Abstract
Tubulointerstitial fibrosis and tubular atrophy play a crucial role in the pathogenesis of chronic kidney disease (CKD). They are also major determinants in chronic kidney disease development and progression in patients with primary renal diseases characterized by persistent or recurrent proteinuria. The purpose of the study was to assess urinary excretion of alpha-glutathione S-transferase (alpha-GST), pi-glutathione S-transferase (pi-GST), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and serum NGAL level in children with idiopathic nephrotic syndrome (INS). Patients and methods: the study group comprised of 39 children with INS and the control group consisted of 20 healthy children. A total of 23 patients were affected with steroid-dependent nephrotic syndrome (SDNS) and 16 with steroid-resistant nephrotic syndrome (SRNS). In the majority of patients, a histopathologic examination revealed minimal change disease (MCD)—25 (64%). Focal segmental glomerulosclerosis (FSGS), mesangioproliferative glomerulonephritis (MesPGN), membranoproliferative glomerulonephritis (MPGN), and membranous glomerulonephritis (MGN) were diagnosed in 4 (10.3 %), 6 (15.5%), 2 (5.1%), and 2 (5.1%) children, respectively. Urinary alpha-GST, urinary pi-GST, urinary KIM-1, and urinary and serum NGAL concentrations were measured using specific enzyme-linked immunosorbent assay. The urinary results were expressed in nanograms per milligram of creatinine (ng/mg). Results: The authors observed significantly higher levels of urinary alpha-GST/creatinine ratio (P = 0.03), urinary KIM-1/creatinine ratio (P < 0.02), serum NGAL level (P < 0.01), and urinary NGAL/creatinine ratio (P = 0.02) in children with INS compared with controls. The median values of urinary pi-GST/creatinine ratio in children with INS and controls did not differ significantly. In children with SRNS, the median values of urinary NGAL/creatinine ratio (P = 0.02) and urinary KIM-1/creatinine ratio (P = 0.02) were significantly higher compared with children with SDNS. The authors noted significant positive correlation between KIM-1/creatinine ratio and proteinuria (r = 0.56, P < 0.05). The analysis of alpha-GST/creatinine ratio, pi-GST/creatinine ratio, sNGAL, and uNGAL/creatinine ratio concerning the histopathologic examination, the duration of the disease, and number of relapses did not show any significant differences. Conclusions: 1. Both children with SDNS and those with SRNS were characterized by increased tubular injury marker levels. 2. Patients with SRNS and higher proteinuria are more susceptible to early kidney damage.
INTRODUCTION
Tubulointerstitial fibrosis and tubular atrophy play a crucial role in the pathogenesis of chronic kidney disease (CKD).1 They are also major determinants in CKD development and progression in patients with primary renal diseases characterized by persistent or recurrent proteinuria. Early detection of tubulointerstitial fibrosis may result in more favorable outcome of CKD, because nephroprotective treatment may be instituted in due course. In recent years, numerous studies on novel early markers of tubulointerstitial fibrosis have been published.
One of early markers of tubulointerstitial fibrosis is glutathione S-transferase (GST). It is a cytosolic enzyme. There are many isoforms of GST. The alpha and pi isoforms of GST (alpha-GST, pi-GST) are typical of human kidney.2 The alpha-GST is expressed in proximal tubular epithelial cells, whereas the pi-GST is specific of distal tubular epithelial cells. Consequently, positive correlations between urinary alpha-GST or pi-GST excretions and severity of proximal or distal tubular injury were observed.3 Increased urinary GSTs4 excretions were reported in an early phase of acute tubular injury caused by various toxic substances5–7 and after cardiac surgery.8,9 Recent studies showed that elevated urinary GSTs excretions are also an early marker of tubular injury in diabetic nephropathy,10,11 glomerulopathies,12 and obesity-related nephropathy.13
Another early and sensitive marker of tubulointerstitial fibrosis is neutrophil gelatinase-associated lipocalin (NGAL). Neutrophil gelatinase-associated lipocalin is a small (25 kd) protein released from renal tubular epithelial cells following acute kidney injury (AKI). Recent studies, however, demonstrated that serum NGAL level and its urinary excretion were also higher in patients with various chronic nephropathies and correlated with their activity and renal function.14–16 In addition, it was revealed that elevated baseline serum NGAL level was a risk factor for CKD progression.17–20 Neutrophil gelatinase-associated lipocalin was also documented to be an early and sensitive marker of tubulointerstitial fibrosis in glomerular diseases.14,20
Recently, much of an interest was taken in kidney injury molecule-1 (KIM-1). It is a transmembrane tubular cell protein of uncertain function. It is not expressed in normal kidney but is markedly upregulated in proximal tubular epithelial cells in experimental and clinical kidney damage.21,22 An elevated urinary KIM-1 excretion was observed in AKI and CKD, and in patients with renal transplant rejection. Kidney injury molecule-1 was also suggested to be an indicator of conversion of AKI to CKD.23 Waanders et al24 reported increased urinary KIM-1 excretion in proteinuric nondiabetic patients. In addition, clinical studies revealed that KIM-1 was an early marker of tubulointerstitial fibrosis in patient with primary and secondary glomerulopathies22,25,26 and in those with congenital hydronephrosis.27 Elevated urinary KIM-1 excretion was also observed in patients with renal scarring because of vesicoureteral reflux.30 Vaidya et al25,26 showed that urinary KIM-1 excretion reflected better severity of tubulointerstitial fibrosis than urinary N-acetyl-β-d-glucosaminidase excretion.
PURPOSE OF THE STUDY
The purpose of the study was to assess urinary excretion of alpha-GST, pi-GST, NGAL, KIM-1, and serum NGAL level in children with idiopathic nephrotic syndrome (INS). The correlations of measured parameters with histopathologic diagnosis, magnitude of proteinuria, duration of the disease, and number of relapses were determined. Steroid-dependent and steroid-resistant patients were compared with respect to measured parameters. Similar comparison was made between children treated and those nontreated with cyclosporine A.
Patients, Material, and Methods
Patients
Baseline characteristics of patients and controls are presented in Table 1. The study comprised 39 children (33 boys and 6 girls) aged 4 to 18 years (median: 13.7 years) with INS treated in the Department of Pediatric Nephrology, Children's University Hospital in Lublin, Poland. A total of 23 patients were affected with steroid-dependent nephrotic syndrome (SDNS) and 16 with steroid-resistant nephrotic syndrome (SRNS). Pathologic proteinuria was observed in 12/16 (75%) children with SRNS. Patients with SDNS were in remission of proteinuria. In the majority of patients, a histopathologic examination revealed minimal change disease (MCD)—25 (64%). Focal segmental glomerulosclerosis (FSGS), mesangioproliferative glomerulonephritis (MesPGN), membranoproliferative glomerulonephritis (MPGN), and membranous glomerulonephritis (MGN) were diagnosed in 4 (10.3 %), 6 (15.5%), 2 (5.1%), and 2 (5.1%) children, respectively. All patients received glucocorticosteroids at typical doses. There were no significant correlations between corticosteroid dose and urinary excretion of enzymes (r = 0.2, P > 0.05). A total of 18 patients were treated with cyclosporine A for 2 to 4 years. The median duration of INS was 6 years (0.25–14 years) and the median numbers of relapses were 6 (2–13). All children had normal estimated glomerular filtration rate calculated by the Schwartz formula: 0.55 × body height (cm)/serum creatinine level (mg/dL).
TABLE 1.
Characteristics of Study and Control Groups

The age- and sex-matched 20 healthy children (median: 12.3 years) were controls. They were observed in the outpatient clinic of Children's University Hospital in Lublin, Poland.
Methods
The midstream first morning urine specimen and serum sample were collected from each study participant on the same day.
The urinary enzyme excretions were expressed as enzyme/creatinine ratio in nanograms per milligram of creatinine (ng/mg). Similarly, the urinary protein excretion was expressed as protein/creatinine ratio in milligram per milligram of creatinine (mg/mg).
Routine laboratory techniques were used to measure proteinuria and concentrations of serum and urinary creatinine. Urinary alpha-GST (USCNK, China), urinary pi-GST (Immundiagnostik AG, Germany), urinary KIM-1, and urinary and serum NGAL (USCNK, China) concentrations were measured using specific enzyme-linked immunosorbent assay kits after prior preparation of urine and serum samples following the manufacturer's instructions.
The statistical analysis was performed using STATISTICA 7.1. Differences between groups were assessed using Mann–Whitney test and correlation coefficients were calculated using Spearman test. P ≤ 0.05 was considered significant.
RESULTS
The results of urinary excretion of alpha-GST, pi-GST, NGAL, KIM-1, and serum NGAL levels are shown in Table 2.
TABLE 2.
The Results of Urinary Excretion of Alpha-GST, Pi-GST, NGAL, KIM-1, and Serum NGAL Levels in Study and Control Groups
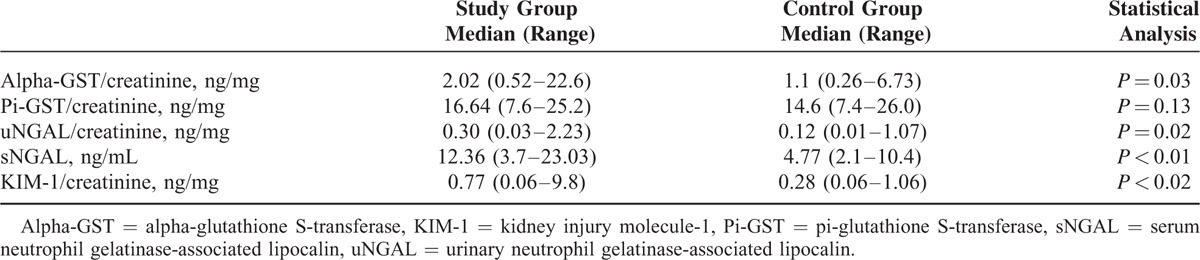
The median value of urinary alpha-GST/creatinine ratio was significantly higher in children with INS than that in controls (P = 0.03). The median values of urinary pi-GST/creatinine ratio in children with INS and controls did not differ significantly.
The median values of urinary NGAL/creatinine ratio (P = 0.02) and serum NGAL level (P < 0.01) were significantly higher in children with INS than those in controls.
Children with INS were characterized by significantly higher median value of urinary KIM-1/creatinine ratio (P < 0.02) compared with controls.
There were no significant differences in the median values of serum NGAL level and urinary excretions of alpha-GST, pi-GST, NGAL, and KIM-1 between children with MCD and those with other glomerulopathies (P = 0.1, P = 0.09, P = 0.1, P = 0.07, and P = 0.07, respectively).
The median values of protein/creatinine ratios in children with SDNS and those with SRNS were 0.13 (0.09–0.19 mg/mg) and 1.1 (0.4–3.5 mg/mg), respectively. There was a significant positive correlation between urinary KIM-1/creatinine ratio and protein/creatinine ratio in patients with pathologic proteinuria (r = 0.84, P < 0.01; Fig. 1). Urinary alpha-GST/creatinine ratio, urinary pi-GST/creatinine ratio, urinary NGAL/creatinine ratio, and serum NGAL level did not correlate significantly with protein/creatinine ratio (Table 3). In patients with pathologic proteinuria, significant correlations between duration of INS and urinary alpha-GST/creatinine ratio, urinary pi-GST/creatinine ratio, urinary NGAL/creatinine ratio, urinary KIM-1/creatinine ratio, and serum NGAL level were not observed. The duration of INS and number of relapses did not correlate significantly with urinary alpha-GST/creatinine ratio, urinary pi-GST/creatinine ratio, urinary NGAL/creatinine ratio, serum NGAL level, and urinary KIM-1/creatinine ratio (Table 3).
FIGURE 1.

The correlation between urinary kidney injury molecule-1/creatinine ratio and magnitude of proteinuria in steroid-resistant nephrotic syndrome patients.
TABLE 3.
The Correlation Between Concentration of Alpha-GST, Pi-GST, KIM-1, and NGAL and Magnitude of Proteinuria, Duration of the Disease, and Number of Relapses in Children with INS

There were also no significant differences in the median values of urinary alpha-GST/creatinine ratio, urinary NGAL/creatinine ratio, urinary KIM-1/creatinine ratio, and serum NGAL level between children with MCD and those with other glomerulopathies (P > 0.05). In children with SRNS, the median values of urinary alpha-GST/creatinine ratio, urinary NGAL/creatinine ratio, urinary KIM-1/creatinine ratio, and serum NGAL level were significantly higher than those in controls whereas patients with SDNS displayed significantly elevated median values of urinary alpha-GST/creatinine ratio and urinary KIM-1/creatinine ratio compared with controls (Table 4). In addition, in children with SRNS, the median values of urinary NGAL/creatinine ratio (P = 0.02) and urinary KIM-1/creatinine ratio (P = 0.02) were significantly higher compared with those with SDNS (Figs. 2 and 3; Table 4), whereas the median values of urinary alpha-GST/creatinine ratio and urinary pi-GST/creatinine ratio in these 2 groups of children did not differ significantly. Similarly, there was no significant difference between the median values of serum NGAL level in children with SRNS and those with SDNS (Table 4).
TABLE 4.
The Results of Concentrations of Alpha-GST, Pi-GST, KIM-1, and NGAL in SDNS, SRNS, and Controls
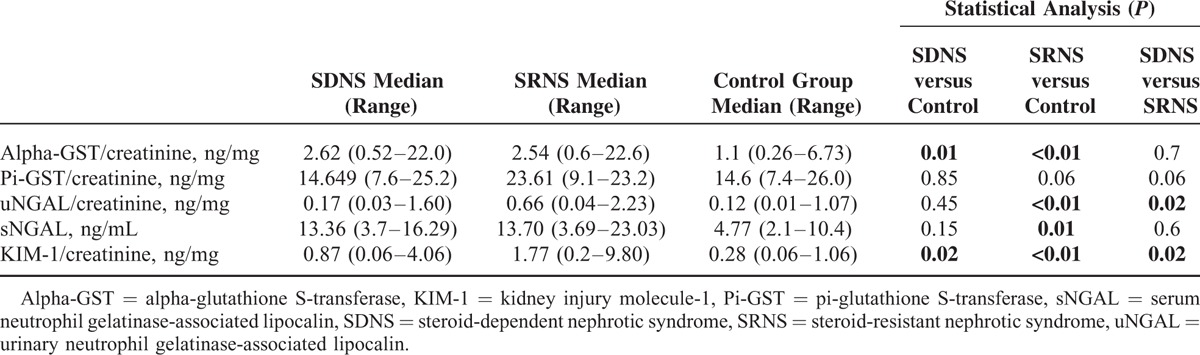
FIGURE 2.
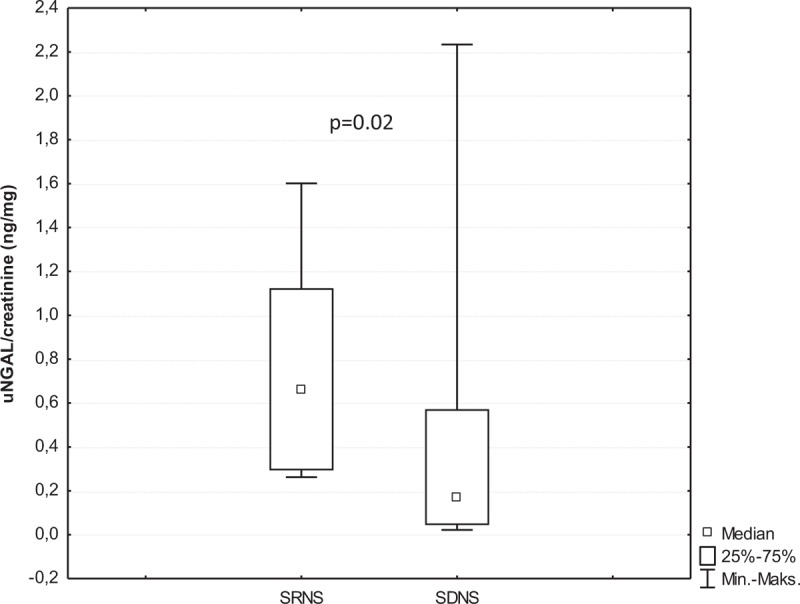
The comparison of urinary neutrophil gelatinase-associated lipocalin /creatinine ratios between patients with steroid-resistant nephrotic syndrome and steroid-dependent nephrotic syndrome.
FIGURE 3.
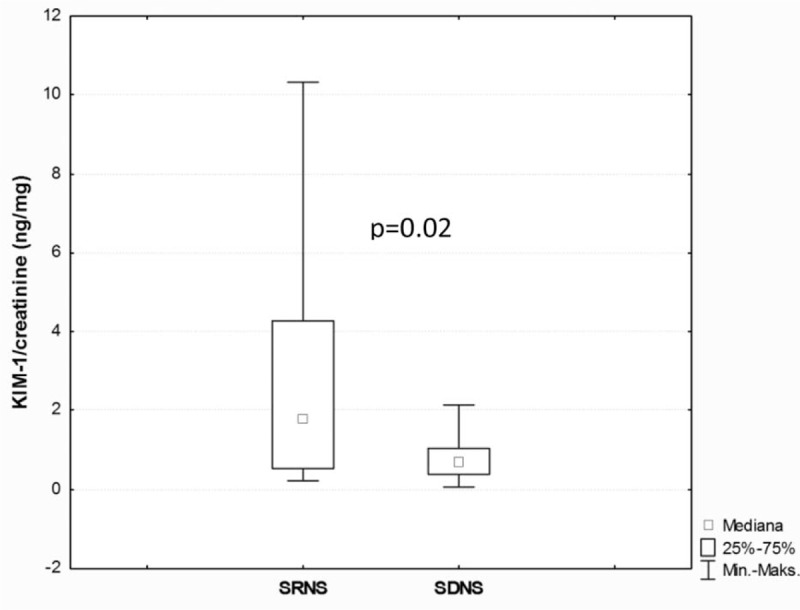
The comparison of urinary kidney injury molecule-1/creatinine ratios between patients with steroid-resistant nephrotic syndrome and steroid-dependent nephrotic syndrome.
There were no significant differences in median values of urinary alpha-GST/creatinine ratio, urinary pi-GST/creatinine ratio, urinary NGAL/creatinine ratio, and urinary KIM-1/creatinine ratio between children treated and those nontreated with cyclosporine A. Similarly, the median values of serum NGAL level in these 2 groups of children did not differ significantly.
Ethical Aspects
The study was approved by Ethics Committee of the Medical University of Lublin.
DISCUSSION
Each glomerulopathy associated with recurrent or persistent proteinuria may lead to progressive tubulointerstitial fibrosis. Initially, GSTs were believed to be early and sensitive markers of AKI. Recent studies, however, revealed that GSTs could also be helpful in detection of tubulointerstitial fibrosis in patient with CKD. Cawood et al10 observed that in diabetic proteinuric patients urinary alpha- and pi-GST markers appeared to identify renal damage, which was related to but distinct from albuminuria. In our patients, elevated urinary alpha-GST excretion was demonstrated, whereas urinary pi-GST excretion was normal. There was no correlation between urinary alpha-GST excretion and magnitude of proteinuria. This observation suggests that renal tubular injury because of proteinuria seems to be independent of its magnitude. In the study by Bashir et al,12 urinary alpha-GST and pi-GST excretions were suggested to be useful markers of proteinuria-induced renal tubular injury. They also disclosed a positive correlation between urinary alpha-GST excretion and severity of proximal tubular histopathologic changes. They proved that in proteinuric patients the initial injury was localized in proximal tubules. Our results confirmed this observation. In all our patients, an elevated urinary alpha-GST excretion and normal urinary pi-GST excretion were demonstrated. This might indicate that in children with SDNS and SRNS renal tubular injury begins in the region of proximal tubules.
Recent studies suggested that urinary NGAL excretion could be helpful in diagnosis and monitoring of treatment and progression of various renal diseases affecting glomerular filtration barrier, proximal tubule reabsorption, and distal nephrons.28–31 In the study by Bennet et al,32 in patients with SRNS urinary NGAL excretion was higher than that in patients with steroid sensitive nephrotic syndrome (SSNS) and was independent of the degree of proteinuria. Nishida et al33 observed an elevated urinary NGAL excretion in patients with CKD, but not in the patients with SSNS. In addition, they revealed a positive correlation between urinary NGAL excretion and magnitude of proteinuria. Ding et al29 showed that in proteinuric patients with Lee grade III IgA nephropathy, urinary NGAL excretion correlated positively with progressive glomerular mesangial proliferation and tubulointerstitial fibrosis. Similarly, Pitashny et al34 disclosed a positive correlation between urinary NGAL excretion and activity of nephritis associated with proteinuria in adult patients with systemic lupus erythematosus. In other studies,19,35,36 urinary NGAL excretions was used as a marker of lupus nephritis activity in children. In our study, a higher urinary NGAL excretion was also observed in children with SRNS compared with those with SDNS. Nevertheless, we did not observe any significant correlation between urinary NGAL excretion and magnitude of proteinuria.
In retrospective study by Waanders et al,24 proteinuric patients were characterized by an increased urinary KIM-1 excretion. In our study, children with INS also displayed elevated urinary KIM-1 excretion. In addition, Waanders at al24 demonstrated a significant decrease in urinary KIM-1 excretion after a short-term antiproteinuric treatment. Similarly, Vaidya et al26 demonstrated that resolution of proteinuria was accompanied by a decrease in urinary KIM-1 excretion. In our study, positive correlation between urinary KIM-1 excretion and magnitude of proteinuria seems to confirm these observations. Our nephrotic patients did not display significant positive correlations between magnitude of proteinuria and the remaining renal tubular injury markers that are serum NGAL level and urinary excretions of alpha-GST, pi-GST, and NGAL. This might indicate that urinary KIM-1 excretion is of higher diagnostic value. In addition, in our patients with SRNS, urinary KIM-1 excretion was significantly higher compared with those with SDNS. This might suggest that renal tubular injury was more severe in children with SRNS.
CONCLUSIONS
In conclusion, the results of our study suggest that tubulointerstitial fibrosis develops early in the course INS not only in steroid-resistant patients but also in those steroid-dependent and initially does not impair renal function. Higher urinary NGAL and KIM-1 excretions observed in our children with SRNS compared with those with SDNS may indicate that the steroid-resistant patients are more susceptible to early kidney damage. In addition, nephrotic children seem to be at risk for proximal tubule injury because our patients displayed elevated urinary alpha-GST and KIM-1 excretions associated with normal urinary pi-GST excretion. In children with SRNS, positive correlation between urinary KIM-1 excretion and magnitude of proteinuria seems to confirm higher risk of tubulointerstitial fibrosis in those patients.
Monitoring of early markers of tubulointerstitial injury in patients with glomerulopathies associated with proteinuria may be helpful in early detection of CKD development. In patients at risk for CKD development, the basic and nephroprotective treatment should be intensified.
The main limitation of the current preliminary study is a relatively small number of patients. Further investigations in a larger population of patients with various forms of glomerulopathies are required to estimate utility of urinary GSTs, NGAL, KIM-1 excretions, and serum NGAL level in an early diagnosis of tubulointerstitial injury.
Footnotes
Abbreviations: AKI = acute kidney injury, CKD = chronic kidney disease, ELISA = enzyme-linked immunosorbent assay, FSGS = focal segmental glomerulosclerosis, GST = glutathione S-transferase, INS = idiopathic nephrotic syndrome, KIM-1 = kidney injury molecule-1, MCD = minimal change disease, MesPGN = mesangioproliferative glomerulonephritis, MGN = membranous glomerulonephritis, MPGN = membranoproliferative glomerulonephritis, NAG = N-acetyl-β-d-glucosaminidase, NGAL = neutrophil gelatinase-associated lipocalin, SDNS = steroid-dependent nephrotic syndrome, sNGAL = serum NGAL, SRNS = steroid-resistant nephrotic syndrome, SSNS = steroid-sensitive nephrotic syndrome, uNGAL = urinary NGAL.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 1992; 20:1–17. [DOI] [PubMed] [Google Scholar]
- 2.Sundberg A, Appelkvist EL, Dallner G, et al. Glutathione transferases in the urine: sensitive methods for detection of kidney damage induced by nephrotoxic agents in humans. Environ Health Perspect 1994; 102:293–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison DJ, Kharbanda R, Cunningham DS, et al. Distribution of glutathione S-transferase isoenzymes in human kidney: basis for possible markers of renal injury. J Clin Pathol 1989; 42:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagshaw SM, Langenberg C, Haase M, et al. Urinary biomarkers in septic acute kidney injury. Intensive Care Med 2007; 33:1285–1296. [DOI] [PubMed] [Google Scholar]
- 5.Laisalmi M, Teppo AM, Koivusalo AM, et al. The effect of ketorolac and sevoflurane anesthesia on renal glomerular and tubular function. Anesth Analg 2001; 93:1210–1213. [DOI] [PubMed] [Google Scholar]
- 6.Marchewka Z, Przewłocki M, Lepka M, et al. Selected biochemical parameters of urine in the evaluation of paracetamol nephrotoxicity. Przegl Lek 2006; 63:1299–1303. [PubMed] [Google Scholar]
- 7.Prozialeck WC, Edwards JR, Lamar PC, et al. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol 2009; 238:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boldt J, Brenner T, Lang J, et al. Kidney-specific proteins in elderly patients undergoing cardiac surgery with cardiopulmonary bypass. Anesth Analg 2003; 97:1582–1589. [DOI] [PubMed] [Google Scholar]
- 9.Susantitaphong P, Perianayagam MC, Tighiouart H, et al. Urinary α- and π-glutathione s-transferases for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers 2013; 18:331–337. [DOI] [PubMed] [Google Scholar]
- 10.Cawood TJ, Bashir M, Brady J, et al. Urinary collagen IV and πGST: potential biomarkers for detecting localized kidney injury in diabetes-a pilot study. Am J Nephrol 2010; 32:219–225. [DOI] [PubMed] [Google Scholar]
- 11.Holmquist P, Torffvit O. Tubular function in diabetic children assessed by Tamm-Horsfall protein and glutathione S-transferase. Pediatr Nephrol 2008; 3:1079–1083. [DOI] [PubMed] [Google Scholar]
- 12.Bashir M, Cawood T, O'Shea D, et al. Obesity-related nephropathy: evidence of proximal tubular damage. Endocrine Abstracts 2008; 15:123. [Google Scholar]
- 13.Branten AJ, Mulder TP, Peters WH, et al. Urinary excretion of glutathione S transferases alpha and pi in patients with proteinuria: reflection of the site of tubular injury. Nephron 2000; 85:120–126. [DOI] [PubMed] [Google Scholar]
- 14.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res 2009; 32:91–98. [DOI] [PubMed] [Google Scholar]
- 15.Brunner HI, Mueller M, Rutherford C, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum 2006; 54:2577–2584. [DOI] [PubMed] [Google Scholar]
- 16.Mitsnefes MM, Kathman TS, Mishra J, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol 2007; 22:101–108. [DOI] [PubMed] [Google Scholar]
- 17.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 2009; 4:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl 2008; 241:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viau A, El Karoui K, Laouari D, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest 2010; 120:4065–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Su T, Yang L, et al. Urinary neutrophil gelatinase-associated lipocalin: a potential biomarker for predicting rapid progression of drug-induced chronic tubulointerstitial nephritis. Am J Med Sci 2010; 339:537–542. [DOI] [PubMed] [Google Scholar]
- 21.van Timmeren MM, Bakker SJ, Vaidya VS, et al. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol 2006; 291:F456–F464. [DOI] [PubMed] [Google Scholar]
- 22.van Timmeren MM, van den Heuvel MC, Bailly V, et al. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 2007; 212:209–217. [DOI] [PubMed] [Google Scholar]
- 23.Ko GJ, Grigoryev DN, Linfert D, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol 2010; 298:F1472–F1483. [DOI] [PubMed] [Google Scholar]
- 24.Waanders F, Vaidya VS, van Goor H, et al. Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: a post hoc analysis of a randomized controlled trial. Am J Kidney Dis 2009; 53:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 2010; 28:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaidya VS, Niewczas MA, Ficociello LH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int 2011; 79:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasilewska A, Taranta-Janusz K, Dębek W, et al. KIM-1 and NGAL: new markers of obstructive nephropathy. Pediatr Nephrol 2011; 26:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolignano D, Coppolino G, Campo S, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transplant 2008; 23:414–416. [DOI] [PubMed] [Google Scholar]
- 29.Ding H, He Y, Li K, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol 2007; 123:227–234. [DOI] [PubMed] [Google Scholar]
- 30.Ichino M, Kusaka M, Kuroyanagi Y, et al. Urinary neutrophil-gelatinase associated lipocalin is a potential noninvasive marker for renal scarring in patients with vesicoureteral reflux. J Urol 2010; 183:2001–2007. [DOI] [PubMed] [Google Scholar]
- 31.Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int 2009; 75:285–294. [DOI] [PubMed] [Google Scholar]
- 32.Bennett MR, Piyaphanee N, Czech K, et al. NGAL distinguishes steroid sensitivity in idiopathic nephrotic syndrome. Pediatr Nephrol 2012; 27:807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishida M, Kawakatsu H, Okumura Y, et al. Serum and urinary neutrophil gelatinase-associated lipocalin levels in children with chronic renal diseases. Pediatr Int 2010; 52:563–568. [DOI] [PubMed] [Google Scholar]
- 34.Pitashny M, Schwartz N, Qing X, et al. Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum 2007; 56:1894–1903. [DOI] [PubMed] [Google Scholar]
- 35.Rubinstein T, Pitashny M, Levine B, et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology 2009; 9:960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki M, Wiers KM, Klein-Gitelman MS, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol 2008; 23:403–412. [DOI] [PubMed] [Google Scholar]


