Abstract
Behcet's disease (BD) is a multisystemic disorder of unknown etiology characterized by relapsing oral–genital ulcers, uveitis, and involvement of vascular, gastrointestinal, neurological, and musculoskeletal system. Although disease pathogenesis is still unclear, both innate and adaptive immunity have shown to play a pivotal role, and multiple proinflammatory cytokines seem to be involved in different pathogenic pathways that eventually lead to tissue damage.
The aims of our study were to evaluate serum cytokines levels of IL-8, IL-18, IFN-α2a, IL-6, IFN-γ, CXCL10, CXCL11, CXCL9, and SAA levels in patients with BD, in comparison to healthy controls (HC), and to correlate their levels to disease activity.
We included 78 serum samples obtained from 58 BD patients and analyzed a set of proinflammatory cytokines including IL-8, IL-18, IFN-α2a, IL-6, IFN-γ, CXCL10, CXCL11, and CXCL9 by multiplex bead analysis as well as SAA by enzyme-linked immunosorbent assay.
Compared to HC, BD patients showed elevated cytokine levels of IL-8, IL-18, IFN-α2a, and IL-6, and low levels of CXCL11. BD patients with SAA serum levels >20 mg/L showed higher levels of proinflammatory markers than HC or group with SAA ≤20 mg/L. IL-18, IFN-α2a, and IL-6 were higher in BD group with SAA >20 mg/L than HC, while IL-8 and CXCL9 levels were higher than in patients with SAA ≤20 mg/L and HC.
Active BD patients with SAA >20 mg/L exhibited elevated levels of inflammatory mediators, suggesting that may exist a relationship between SAA and proinflammatory cytokines in the intricate scenario of BD pathogenesis.
INTRODUCTION
Behcet's disease (BD) is a chronic systemic inflammatory syndrome at the crossroad between autoimmune and autoinflammatory diseases.1 Beside the classical symptoms represented by muco-cutaneous lesions and chronic relapsing bilateral uveitis, vascular, gastro-enteric, neurological, and joint involvement may also occur.2 Although disease pathogenesis is still unclear, both innate and adaptive immunity have shown to play a pivotal role.3–5 The recent understandings on cellular and molecular biology seem to suggest that an imbalance of the Th1/Th2 polarization in favor of Th1 would be able to generate an inflammatory process leading to a CD4+ T lymphocytes clonal expansion, producing high concentrations of both proinflammatory cytokines (such as IFN-γ, IL-6, and IL-8) and cytotoxic CD8+ cells. Moreover, enhanced circulating Th17 lymphocytes as well as increased levels of IL-17A have also been reported in BD.6 In addition, high levels of the classical Th1 type cytokine IFN-γ have been found in patients with ocular involvement.7 Similarly, IFN-γ secretion is stimulated by natural killer cells through IL-18 action closely relating to BD disease activity and local inflammatory response.8 Also, IFN-γ leads to IFN-γ-inducible CXCR3 ligands production (including CXCL9, CXCL10, and CXCL11), known to be involved in various angiogenesis-related disease as well as immunological disorders by inducing the recruitment of activated Th1 cells.9 In this regard, few data are available on the role of these chemokines in BD pathogenesis with the exception of CXCL10 chemokine, known to be involved in inducing inflammation in nervous system of BD patients.10–16 Not least in importance is the action of antigen-presenting cells (APCs), leading to inflammatory T-cell response as well as neutrophils activation which through the secretion of IL-18, IL-1, IL-6, IL-8, and TNF-a.6,13,16,17
To date, there are no specific laboratory clues to assess disease activity.18 In this regard, the acute phase protein serum amyloid-A (SAA), synthesized by the liver under the stimulus of proinflammatory cytokines such as IL-6, has been investigated as a potential laboratory marker for several rheumatologic disorders.19
Recently the role of SAA levels as a potential marker of disease activity in BD patients has been explored, and it has been reported that the occurrence of oral aphthosis, neurological and ocular disease are significantly associated with high serum levels of SAA.20
Therefore, the present study was aimed at investigating any potential correlation among cytokines profile, serum levels of SAA, and disease activity in BD patients.
MATERIALS AND METHODS
Patients
Seventy-eight serum samples were routinely collected from 58 BD patients (28 males, 30 females, mean age 44.7 ± 12.2 years) who met the International Study Group classification criteria for BD;21 the samples were collected every 3 to 4 months and in case of disease relapse.
Of these, 37 samples were obtained from patients with active disease and 41 from patients with inactive disease.
The primary aim of the study was to compare cytokines profile among patients with active BD, patients with inactive BD and healthy controls (HC); the secondary aims were to evaluate the levels of SAA according to BD activity; explore any potential correlation among levels of cytokines, SAA, and status of disease activity.
Patients were included in the active-BD group if they had at least 2 of the following BD-related clinical findings: uveitis, oral aphthosis, genital aphthosis, cutaneous disease, central nervous system involvement, vascular involvement, and gastrointestinal involvement. Samples were also obtained from 32 HC (6 males, 26 females, mean age 41 ± 8.3 years). Table 1 summarizes the clinical and demographic characteristics of BD patients.
TABLE 1.
Demographic, Laboratory, and General Clinical Characteristics of Patients Affected by Behcet's Disease Recruited in Our Study
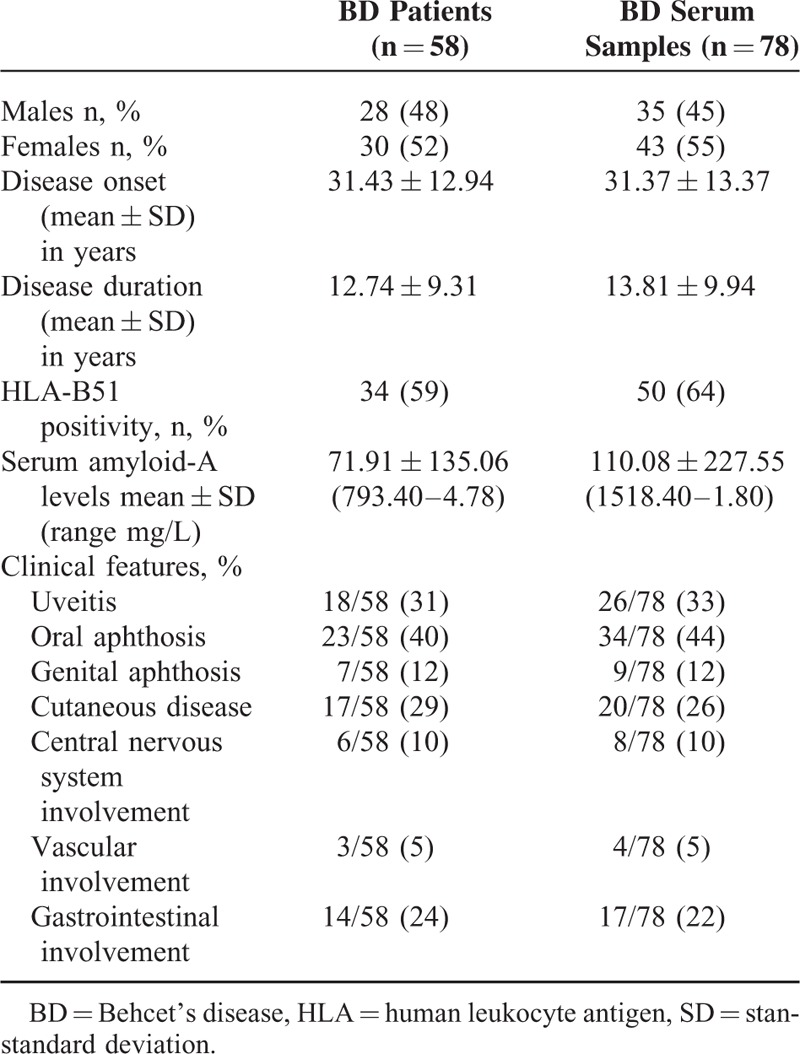
The endpoints of the study were: to determine serum levels of IL-8, IL-18, IFN-α2a, IL-6, IFN-γ, CXCL10, CXCL11, CXCL9, and SAA serum concentration every 3 to 4 months and in case of disease relapse; to correlate IL-8, IL-18, IFN-α2a, IL-6, IFN-γ, CXCL10, CXCL11, CXCL9, and SAA serum concentration with the status of disease activity.
Written informed consent was obtained both from patients and HC. The study protocol was reviewed and approved by the Ethical Committee of the Medical University of Bari. Demographic and clinical information was obtained through structured interview, review of medical records, physical examination, and laboratory tests.
Multiplex Bead Analysis
Serum cytokine levels of IL-8, IL-18, IFN-α2a, IL-6, IFN-γ, CXCL10, CXCL11, and CXCL9 were determined, using a Bio-Rad cytokine bead arrays according to the manufacturers’ instructions. Data analysis was performed using the Bioplex manager software 6.0 and results displayed as mean ± SD.
SAA Enzyme-Linked Immunosorbent Assay
SAA serum concentration was determined with a commercial solid phase sandwich enzyme-linked immunosorbent assay (Human SAA, BioSource Europe S.A., Belgium) according to the manufacturer's protocol. Although literature data suggest that SAA levels are elevated over the threshold of 10 mg/L,22 we referred in our analysis to a cut-off of 20 mg/L, since it represents the threshold of our laboratory.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5 software. Results were expressed as mean ± standard deviation. Analysis of variance was used to analyze differences between groups. Two-tailed Mann–Whitney U test (for 2 nonparametric groups) and Student's t-test (for 2 parametric groups) were used for statistical comparisons between groups. Significance was defined as P < 0.05. Correlations were calculated using Spearman correlation (2-tailed P-value) as well as Pearson correlation test when required.
RESULTS
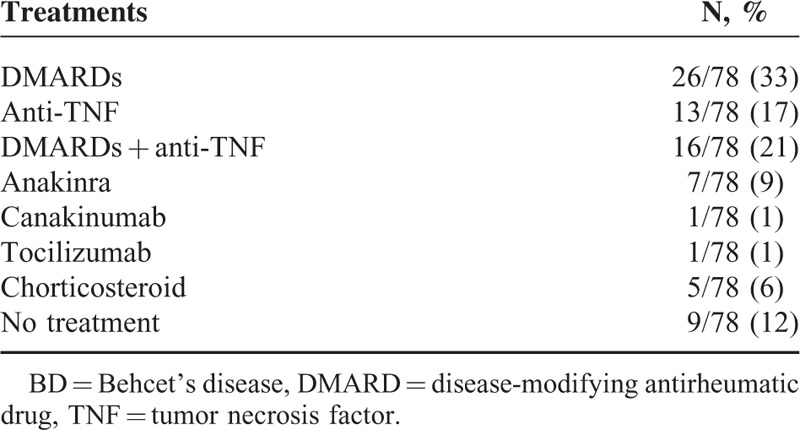
The characteristics of 78 serum samples obtained from 58 BD patients are summarized in Table 1. Specifically, 37/78 (47%) were collected from patients with active disease; moreover, 69/78 were treated at the time of serum collection. Anti-TNF as monotherapy (13/78, 17%) or in combination with disease-modifying antirheumatic drugs (16/78, 21%) and disease-modifying antirheumatic drugs monotherapy (26/78, 33%) were the commonest treatments at the time of serum collection. Table 2 summarizes the treatment at the time of serum collection.
TABLE 2.
BD Patients’ Therapy at the Time of Serum Collection
Cytokine levels of IL-8, IL-18, IFN-α2a, IL-6, IFN-γ, CXCL10, CXCL11, and CXCL9 were analyzed in 78 serum samples from BD patients and 32 HC. In BD patients serum concentrations of IL-8 (P = 0.0001), IL-18 (P = 0.0058), IFN-α2a (P = 0.0181), and IL-6 (P = 0.0233) were significantly higher than in HC. Conversely, CXCL11 resulted lower in BD than in HC (P = 0.0369), while no differences in CXCL10 and CXCL9 levels between BD and HC were found (Figure 1, Table 3). Detectable levels of IFN-γ were found in few samples both in HC and BD subjects, for this reason this cytokine was not included in the analysis. In addition statistical analysis by Spearman rho test showed significant correlations between serum concentration of IL-8 and IL-18 (r = 0.337, P = 0.002), IL-6 (r = 0.333, P = 0.004) serum levels, and strong correlation was found between IL-8 and IFN-α2a (r = 0.661, P < 0.0001). Moreover, also IL-6 serum levels positively correlated with IFN-α2a (r = 0.356, P = 0.0011) serum concentrations. In order to understand whether disease activity may affect cytokine profiles, we compared serum cytokines levels between active-BD (n = 37), inactive-BD (n = 41), and HC (n = 32) subjects. IL-8 and IL-18 resulted higher in both active-BD (P = 0.0001 and 0.012, respectively) and inactive-BD (P = 0.0001 and 0.0128, respectively) than in HC. No differences were observed in serum levels of CXCL10 and CXCL9 between patients and HC. Interestingly IFN-α2a (P = 0.0141) and IL-6 (P = 0.0332) serum levels were significantly higher in active-BD than HC. Moreover, CXCL11 serum concentration was significantly lower in inactive-BD than HC (P = 0.0154) (Figure 2, Table 3).
FIGURE 1.
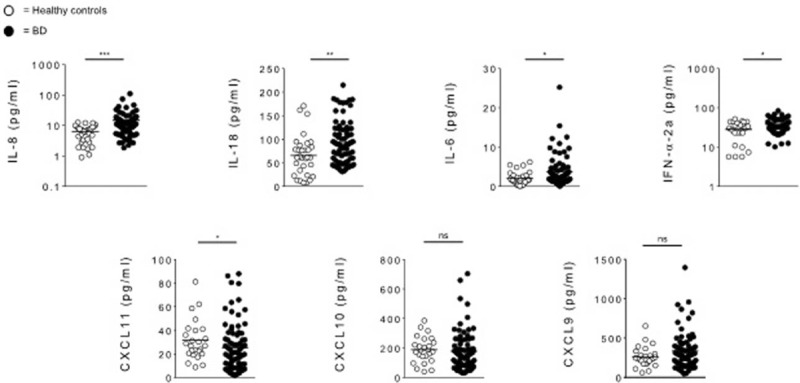
Serum cytokines levels in BD patients and HC. Circulating level of IL-8, IL-18, IL-6, IFN-α2a, CXCL11, CXCL10, and CXCL9 were analyzed in serum samples obtained from BD patients (n = 78) and HC (n = 32). Data are expressed as mean ± SD. Mann–Whitney U test and Student's t-test were carried out to check for statistical significance between groups (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.01, ns = not significant). BD = Behcet's disease, HC = healthy controls, SD = standard deviation.
TABLE 3.
Levels of Cytokines in HC and BD Patients
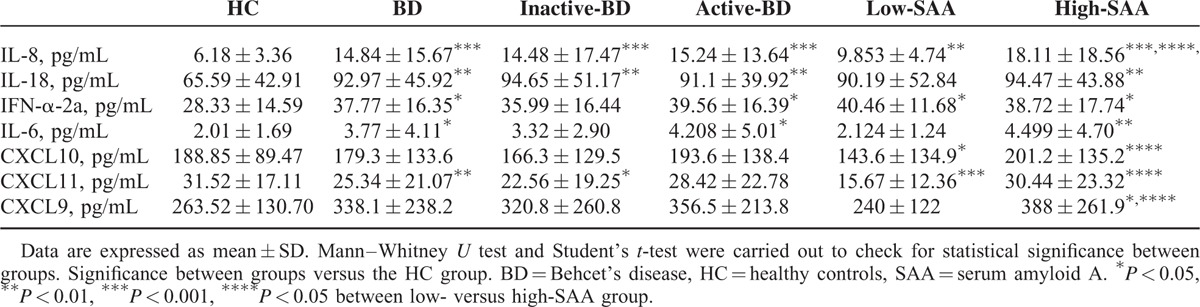
FIGURE 2.
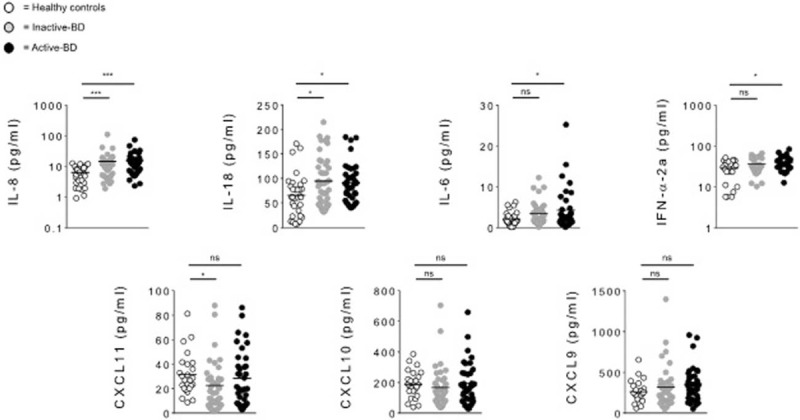
Serum cytokines levels in inactive-BD, active-BD, patients and HC. Circulating level of IL-8, IL-18, IL-6, IFN-α2a, CXCL11, CXCL10, and CXCL9 were analyzed in serum samples obtained from active-BD (n = 37), inactive-BD (n = 41), and HC (n = 32) subjects. Data are expressed as mean ± SD. Mann–Whitney U test and Student's t-test were carried out to check for statistical significance between groups (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.01, ns = not significant). BD = Behcet's disease, HC = healthy controls, SD = standard deviation.
When cytokines were evaluated in the active-BD group, serum IL-8 concentration positively correlated with IL-18 (r = 0.381, P = 0.02) and a strong correlation was found with IFN-α2a (r = 0.737, P < 0.0001) serum levels. In addition, IFN-α2a serum concentration also correlated positively with serum concentration of IL-6 (r = 0.513, P = 0.007).
When IL-8, IL-18, IFN-α2a, and IL-6 serum levels were compare between BD patients with SAA serum levels ≤20 mg/L (n = 20), >20 mg/L (n = 50) and HC (n = 32), patients with SAA levels below 20 mg/L showed significantly increased IL-8 (P = 0.002), IFN-α2a (P = 0.0213) and significantly decreased CXCL10 (P = 0.0205) and CXCL11 (P = 0.0004) serum levels compared to HC. Interestingly, no differences were observed in IL-18, IL-6, and CXCL9 serum levels between patients with SAA below 20 mg/L and HC. Patients with SAA >20 mg/L showed serum IL-8 levels significantly higher than patients with SAA ≤20 mg/L (P = 0.0298) and HC (P = 0.0001). Serum IL-18 (P = 0.0044), IFN-α2a (P = 0.0212), and IL-6 (P = 0.0042) levels were significantly higher than in HC, but comparable to BD subjects with SAA ≤20 mg/L. In addition, CXCL9 resulted higher when SAA was higher than 20 mg/L compared to HC (P = 0.032) and patients with serum levels ≤20 mg/L (P = 0.0242). Furthermore, we observed that CXCL10 and CXCL11 serum levels showed to be significantly higher when SAA levels were >20 mg/L than in patients with lower SAA levels (P = 0.0239 and 0.0123, respectively), but were comparable to HC group (Figure 3, Table 3).
FIGURE 3.
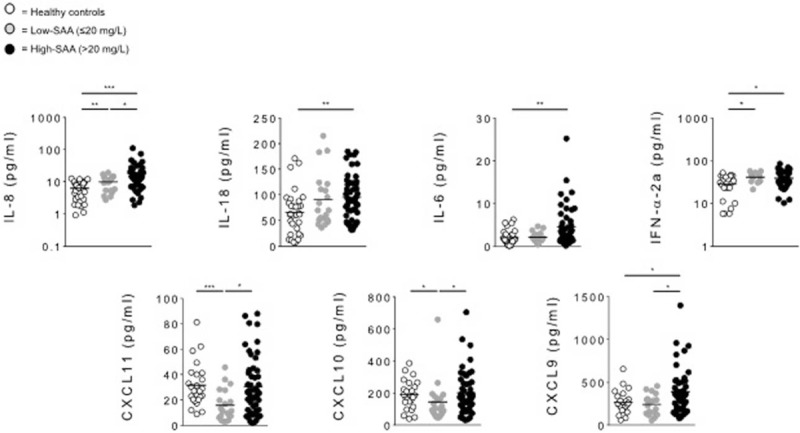
Serum cytokines levels in low-SAA BD, high-SAA BD patients, and HC. Circulating level of IL-8, IL-18, IL-6, IFN-α2a, CXCL11, CXCL10, and CXCL9 were analyzed in serum samples obtained from BD patients with SAA serum levels ≤20 mg/L (low-SAA, n = 20), >20 mg/L (high-SAA, n = 50), and HC (n = 32). Data are expressed as mean ± SD. Mann–Whitney U test and Student's t-test were carried out to check for statistical significance between groups (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.01). BD = Behcet's disease, HC = healthy controls, SAA = serum amyloid-A.
Finally, we found SAA serum levels positively correlated with IL-8 (r = 0.273, P = 0.022), IL-6 (r = 0.421, P = 0.0005), CXCL10 (r = 0.293, P = 0.014), CXCL11 (r = 0.318, P = 0.007), and CXCL9 (r = 0.336, P = 0.006) serum levels.
DISCUSSION
Our study was aimed at exploring any potential correlation among cytokines profile, serum levels of SAA and disease activity in BD patients. First of all, we analyzed serum levels of a core set of cytokines (IL-8, IL-18, IFN-α2a, IL-6, IFN-γ, CXCL10, CXCL11, and CXCL9) and SAA serum concentration.
BD is a complex inflammatory disorder characterized by an abnormal innate and adaptive immune response leading to hyper-activation of proinflammatory mediators. In the present study, in line with previously reported data,11,23,24 we found elevated levels of several inflammatory markers in BD patients compared to HC. In particular, BD appears associated to high levels of IL-8, IL-18, IL-6, and IFN-α especially in patients with high disease activity and SAA serum levels higher than 20 mg/L.
More specifically, IL-8 is known to be involved in the neutrophils activation as well as in leukocytes migration. In addition, it promotes the differentiation of mononuclear to granulocytic infiltration and the adhesion of peripheral blood leukocytes to endothelial cells. Several studies reported elevated IL-8 levels in serum from BD patients compared to HC, but discordant results were obtained when a relation to disease activity was investigated. In particular, IL-8 significantly correlate to specific BD clinical characteristic such as oral ulcers, skin lesions, and ocular involvement due to its role in the inflammatory response and its capability to attract polymorphonuclear cells into the lesions.11–16 However, these findings are controversial.15
IL-18 is a proinflammatory cytokines over-expressed in BD.24 It is principally produced by APCs and is involved in Th1 polarized immune response as well as in IL-6, IFN-α, and IL-12 production.6 Moreover, IL-18 seems to enhance chemokine productions, especially CXC, and induce INF-γ release by NK cells, suggesting a role also in innate responses. Although serum IL-18 levels have been observed to be not significantly different between active and inactive BD patients,25 we found that IL-18 correlates with disease activity. Notably, the detection of increased levels of IL-18 in patients with inactive disease might be explained by a smouldering subclinical inflammation during intercritical periods.
Interestingly, in our study serum IL-6 and IFN-α levels were significantly higher exclusively in patients with active disease compared to HC. IFN-α is a cytokine involved in BD pathogenesis presenting pleiotropic effects. Especially, IFN-α may significantly increase IFN-γ levels in memory CD4+ T cells and inhibit Th1 and Th17 cells.26 Several studies suggest that IFN-α promotes a regulatory Th1 response causing antiinflammatory effects mainly through an increase in soluble cytokine receptors, cytokine antagonists, and soluble adhesion molecules.27–29 Few data are available concerning IFN-α levels in BD. Kötter et al30 observed increased IFN-α serum levels in a group of BD patients with ocular involvement. More recently, it has been suggested that high serum IFN-α levels in BD patients might be due to increased frequency and activation of IFN-α+ plasmacytoid dendritic cells (pDC) resulting in Th1 type immune response.27
With regard to IL-6, it is mainly produced by activated macrophages and monocytes, but is also released by other cell types such as APCs and lymphocytes. IL-6, known to be associated to monocyte differentiation and CD4+ T cells differentiation into Th17 cells, is linked to BD activity.6,31,32 In addition, it also seems to play a critical role in BD patients presenting with central nervous system involvement,33 albeit it might have a protective role on mucocutaneous BD features.34
Since we recently demonstrated a correlation between increased SAA serum levels and various typical BD clinical manifestations;20 in the present, study we also looked for possible different cytokine patterns in patients with low and high SAA serum levels.
Noteworthy, we observed that BD patients with SAA higher than 20 mg/L showed intriguing characteristics in terms of proinflammatory cytokines serum levels. In particular, IL-18, IFN-α2a, and IL-6 were higher in patients with at least 20 mg/L SAA levels than in HC, while IL-8 and CXCL9 levels were higher in patients with SAA >20 mg/L than in patients with SAA ≤20 mg/L and in HC. Although SAA is mainly produced in the liver by proinflammatory stimuli such as IL-6, IL-1, and TNFα,35 also other cell types are known to produce SAA-including activated endothelial cells and monocytes/macrophages.36,37 Moreover, monocytes and monocytes derived macrophages have been reported to produce proinflammatory cytokines after SAA stimulation.38 Indeed, it has been recently demonstrated that SAA may stimulate the release of mature IL-1β from neutrophils, macrophages, and fibroblasts through inflammasome activation.39–41 Interestingly, the release of IL-8 as well as TNF-a from SAA-stimulated neutrophils was also reported.42 In addition, it has been observed that SAA induces neutrophil transmigration, monocyte migration, and peripheral blood mononuclear cells adhesion in an IL-8 and monocyte chemoattractant protein-1 dependent manner.43 The main limitations of our study are represented by the sample size that did not allow us to compare data according to specific organ involvement, and the absence of a disease control group, that could add more information regarding the disease specificity of the alterations observed. Moreover, at the time of samples collection, all of the patients were already taking corticosteroids and immunosuppressive agents, which might have affected cytokines serum levels.
In conclusion, our findings corroborate that BD patients exhibit elevated levels of specific inflammatory mediators, especially during active disease periods and in patients with increased SAA serum levels. Notably the increased levels of proinflammatory cytokines found in our study show that the immune response in BD is skewed toward a Th1 pathway. However, no obvious differences in cytokine profile between active and inactive-BD patients were found. This lack of differences may be due to various grounds such as different subsets of patients, admission criteria, besides the treatment regimens. For the above reason it is difficult to attribute an actual pathogenic role for each cytokine as regards the cytokines profile between active and inactive patients. Together with all the results from studies aimed at exploring, the pathogenetic role of cytokines in BD, these findings, may certainly contribute to improve our knowledge regarding specific target for novel therapies or for a different and more specific use of biologic drug currently available.44–48 However, although our suggestion of a possible SAA role in the induction of BD inflammatory manifestations is intriguing, further studies are needed both to better understand this pathogenetic perspective and to investigate the possible role of SAA inhibition in such patients. In order to confirm the results of our proof of concept study on the role of SAA, cytokines profile, and disease activity, a validation study on a larger cohort is ongoing.
Footnotes
Abbreviations: APC = antigen-presenting cell, BD = Behcet's disease, HC = healthy controls, PBMC = peripheral blood mononuclear cell, SAA = serum amyloid-A.
GL, OML, LC, and FI equally contributed to the work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Hatemi G, Yazici Y, Yazici H. Behcet's syndrome. Rheum Dis Clin North Am 2013; 39:245–261. [DOI] [PubMed] [Google Scholar]
- 2.Cantarini L, Lopalco G, Caso F, et al. Effectiveness and tuberculosis-related safety profile of interleukin-1 blocking agents in the management of Behcet's disease. Autoimmun Rev 2015; 14:1–9. [DOI] [PubMed] [Google Scholar]
- 3.Direskeneli H. Behcet's disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis 2001; 60:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yavuz S, Elbir Y, Tulunay A, et al. Differential expression of toll-like receptor 6 on granulocytes and monocytes implicates the role of microorganisms in Behcet's disease etiopathogenesis. Rheumatol Int 2008; 28:401–406. [DOI] [PubMed] [Google Scholar]
- 5.Nara K, Kurokawa MS, Chiba S, et al. Involvement of innate immunity in the pathogenesis of intestinal Behcet's disease. Clin Exp Immunol 2008; 152:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamzaoui K, Hamzaoui A, Guemira F, et al. Cytokine profile in Behcet's disease patients. Relationship with disease activity. Scand J Rheumatol 2002; 31:205–210. [DOI] [PubMed] [Google Scholar]
- 7.Guenane H, Hartani D, Chachoua L, et al. Production of Th1/Th2 cytokines and nitric oxide in Behcet's uveitis and idiopathic uveitis. J Fr Ophtalmol 2006; 29:146–152. [DOI] [PubMed] [Google Scholar]
- 8.Ben Ahmed M, Houman H, Miled M, et al. Involvement of chemokines and Th1 cytokines in the pathogenesis of mucocutaneous lesions of Behcet's disease. Arthritis Rheum 2004; 50:2291–2295. [DOI] [PubMed] [Google Scholar]
- 9.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 2011; 89:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saruhan-Direskeneli G, Yentür SP, Akman-Demir G, et al. Cytokines and chemokines in neuro-Behcet's disease compared to multiple sclerosis and other neurological diseases. J Neuroimmunol 2003; 145:127–134. [DOI] [PubMed] [Google Scholar]
- 11.Gür-Toy G, Lenk N, Yalcin B, et al. Serum interleukin-8 as a serologic marker of activity in Behcet's disease. Int J Dermatol 2005; 44:657–660. [DOI] [PubMed] [Google Scholar]
- 12.Mantas C, Direskeneli H, Oz D, et al. IL-8 producing cells in patients with Behcet's disease. Clin Exp Rheumatol 2000; 18:249–251. [PubMed] [Google Scholar]
- 13.Zouboulis CC, Katsantonis J, Ketteler R, et al. Adamantiades-Behcet's disease: interleukin-8 is increased in serum of patients with active oral and neurological manifestations and is secreted by small vessel endothelial cells. Arch Dermatol Res 2000; 292:279–284. [DOI] [PubMed] [Google Scholar]
- 14.Wang LM, Kitteringham N, Mineshita S, et al. The demonstration of serum interleukin-8 and superoxide dismutase in Adamantiades-Behcet's disease. Arch Dermatol Res 1997; 289:444–447. [DOI] [PubMed] [Google Scholar]
- 15.Sahin S, Akoğlu T, Direskeneli H, et al. Neutrophil adhesion to endothelial cells and factors affecting adhesion in patients with Behcet's disease. Ann Rheum Dis 1996; 55:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsantonis J, Adler Y, Orfanos CE, et al. Adamantiades-Behcet's disease: serum IL-8 is a more reliable marker for disease activity than C-reactive protein and erythrocyte sedimentation rate. Dermatology 2000; 201:37–39. [DOI] [PubMed] [Google Scholar]
- 17.Carletto A, Pacor ML, Biasi D, et al. Changes of neutrophil migration without modification of in vitro metabolism and adhesion in Behcet's disease. J Rheumatol 1997; 24:1332–1336. [PubMed] [Google Scholar]
- 18.Müftüoğlu AU, Yazici H, Yurdakul S, et al. Behcet's disease. Relation of serum C-reactive protein and erythrocyte sedimentation rates to disease activity. Int J Dermatol 1986; 25:235–239. [DOI] [PubMed] [Google Scholar]
- 19.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 1999; 265:501–523. [DOI] [PubMed] [Google Scholar]
- 20.Vitale A, Rigante D, Lopalco G, et al. Serum amyloid-A in Behcet's disease. Clin Rheumatol 2014; 33:1165–1167. [DOI] [PubMed] [Google Scholar]
- 21.Criteria for diagnosis of Behcet's disease. International Study Group for Behcet's Disease. Lancet 1990; 335:1078–1080. [PubMed] [Google Scholar]
- 22.Obici L, Merlini G. AA amyloidosis: basic knowledge, unmet needs and future treatments. Swiss Med Wkly 2012; 142:w13580. [DOI] [PubMed] [Google Scholar]
- 23.Curnow SJ, Pryce K, Modi N, et al. Serum cytokine profiles in Behcet's disease: is there a role for IL-15 in pathogenesis? Immunol Lett 2008; 121:7–12. [DOI] [PubMed] [Google Scholar]
- 24.Oztas MO, Onder M, Gurer MA, et al. Serum interleukin 18 and tumour necrosis factor-alpha levels are increased in Behcet's disease. Clin Exp Dermatol 2005; 30:61–63. [DOI] [PubMed] [Google Scholar]
- 25.Musabak U, Pay S, Erdem H, et al. Serum interleukin-18 levels in patients with Behcet's disease. Is its expression associated with disease activity or clinical presentations? Rheumatol Int 2006; 26:545–550. [DOI] [PubMed] [Google Scholar]
- 26.Touzot M, Cacoub P, Bodaghi B, et al. IFN-α induces IL-10 production and tilt the balance between Th1 and Th17 in Behçet disease. Autoimmun Rev 2015; 14:370–375. [DOI] [PubMed] [Google Scholar]
- 27.Pay S, Pekel A, Simsek I, et al. Pronounced interferon-alpha production from plasmacytoid dendritic cells in patients with Behcet's disease following CpG D ODN stimulation. Clin Exp Rheumatol 2009; 27:S37–S42. [PubMed] [Google Scholar]
- 28.Tugal-Tutkun I, Güney-Tefekli E, Urgancioglu M. Results of interferon-alfa therapy in patients with Behcet uveitis. Graefes Arch Clin Exp Ophthalmol 2006; 244:1692–1695. [DOI] [PubMed] [Google Scholar]
- 29.Deuter CM, Zierhut M, Möhle A, et al. Long-term remission after cessation of interferon-α treatment in patients with severe uveitis due to Behcet's disease. Arthritis Rheum 2010; 62:2796–2805. [DOI] [PubMed] [Google Scholar]
- 30.Kötter I, Koch S, Vonthein R, et al. Cytokines, cytokine antagonists and soluble adhesion molecules in patients with ocular Behcet's disease treated with human recombinant interferon-alpha2a. Results of an open study and review of the literature. Clin Exp Rheumatol 2005; 23:S20–S26. [PubMed] [Google Scholar]
- 31.Yamakawa Y, Sugita Y, Nagatani T, et al. Interleukin-6 (IL-6) in patients with Behcet's disease. J Dermatol Sci 1996; 11:189–193. [DOI] [PubMed] [Google Scholar]
- 32.Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003; 374:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akman-Demir G, Tüzün E, Içöz S, et al. Interleukin-6 in neuro-Behcet's disease: association with disease subsets and long-term outcome. Cytokine 2008; 44:373–376. [DOI] [PubMed] [Google Scholar]
- 34.Cantarini L, Lopalco G, Vitale A, et al. Paradoxical mucocutaneous flare in a case of Behçet's disease treated with tocilizumab. Clin Rheumatol 2015; 34:1141–1143. [DOI] [PubMed] [Google Scholar]
- 35.Uhlar CM, Grehan S, Steel DM, et al. Use of the acute phase serum amyloid A2 (SAA2) gene promoter in the analysis of pro- and anti-inflammatory mediators: differential kinetics of SAA2 promoter induction by IL-1 beta and TNF-alpha compared to IL-6. J Immunol Methods 1997; 203:123–130. [DOI] [PubMed] [Google Scholar]
- 36.Meek RL, Urieli-Shoval S, Benditt EP. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc Natl Acad Sci USA 1994; 91:3186–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada T, Wada A, Itoh K, et al. Serum amyloid A secretion from monocytic leukaemia cell line THP-1 and cultured human peripheral monocytes. Scand J Immunol 2000; 52:7–12. [DOI] [PubMed] [Google Scholar]
- 38.Song C, Hsu K, Yamen E, et al. Serum amyloid A induction of cytokines in monocytes/macrophages and lymphocytes. Atherosclerosis 2009; 207:374–383. [DOI] [PubMed] [Google Scholar]
- 39.Migita K, Izumi Y, Jiuchi Y, et al. Serum amyloid A induces NLRP-3-mediated IL-1β secretion in neutrophils. PLoS One 2014; 9:e96703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niemi K, Teirilä L, Lappalainen J, et al. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol 2011; 186:6119–6128. [DOI] [PubMed] [Google Scholar]
- 41.Migita K, Koga T, Satomura K, et al. Serum amyloid A triggers the mosodium urate -mediated mature interleukin-1β production from human synovial fibroblasts. Arthritis Res Ther 2012; 14:R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 2003; 101:1572–1581. [DOI] [PubMed] [Google Scholar]
- 43.Connolly M, Marrelli A, Blades M, et al. Acute serum amyloid A induces migration, angiogenesis, and inflammation in synovial cells in vitro and in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol 2010; 184:6427–6437. [DOI] [PubMed] [Google Scholar]
- 44.Selmi C, Ceribelli A, Naguwa SM, et al. Safety issues and concerns of new immunomodulators in rheumatology. Expert Opin Drug Saf 2014; 18:1–11. [DOI] [PubMed] [Google Scholar]
- 45.Caso F, Costa L, Rigante D, et al. Biological treatments in Behcet's disease: beyond anti-TNF therapy. Mediators Inflamm 2014; 2014:107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitale A, Rigante D, Caso F, et al. Inhibition of interleukin-1 by canakinumab as a successful mono-drug strategy for the treatment of refractory Behcet's disease: a case series. Dermatology 2014; 228:211–214. [DOI] [PubMed] [Google Scholar]
- 47.Cantarini L, Vitale A, Scalini P, et al. Anakinra treatment in drug-resistant Behcet's disease: a case series. Clin Rheumatol 2015; 34:1293–1301. [DOI] [PubMed] [Google Scholar]
- 48.Saygin C, Uzunaslan D, Hatemi G. Currently used biologic agents in the management of Behcet's syndrome. Curr Med Chem 2015; 22:1976–1985. [DOI] [PubMed] [Google Scholar]


