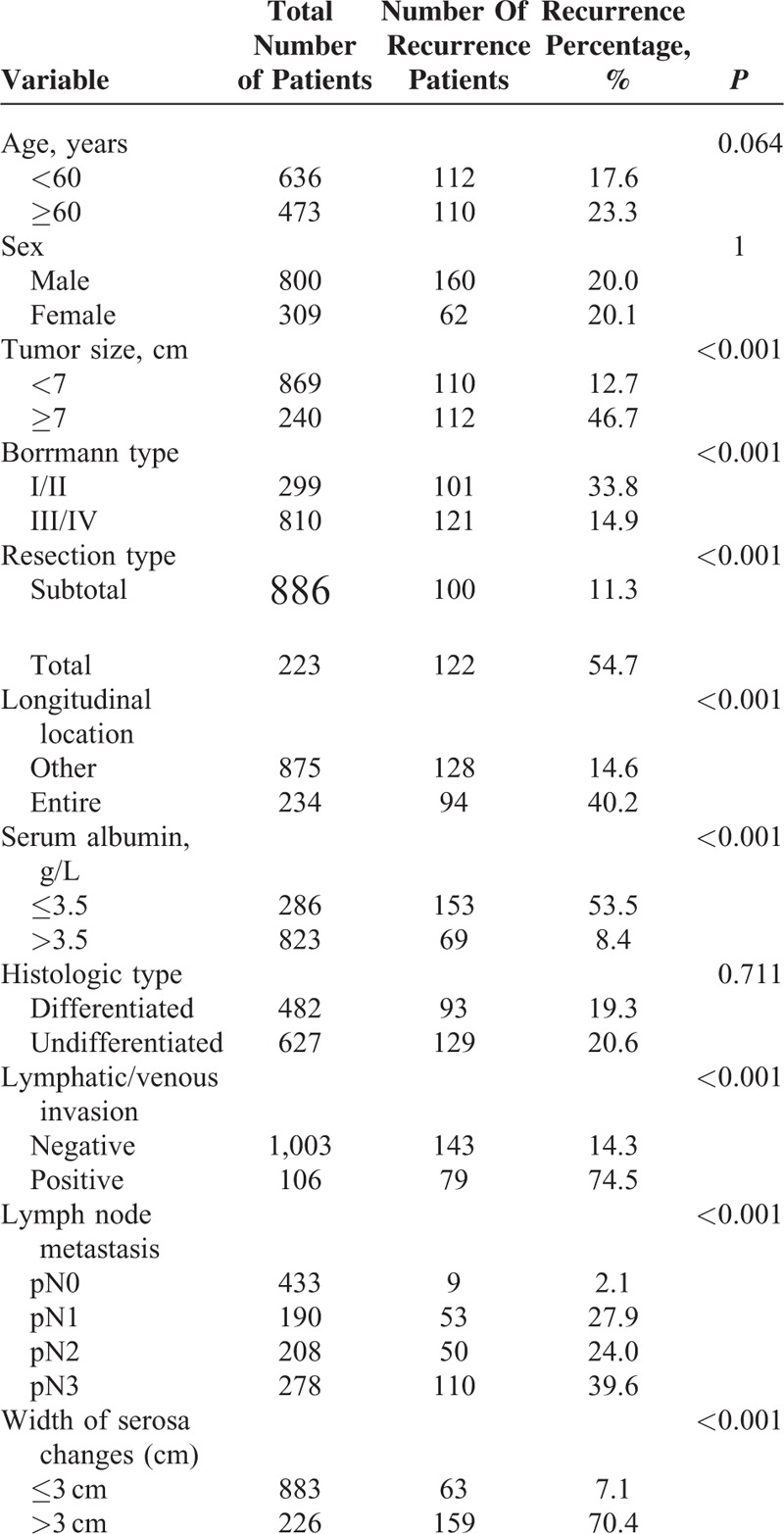
Peritoneal recurrence occurred in 222 of 1109 patients (20%). As shown in Table 2, peritoneal recurrence rates were correlated with tumor size, resection type, longitudinal location, serum albumin concentration, lymphatic/venous invasion, and lymph node metastasis status as well as the lesion size, but not with the histologically characterized type of tumor. Positive lymphatic/venous invasion and gastric lesion sizes >3 cm were strong indicators for the risk of recurrence. There was also a correlation between the recurrence rates, with Borrmann cancer types I and II being the most frequently detected cancers (P < 0.001).
TABLE 2.
Gastric Cancer Parameters and Peritoneal Recurrence Rates
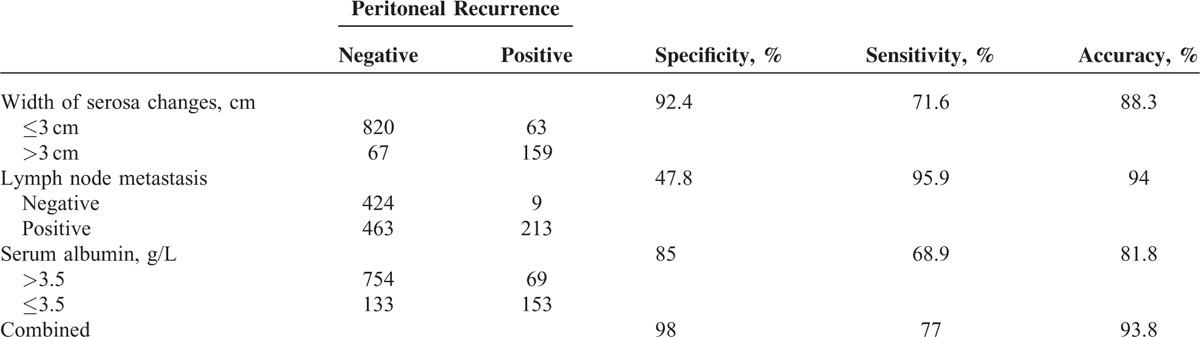
When comparing predictive values of serosa changes >3 cm, lymph node metastasis, and serum albumin concentrations ≤3.5 g/L for peritoneal recurrence, the specificity of serosa changes >3 cm was highest (92.4%), and the sensitivity (71.6%) and accuracy (88.3%) were higher than the sensitivity (68.9%) and accuracy (81.8%) of serum albumin levels, but lower than the sensitivity values (95.5%) and accuracy levels (94%) of lymph node metastasis. Combination of all 3 parameters for peritoneal recurrence prediction led to an accuracy of 93.8%, a sensitivity of 77%, and a specificity of 98% (Table 3).
TABLE 3.
Predictive Specificity, Sensitivity, and Accuracy Levels of Serosa Lesion Size, Lymph Node Metastasis, and Serum Albumin Concentrations for Predicting Peritoneal Recurrence
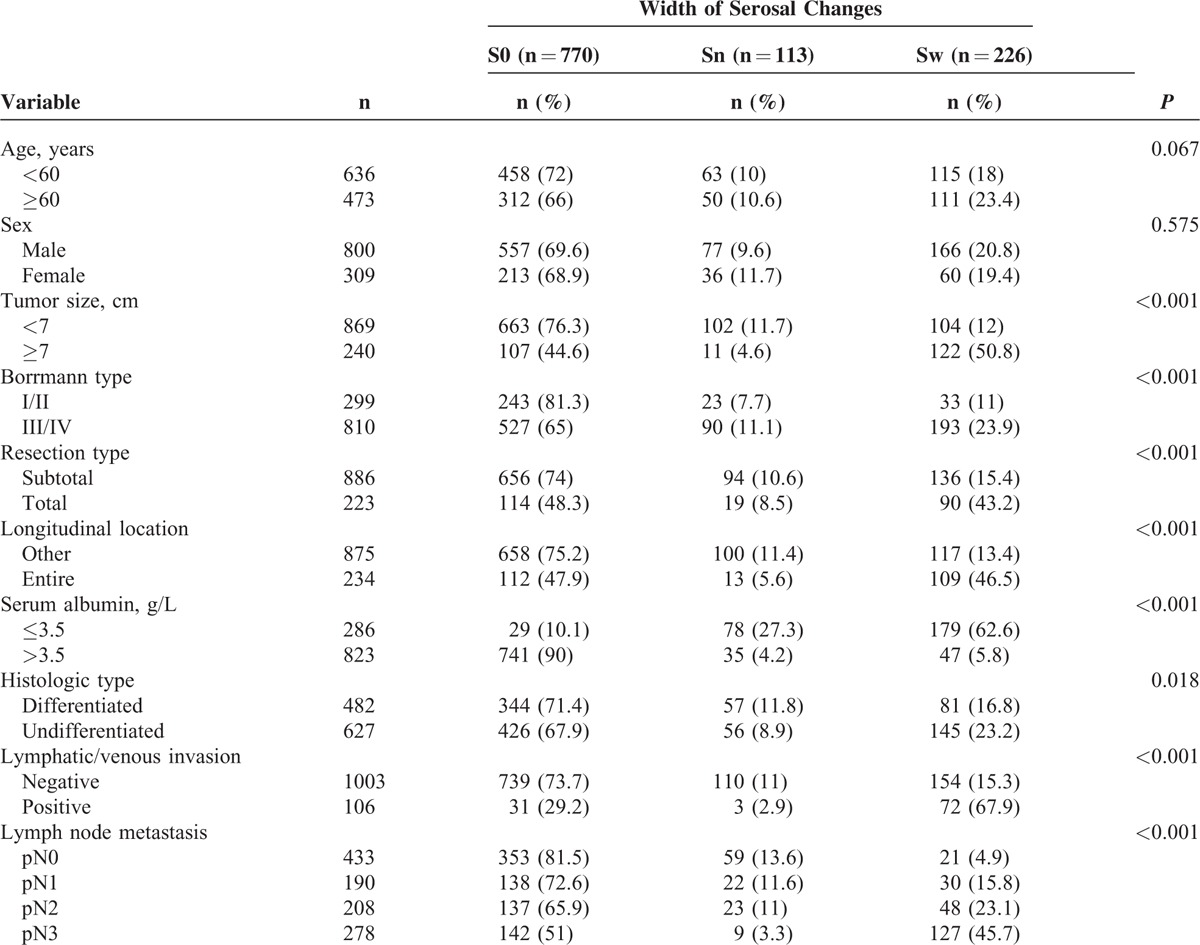
Width of Serosal Changes and Clinicopathological Parameters
When the S0 with the Sw patients were compared, all analyzed clinicopathological parameters correlated with the width of the serosal changes, indicating that serosal changes reflect the severity of gastric cancers (Table 4).
TABLE 4.
Associations Between Clinicopathologic Parameters and the Width of Serosal Changes in Patients With Advanced Gastric Cancer Who Underwent Curative Gastrectomy
Prognostic Factors for Patients With Advanced Stage gastric Cancers
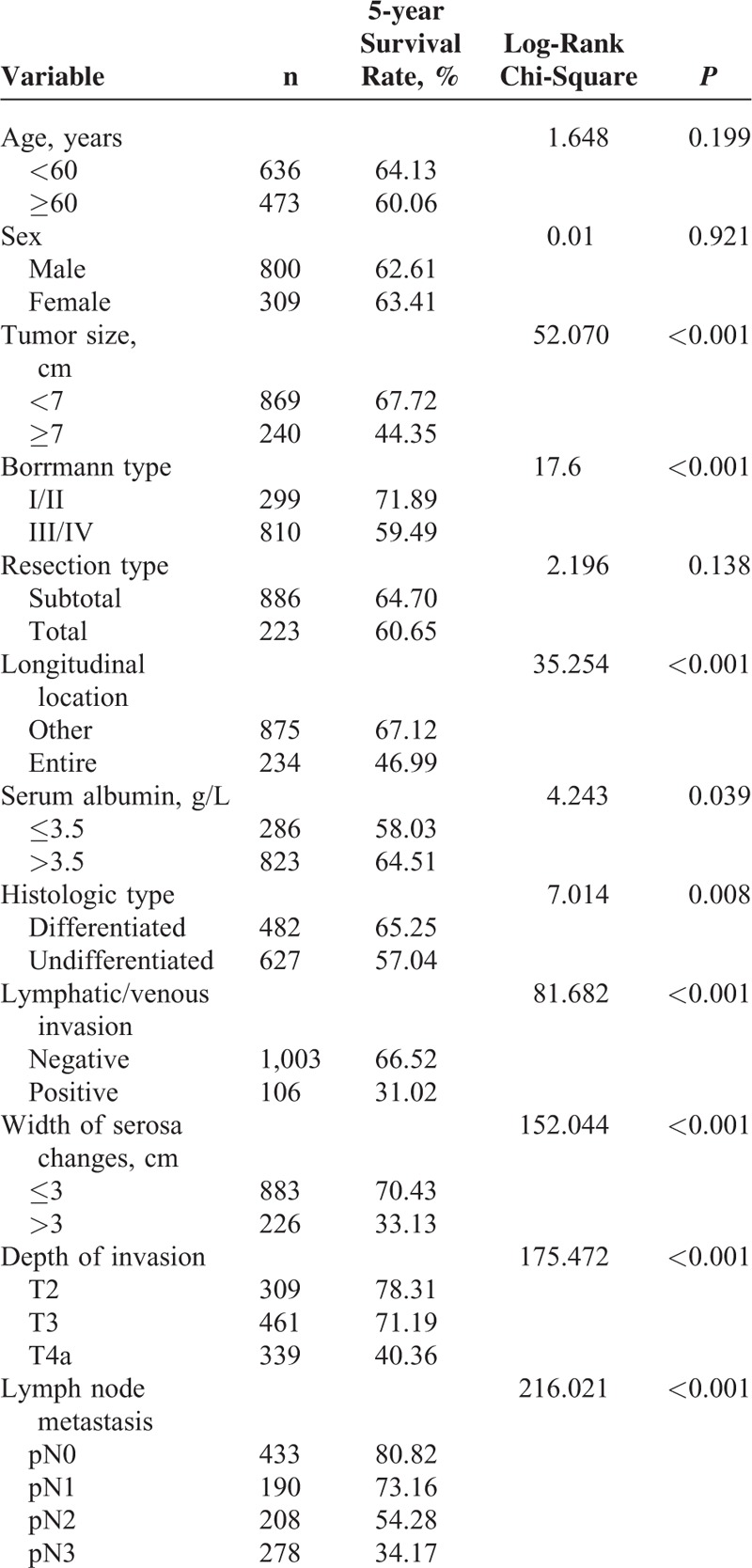
Single factor analysis for 5-year survival rates revealed that tumor size, the Borrmann cancer type, longitudinal location, serum albumin concentrations, histology type, lymphatic/venous invasion, width of the serosa changes, T staging, and lymph node metastasis staging, but not the resection type, correlated with the 5-year survival rate (Table 5).
TABLE 5.
Single Factor Analysis of Different Variables for 5-year Survival Rates of Patients With Advanced Gastric Cancer Who Underwent Curative Gastrectomy
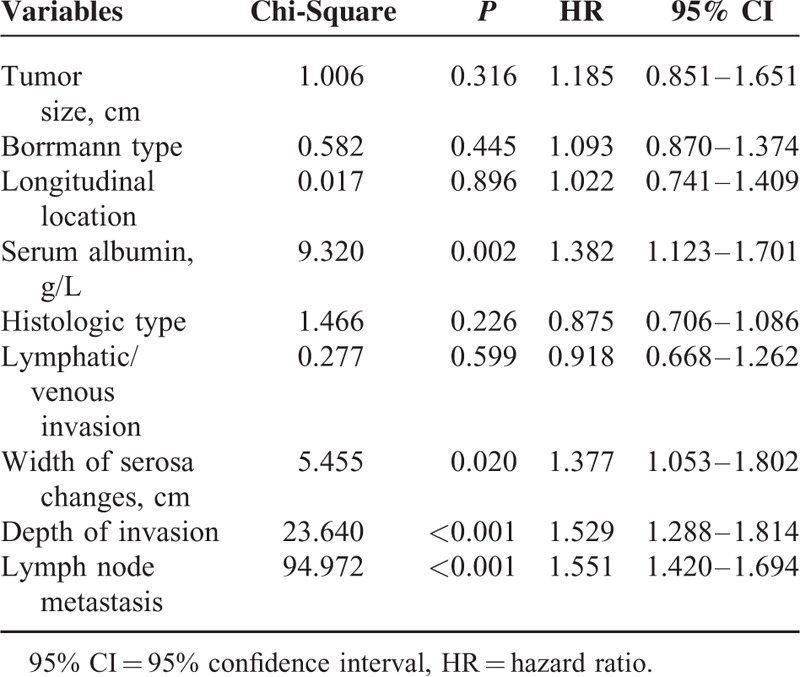
A further multivariate analysis revealed that only the serum albumin concentration, width of serosa changes, depth of tumor invasion, and lymph node metastasis correlated with the 5-year survival rate (Table 6).
TABLE 6.
Multivariate Analysis of Different Variables for the 5-year Survival Rates of Patients with Advanced Gastric Cancer Who Underwent Curative Gastrectomy
Prognostic Factors for Patients With Peritoneal Recurrence
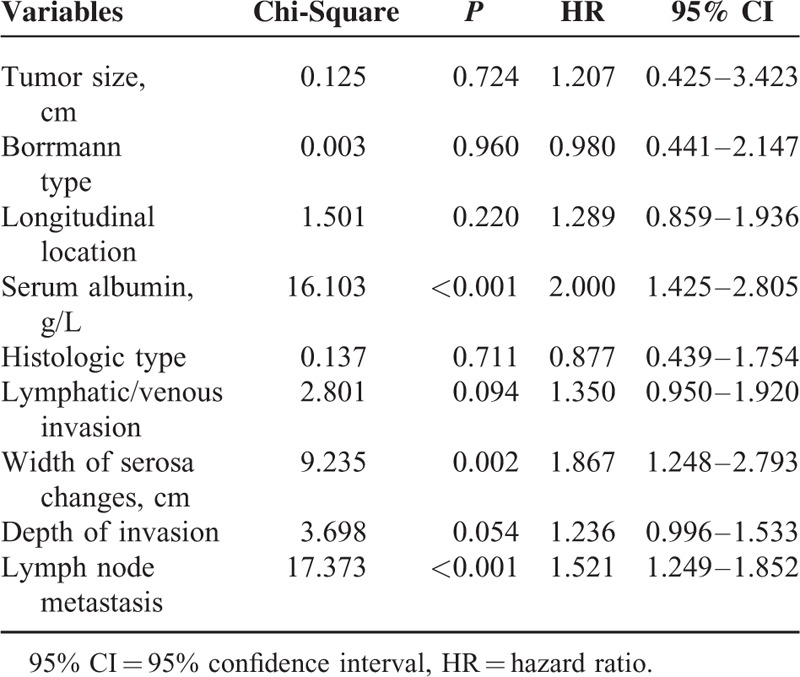
For peritoneal recurrence patients, single factor analyses for the 5-year survival rate revealed that the tumor size, Borrmann cancer type, longitudinal location, serum albumin concentrations, histology type, lymphatic/venous invasion, width of serosa changes, T staging, and lymph node metastasis staging, but not the resection type, correlated with the 5-year survival rate (Table 7). A multivariate analysis of the factors influencing the 5-year survival rate for patients with peritoneal recurrence showed that only the serum albumin concentration, lymph node metastasis, and width of the serosa changes correlated with 5-year survival (Table 8).
TABLE 7.
Single Factor Analysis of 5-year Survival Rate Factors for Patients With Advanced Gastric Cancer Who Underwent Curative gastrecTomy and Developed Peritoneal Recurrence
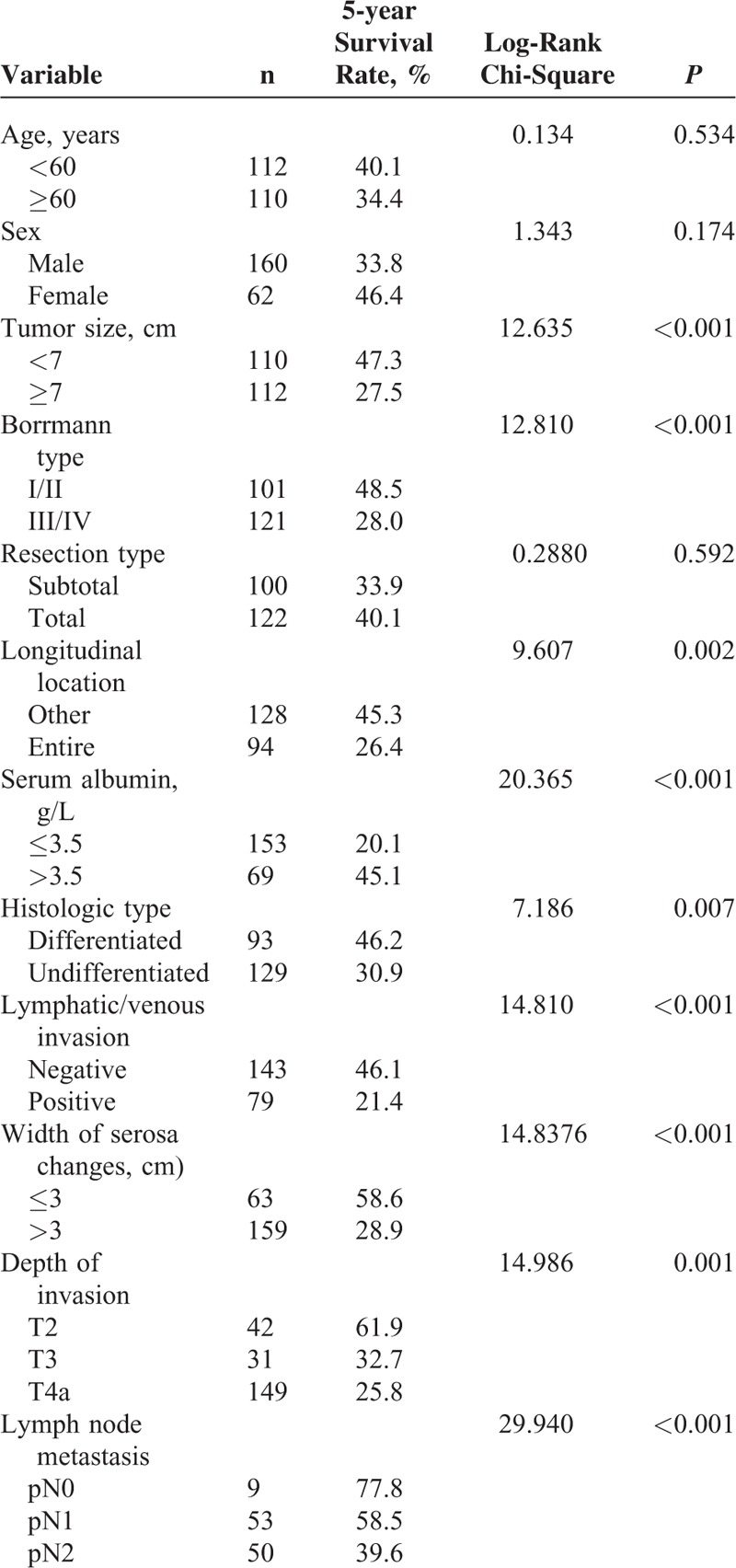
TABLE 8.
Multivariate Analysis of Factors for 5-year Survival Rates in Patients With Advanced Gastric Cancer That Underwent Curative Gastrectomy and Developed Peritoneal Recurrence
Taken together, the recurrence rate was higher in patients with serosal changes >3 cm (70.4%) than in patients with serosal changes ≤3 cm (7.1%) (Table 2), and the degree of the serosal changes indicated the severity of the gastric cancer (Table 4). In addition, our statistical evaluation of factors influencing the 5-year survival rate of patients with advanced stage gastric cancer showed that the width of the serosa changes correlated with the survival of all patients (Table 6), as well as recurrence patients (Table 7), which was revealed by the Kaplan–Meier curves of all patients (Fig. 1) and recurrence patients (Fig. 2).
FIGURE 1.
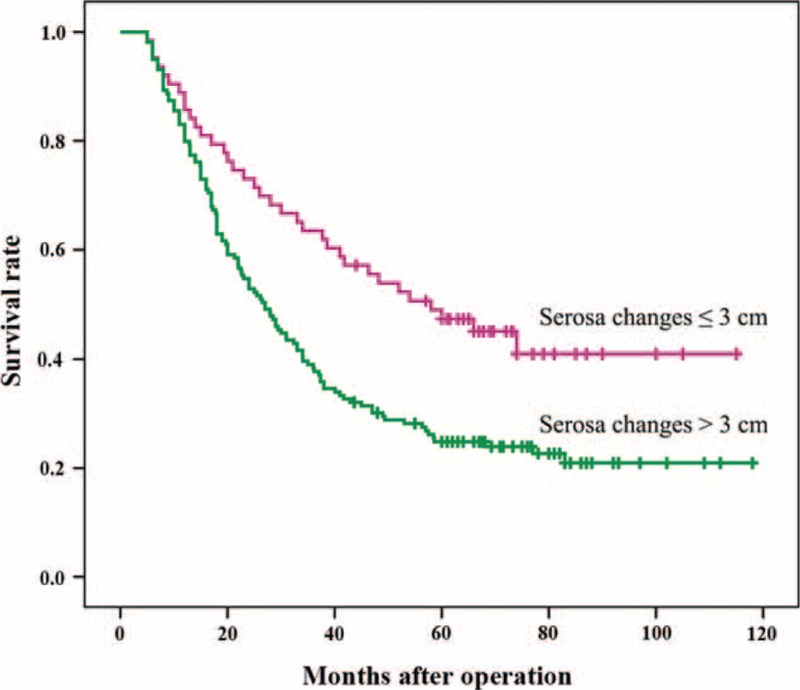
Kaplan–Meier curves of gastric cancer postintervention survival with indicated serosa changes at surgery. Horizontal marks (I) indicate censored values.
FIGURE 2.
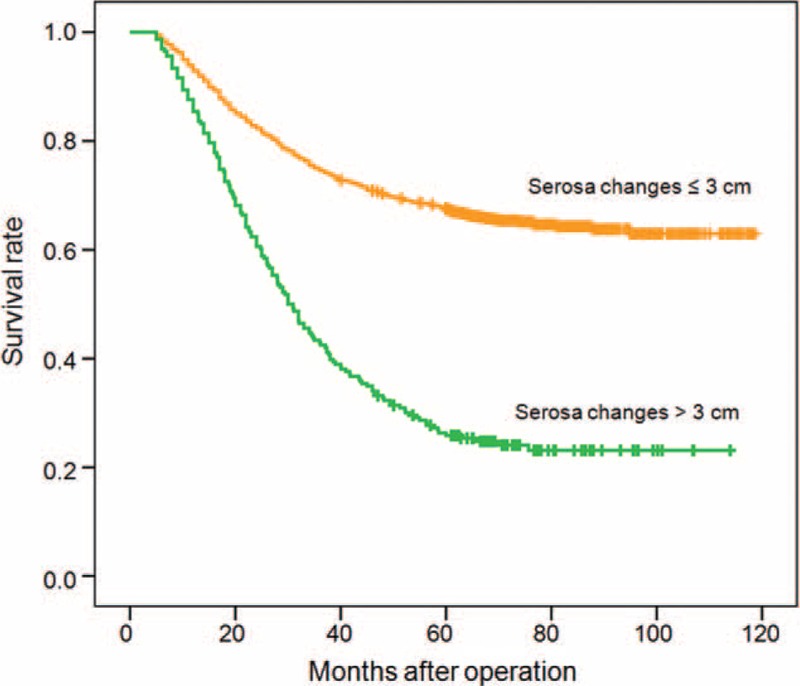
Kaplan–Meier curves of postinterventional survival with indicated serosa changes at surgery and peritoneal recurrence after gastric cancer treatments. Horizontal marks (I) indicate censored values.
DISCUSSION
Although the therapy of patients with locally advanced gastric cancer is continually improving, peritoneal recurrence remains a frequent occurrence in relapsed patients.10,11 When patients develop overt peritoneal metastasis after curative resection, the only therapeutic options are palliative surgery or adjuvant chemotherapy, but these treatments are difficult, demanding, and often unrewarding. Several authors have reported that normothermic intraperitoneal chemotherapy and hyperthermic intraperitoneal chemotherapy (HIPC) have some benefit in the prevention of peritoneal recurrence. One randomized trial of postoperative adjuvant intraperitoneal chemotherapy revealed a significant reduction in local and peritoneal recurrences in patients with advanced tumors who underwent normothermic intraperitoneal chemotherapy.12 Another randomized prospective study demonstrated that HIPC was capable of improving survival times in patients with more advanced stages of the disease.13 Other authors obtained similar results in favor of HIPC.14–17 A proposed strategy to diagnose peritoneal carcinomatosis after gastric cancer surgery is preoperative PLC to identify patients at very high risk before surgery.18 Fukagawa et al (2010)19 reported that if intraoperative lavage cytology is positive in type 4 gastric cancer patients the prognosis becomes so poor that multimodality therapy, including perioperative chemotherapy, is essential. Bonenkamp et al (1996)20 reported that cytological examination of abdominal washings increased the accuracy of staging and improved the selection of patients suitable for curative or palliative resection. In contrast, other studies noted that PLC is insensitive in predicting the development of peritoneal recurrence.5 In 2010, PLC was incorporated into the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system as a specific index of peritoneal dissemination. Patients with positive peritoneal cytology without peritoneal metastases are classified as M1, but the sensitivity of positive peritoneal cytology is low, owing to a considerable false negative rate, with the incidence of peritoneal recurrence ranging from 18% to 35%. It is widely accepted that a correlation exists between peritoneal recurrence and serosal invasion in patients with gastric cancer. Malignant cells exfoliated from the serosal lesions are the main reason for the development of peritoneal metastasis. Jeong et al21 reported that the overall accuracy of macroscopic diagnosis of serosal invasion was 88%, and its sensitivity and specificity was 82% and 89%, respectively, while the extent of macroscopic serosal invasion was proposed to be helpful in planning adjuvant chemotherapy.22 A few reports have shown that the width of serosal changes is a useful indicator for the prediction of peritoneal recurrence. Several authors have used 2.0, 2.5, or 3.0 cm as the cutoff points and reported that the prognosis of patients with extended serosal changes was significantly poorer than those with narrow serosal changes.23–25 Our study revealed that the width of serosal changes in gastric cancer patients is significantly correlated with the recurrence rate (P < 0.001) and the 5-year survival rate (P < 0.001) of surgically treated gastric cancer, as well as the 5-year survival rate of peritoneal recurrence patients (P < 0.001). Other significant correlations between the 5-year survival rate of postoperative gastric cancer patients were the serum albumin concentration ≤3.5 g/L, depth of invasion, and lymph node metastasis, findings in agreement with previously reported 5-year survival rates correlated with smaller serosal invasion and fewer metastatic nodes in stage IIIA gastric cancer patients.1 When data predicting peritoneal recurrence were combined (serosa lesion size >3 cm, lymph node metastasis, and serum albumin concentrations ≤3.5 g/L), the specificity became 98%, the sensitivity 77%, and accuracy 93.8%. Therefore, we suggest, in agreement with a previous study, that serosal changes might serve as a powerful additional indicator for prophylactic adjuvant chemotherapy for gastric cancer patients.26
In conclusion, based on our findings of correlations between the extent of gastric serosal changes and recurrence, as well as the 5-year survival rates in gastric cancer patients, we propose that the width of gastric serosal changes should be used as an additional indicator for prophylactic postoperative measures to prevent peritoneal recurrence, using adjuvant chemotherapies such as HIPC.
Acknowledgements
The authors thank the support from Postdoctoral Science Foundation of Heilongjiang Province (No. LBH-Z14136).
Footnotes
Abbreviations: HIPC = hyperthermic intraperitoneal chemotherapy, PLC = peritoneal lavage cytology.
YK and SL contributed equally to this work.
This work was supported by the Postdoctoral Science Foundation of Heilongjiang Province (No. LBH-Z14136).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer J Int Cancer 2015; 136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Maehara Y, Hasuda S, Koga T, et al. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg 2000; 87:353–357. [DOI] [PubMed] [Google Scholar]
- 3.Zhang XF, Huang CM, Lu HS, et al. Surgical treatment and prognosis of gastric cancer in 2,613 patients. World J Gastroenterol 2004; 10:3405–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe S, Yoshimura H, Tabara H, et al. Curative resection of gastric cancer: limitation of peritoneal lavage cytology in predicting the outcome. J Surg Oncol 1995; 59:226–229. [DOI] [PubMed] [Google Scholar]
- 5.Kang KK, Hur H, Byun CS, et al. Conventional cytology is not beneficial for predicting peritoneal recurrence after curative surgery for gastric cancer: results of a prospective clinical study. J Gastric Cancer 2014; 14:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ki YJ, Ji SH, Min JS, et al. Test execution variation in peritoneal lavage cytology could be related to poor diagnostic accuracy and stage migration in patients with gastric cancer. J Gastric Cancer 2013; 13:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boku T, Nakane Y, Minoura T, et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg 1990; 77:436–439. [DOI] [PubMed] [Google Scholar]
- 8.Deng J, Liang H, Sun D, et al. Suitability of 7th UICC N stage for predicting the overall survival of gastric cancer patients after curative resection in China. Ann Surg Oncol 2010; 17:1259–1266. [DOI] [PubMed] [Google Scholar]
- 9.Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg 1981; 11:127–139. [DOI] [PubMed] [Google Scholar]
- 10.D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004; 240:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roviello F, Marrelli D, de Manzoni G, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg 2003; 90:1113–1119. [DOI] [PubMed] [Google Scholar]
- 12.Yu W, Whang I, Chung HY, et al. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg 2001; 25:985–990. [DOI] [PubMed] [Google Scholar]
- 13.Yonemura Y, de Aretxabala X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology 2001; 48:1776–1782. [PubMed] [Google Scholar]
- 14.Chua TC, Morris DL, Esquivel J. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2010; 17:1330–1336. [DOI] [PubMed] [Google Scholar]
- 15.Witkamp AJ, de Bree E, Van Goethem R, et al. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev 2001; 27:365–374. [DOI] [PubMed] [Google Scholar]
- 16.Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 2007; 14:2702–2713. [DOI] [PubMed] [Google Scholar]
- 17.Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009; 27:6237–6242. [DOI] [PubMed] [Google Scholar]
- 18.Bentrem D, Wilton A, Mazumdar M, et al. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol 2005; 12:347–353. [DOI] [PubMed] [Google Scholar]
- 19.Fukagawa T, Katai H, Saka M, et al. Significance of lavage cytology in advanced gastric cancer patients. World J Surg 2010; 34:563–568. [DOI] [PubMed] [Google Scholar]
- 20.Bonenkamp JJ, Songun I, Hermans J, et al. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg 1996; 83:672–674. [DOI] [PubMed] [Google Scholar]
- 21.Jeong O, Ryu SY, Jeong MR, et al. Accuracy of macroscopic intraoperative diagnosis of serosal invasion and risk of over- and underestimation in gastric carcinoma. World J Surg 2011; 35:2252–2258. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda K, Shiraishi N, Inomata M, et al. Prognostic significance of macroscopic serosal invasion in advanced gastric cancer. Hepatogastroenterology 2007; 54:2028–2031. [PubMed] [Google Scholar]
- 23.Abe S, Shiraishi M, Nagaoka S, et al. Serosal invasion as the single prognostic indicator in stage IIIA (T3N1M0) gastric cancer. Surgery 1991; 109:582–588. [PubMed] [Google Scholar]
- 24.Baba H, Korenaga D, Haraguchi M, et al. Width of serosal invasion and prognosis in advanced human gastric cancer with special reference to the mode of tumor invasion. Cancer 1989; 64:2482–2486. [DOI] [PubMed] [Google Scholar]
- 25.Bando E, Kawamura T, Kinoshita K, et al. Magnitude of serosal changes predicts peritoneal recurrence of gastric cancer. J Am Coll Surg 2003; 197:212–222. [DOI] [PubMed] [Google Scholar]
- 26.Kim JM, Jung H, Lee JS, et al. Clinical implication of serosal change in pathologic subserosa-limited gastric cancer. World J Surg 2012; 36:355–361. [DOI] [PubMed] [Google Scholar]
- 27.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112. [DOI] [PubMed] [Google Scholar]


