Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) infection is an important public health issue. This observational study aimed to characterize clinical features, antibiotic susceptibility, and genotypes of ocular infections caused by MRSA based on the clinical and molecular definitions of community-associated (CA) and healthcare-associated (HA) strains.
Fifty-nine patients with culture-proven S aureus ocular infection were enrolled from January 1, 2010 to December 31, 2011 at Chang Gung Memorial Hospital, Taiwan. Antibiotic susceptibility was verified using disk diffusion/E test. For characterization, staphylococcal cassette chromosome mec (SCCmec), pulsed-field gel electrophoresis (PFGE), multilocus sequence type (MLST), and Panton–Valentine leukocidin (PVL) gene, were performed. MRSA isolates from the patients with HA factors were classified as clinically defined HA-MRSA, and those carrying SCCmec type I to III as molecularly defined HA-MRSA.
Thirty-four patients with MRSA ocular infection were identified. The most common clone of CA-MRSA and HA-MRSA isolates was ST59/PFGE type D/SCCmec IV,VT/PVL (+) (n = 12) and CC 239/PFGE type A/SCCmec III, IIIA/PVL(−) (n = 10), respectively. All the 11 patients with molecularly defined HA-MRSA infections and 50% of the 22 patients with molecularly defined CA-MRSA infections were found to have HA factors (P = .005). CA-MRSA tended to cause lid infections, whereas HA-MRSA tended to cause corneal infections. Contrary to HA-MRSA isolates, nearly all the CA-MRSA isolates were susceptible to trimethoprim/sulfamethoxazole and fluoroquinolones under either clinical or molecular classifications.
In Taiwan, CA-MRSA isolates exhibited considerably higher susceptibility to fluoroquinolones when compared with HA-MRSA isolates. A strong correlation was observed between the HA factors and molecularly defined HA-MRSA isolates.
INTRODUCTION
Staphylococcus aureus has been a common pathogen that causes infectious diseases at various sites in the human body. Since the identification of methicillin-resistant S aureus (MRSA) in 1960s, it has been a crucial concern in several infectious diseases and was initially associated with hospital-acquired pathogens. However, its prevalence has increased in healthy people without risk factors for exposure to healthcare facilities.1,2 Thereafter, MRSA strains were arbitrarily classified into 2 groups, namely community-associated (CA) and healthcare-associated (HA)-MRSA. Compared with HA-MRSA isolates, CA-MRSA isolates had different molecular characteristics in addition to clinical features.3,4 With regard to the antibiotic susceptibility of S aureus, a mobile genetic element, staphylococcal cassette chromosome mec (SCCmec), plays an essential role and is a major molecular hallmark for MRSA classification. MRSA strains with type I to III SCCmec elements, which are responsible for resistance to numerous classes of antibiotics, were associated with HA-MRSA,5 whereas those carrying type IV and V (VT) SCCmec elements were commonly identified in CA-MRSA strains.6 In addition, the gene coding for Panton–Valentine leukocidin (PVL), a cytotoxin that causes leukocyte destruction, is associated with increased virulence of S aureus and is frequently present in CA-MRSA strains.7 Moreover, the primary clinical manifestations of CA-MRSA strains are skin and soft tissue infections.8 Nevertheless, the distinction between CA- and HA-MRSA becomes blurred, whereas CA-MRSA strains are transmitting to hospital settings.9
Until recently, limited studies focused on the issue of MRSA ocular infections stratified by CA- and HA-MRSA strains.10–16 Most studies regarding CA- and HA-MRSA ocular infections, including our previous study, were based on the clinical definitions without molecular characteristics,10–13,16 whereas some with molecular characteristics did not report the specific clinical manifestations.14,15 Meanwhile, the recent increased antibiotic resistance in MRSA ocular infections has become a critical concern.17,18 Hence, we conducted a study to evaluate the clinical features, molecular characterization, and antibiograms of MRSA ocular infections and compare CA- and HA-MRSA isolates based on both clinical and molecular definitions, and to seek for the clinical application of these results.
MATERIALS AND METHODS
Ethics Statement
This study followed the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital (CGMH), Taiwan (IRB102–2184C). All data were deidentified and anonymously reviewed to protect the privacy of the study participants; therefore, the need for informed consent was waived by the IRB.
Study Population and Data Collection
From January 1, 2010 to December 31, 2011, S aureus isolates from the patients with ocular infections were prospectively collected in the microbiology laboratory of CGMH, a 3700-bed medical center in Northern Taiwan. In total, 59 patients with S aureus ocular infections were identified. Medical records of these cases were retrospectively reviewed and collected.
The clinical data, including demographics, underlying disease, ocular history, recent medication history (immunosuppressants and antibiotics), HA factors (described later), primary diagnosis, management, and outcomes, were collected based on the electronic charts of the patients. The recorded underlying diseases included diabetes mellitus, hypertension, pulmonary disease, renal disease, liver disease, malignancy, and current nonocular infections. The ocular history included the use of contact lenses, ocular trauma, ocular surface disease, and ocular surgery. According to the ocular structure involved, the infections were classified in 7 categories based on diagnoses: lid disorder, lacrimal system disorder, conjunctivitis, keratitis, endophthalmitis, wound infection, and others. When a patient was diagnosed with >1 ocular infection, the primary pathology or the most severe diagnosis was considered.
Drug Susceptibility Tests
The antimicrobial susceptibility of all S. aureus isolates to antibiotics, including cefoxitin, penicillin, clindamycin, erythromycin, trimethoprim/sulfamethoxazole (TMP-SMX), teicoplanin, and vancomycin, was routinely performed using the disk diffusion method in our microbiology laboratory according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) antimicrobial susceptibility testing standards. We used cefoxitin instead of oxacillin/methicillin to test for β-lactam antibiotic resistance. In addition, we used an E-test (BioMerieux SA, Marcy-I’Etoile, France) to determine the susceptibility to fluoroquinolones including ciprofloxacin, levofloxacin, gatifloxacin, and moxifloxacin, that were not included in the antibiotic susceptibility profiles for S aureus in our microbiology laboratory.
Molecular Typing and Detection of PVL Gene
The molecular methods used in this study included pulsed-field gel electrophoresis (PFGE) with SmaI digestion, SCCmec typing,19 multilocus sequence type (MLST),20 and S. aureus protein A (spa) gene typing.21 In addition, the presence of PVL genes22 was examined. The details of the procedures have been described previously.19–23 All the MRSA isolates were molecularly characterized based on PFGE, PVL, and SCCmec. The PFGE genotypes were designated in alphabetical order, as in our previous studies; any new type, when identified, was designated consecutively. PFGE patterns with <4-band differences from an existing genotype were defined as subtypes. MLST and spa gene typing were examined for selective isolates of representative PFGE patterns.
CATEGORIZATION
Patients with MRSA infection were classified into 2 groups, CA- and HA-MRSA, based on the molecular and clinical criteria. The molecular criteria were derived from previous MRSA studies conducted in Taiwan6; isolates carrying type I to III SCCmec were defined as molecular HA-MRSA, whereas those carrying type IV or V SCCmec were defined as molecular CA-MRSA. An isolate without a typable SCCmec was excluded for further analysis. The clinical HA criteria were based on the definition proposed by the Centers for Disease Control and Prevention Active Bacterial Core Surveillance sites,24 including specimens obtained after 48 hours of admission, history of hospitalization, surgery, dialysis, or living in a long-term-care facility within 1 year, any permanent indwelling catheter, and any report of prior positive culture for MRSA.
Statistical Analysis
In descriptive statistics, the variables of interest were either presented as mean ± standard deviation or a number with a percentage. The intergroup differences in the variables were compared using t, Pearson χ2, or Fisher exact tests. A 2-tailed P value <0.05 was considered statistically significant. All the data analyses were performed using SPSS Version 19.0 (IBM, Armonk, NY).
RESULTS
Among the S aureus strains isolated from the 59 study patients, 25 (42.4%) were methicillin-sensitive S aureus (MSSA) and 34 (57.6%) were MRSA.
Comparison of Clinical Features and Drug Susceptibility Between MRSA and MSSA
Supplementary Table 1, http://links.lww.com/MD/A452 presents comparisons of clinical features between the MSSA and the MRSA groups. No significant differences were observed in sex composition, mean age, underlying disease, ocular history, diagnosis, outcome, and patients with HA factors between both groups. In both groups, keratitis was the most common ocular diagnosis, followed by conjunctivitis.
Supplementary Table 2, http://links.lww.com/MD/A452 lists comparisons of antibiotic susceptibility between the MSSA and the MRSA groups. The MRSA strains exhibited greater resistance to several antibiotics, including clindamycin, erythromycin, TMP-SMX, and 4 fluoroquinolones than did the MSSA strains, whereas both MRSA and MSSA strains were susceptible to teicoplanin and vancomycin.
Molecular Typing of MRSA
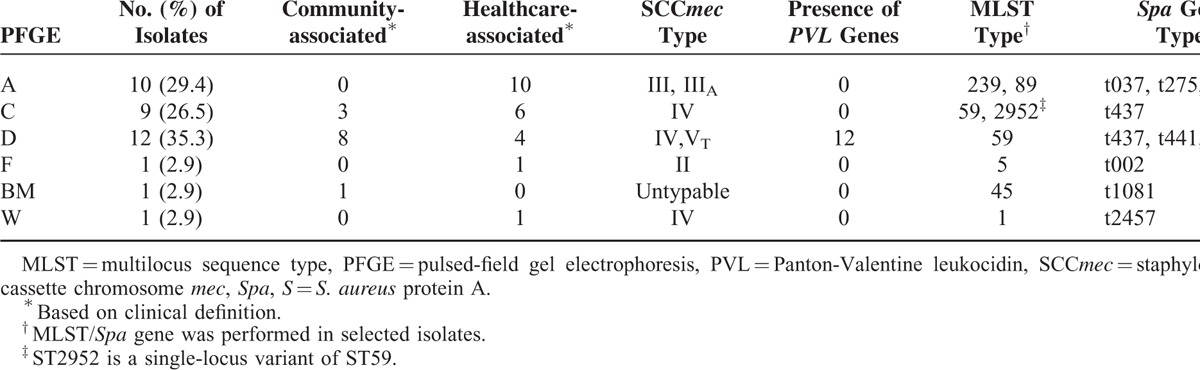
Table 1 summarizes the molecular typing of the 34 MRSA isolates. All but 3 isolates clustered in 3 PFGE patterns, namely types A, C, and D. PVL genes were only detected in the isolates of PFGE type D. The most predominant clone was PFGE type D/SCCmec IV,VT/Sequence type (ST) 59/spa clonal complex (CC) t437 (12 isolates, 35.3%), which is considered as a common endemic CA clone in Taiwan, followed by PFGE A/SCCmec III, IIIA/CC 239/spa CC t037 (10 isolates, 29.4%), which is considered an endemic HA clone in Taiwan. In addition, the isolates with PFGE C/SCCmec IV/CC 59/spa t437, which are the other one common endemic CA clone, were identified in 9 patients (26.5%).
TABLE 1.
Molecular Characteristics of 34 Clinical Methicillin-Resistant S aueus Isolates From Patients With Ocular Infections, Stratified by Pulsed-Field Gel Electrophoresis
Comparison of Clinical Features and Drug Susceptibility Between CA- and HA-MRSA Based on Clinical Definition
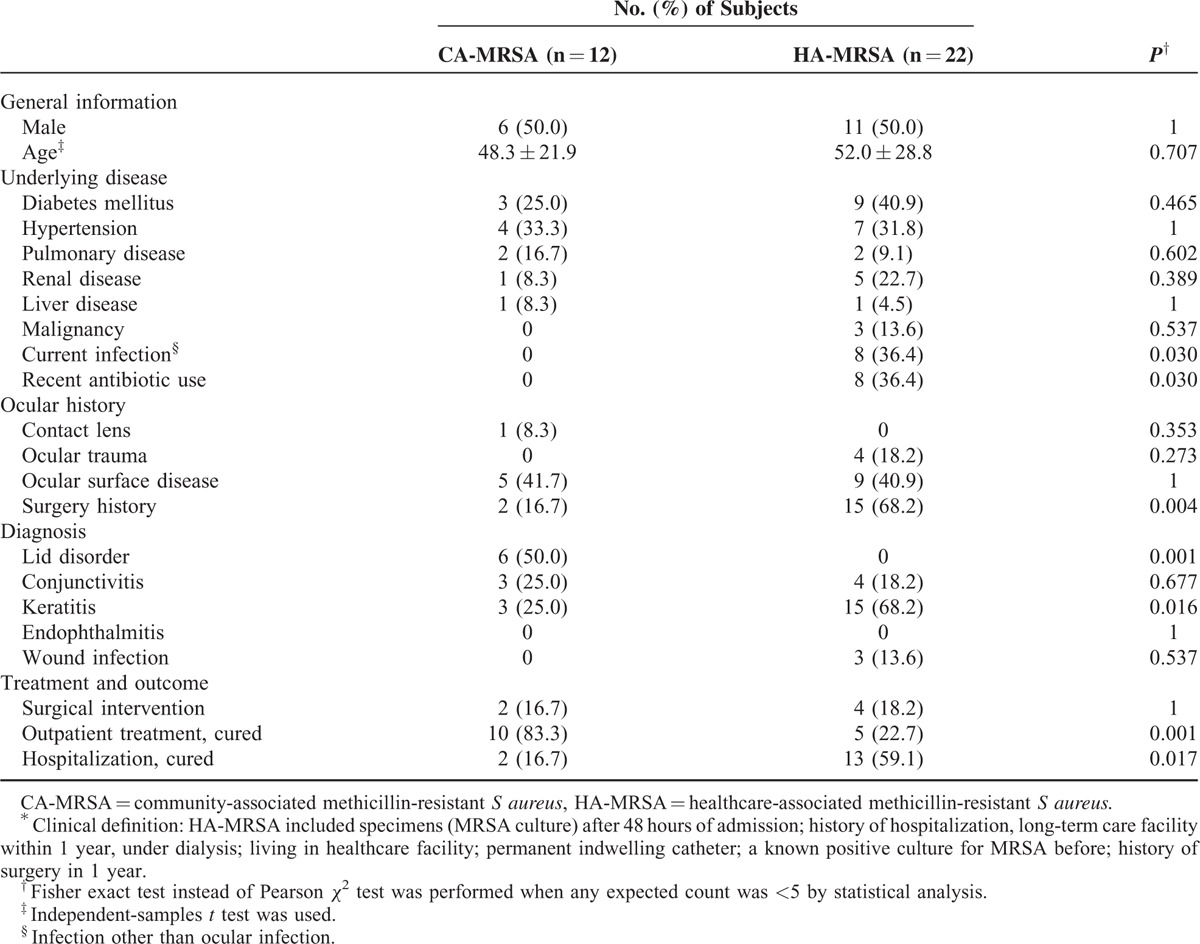
Based on the clinical definition, 12 isolates were classified as CA-MRSA (35.3%) and 22 as HA-MRSA (64.7%). Table 2 illustrates the comparisons between clinically defined CA- and HA-MRSA. No significant differences were observed in terms of demographics and underlying systemic diseases except for current infections (P = .030) between the 2 groups. The rate of recent antibiotic use in the HA-MRSA group was significantly higher than that in the CA-MRSA group (P = .030). Patients with HA-MRSA infection exhibited a higher rate (68.2% vs 16.7%) of ocular surgery history (P = .004). No patient with HA-MRSA infection presented as lid disorder, which were caused predominantly by CA-MRSA strains (50% of CA isolates, P = .001). In contrast, the rate of keratitis caused by HA-MRSA strains was higher than that caused by CA-MRSA strains (68.2% vs 25%, P = .016). Patients with CA-MRSA infection were primarily managed at outpatient clinics (83.3% of CA-MRSA isolates, P = .001), whereas those with HA-MRSA infections were more hospitalized (59.1% of HA isolates, P = .017).
TABLE 2.
Comparison of Clinical Features Between HA-MRSA and CA-MRSA Based on Clinical Definition∗
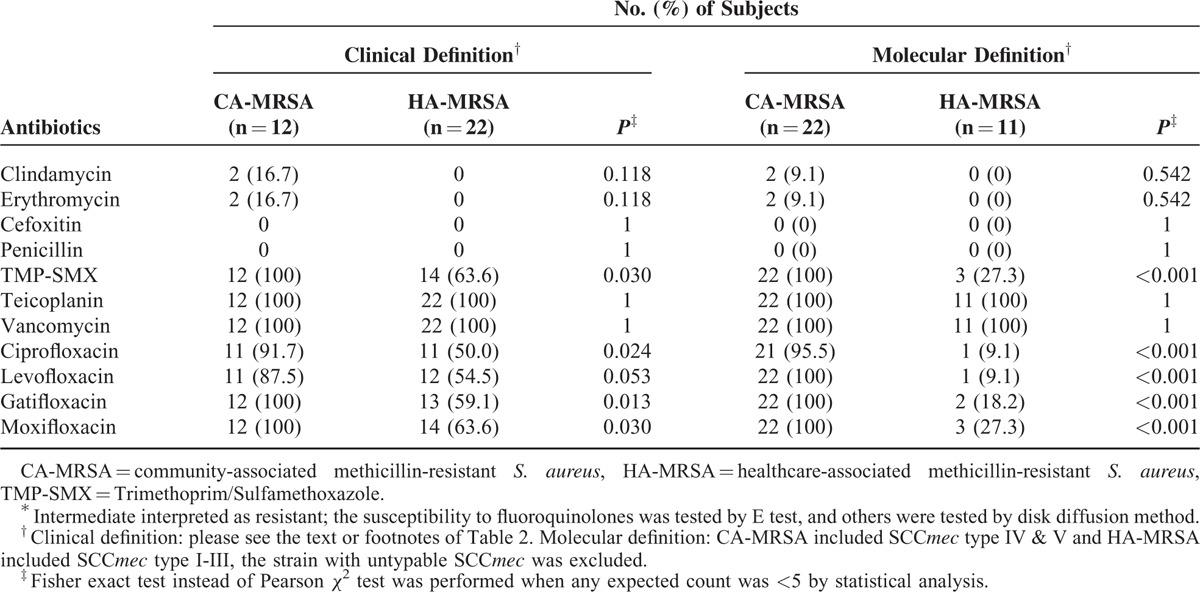
Left portion of Table 3 lists the drug susceptibility of MRSA strains based on clinical definition. All MRSA isolates were susceptible to vancomycin and teicoplanin, but both CA- and HA-MRSA were resistant to erythromycin and clindamycin. All CA-MRSA strains were susceptible to TMP-SMX, whereas the HA-MRSA strains exhibited lower susceptibility to TMP-SMX (63.6%, P = .03). In addition, the CA-MRSA strains were more susceptible to fluoroquinolones (87.5%-100%) than were the HA-MRSA strains (50.0%–63.6%, P = 0.024–0.053).
TABLE 3.
Antibiotic Susceptibility Tests∗ of HA-MRSA and CA-MRSA Isolates for Ocular Infections
Comparison of Clinical Features and Drug Susceptibility Between CA- and HA-MRSA Based on Molecular Definition
Excluding 1 isolate with untypable SCCmec, 22 isolates were classified as CA-MRSA strains (66.7%) and 11 as HA-MRSA strains (33.3%) based on molecular definition.
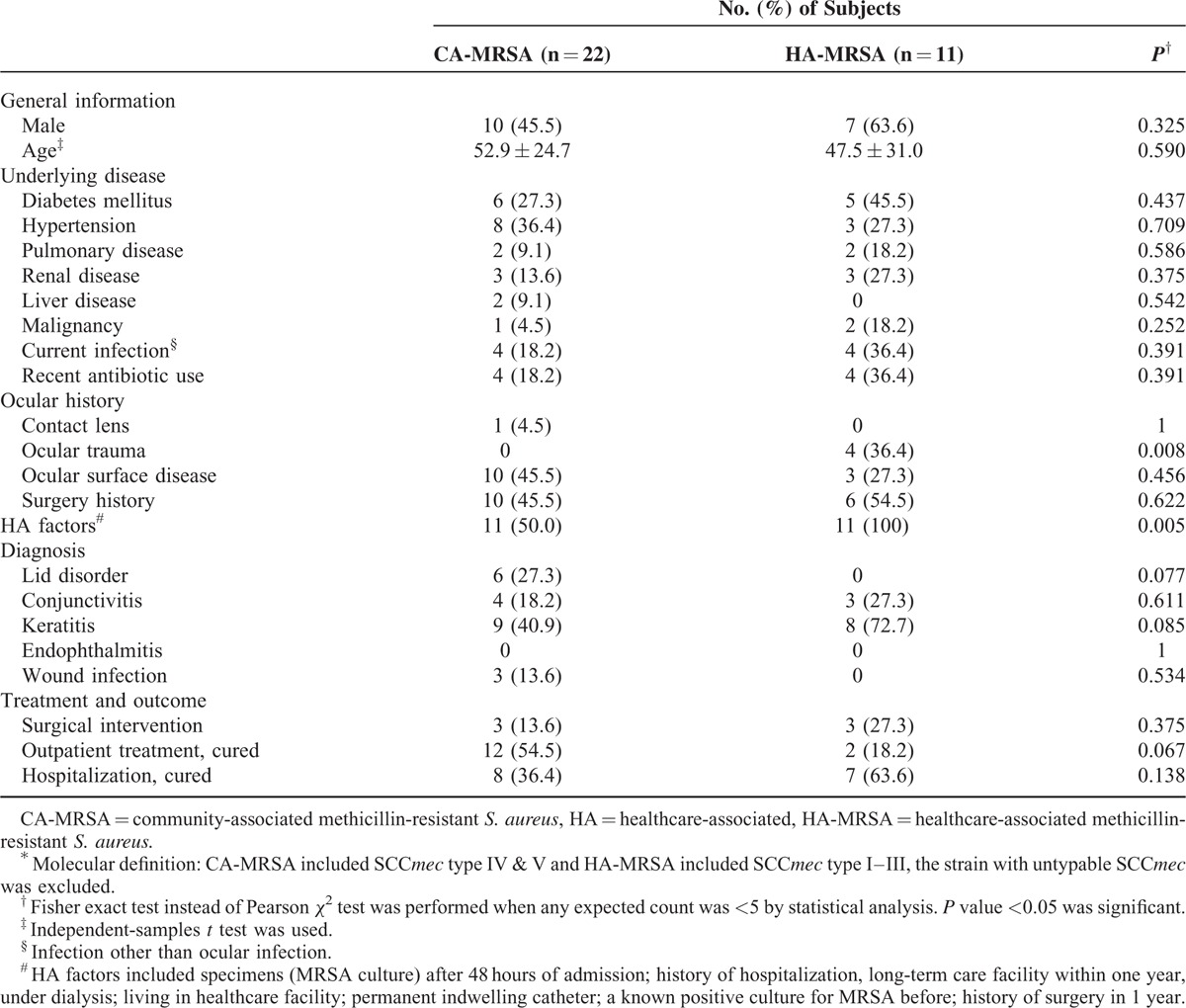
Table 4 summarizes comparisons of clinical features between the patients with molecularly defined CA- and HA-MRSA infections. There were only 2 significant differences found between 2 groups: ocular trauma history (P = 0.008) and HA factors (P = 0.005). All patients with a history of ocular trauma developed HA-MRSA infections. HA factors were identified in all patients with molecularly defined HA-MRSA infections and 50% (11/22) of the patients with molecularly defined CA-MRSA infections.
TABLE 4.
Comparison of Clinical Characteristics Between HA-MRSA and CA-MRSA Based on Molecular Definition∗
Right portion of Table 3 lists the drug susceptibility of molecularly defined CA-MRSA and HA-MRSA isolates, which was similar to that of clinically defined CA- and HA-MRSA isolates, but the differences were more statistically significant. One molecularly defined CA-MRSA isolate was resistant to ciprofloxacin but susceptible to the other 3 fluoroquinolones and TMP-SMX; all the other molecularly defined CA-MRSA strains were susceptible to TMP-SMX and fluoroquinolones. Conversely, the molecularly defined HA-MRSA strains exhibited lower susceptibility to TMP-SMX and fluoroquinolones (27.3% and 9.1%–27.3%, respectively, all P < 0.001).
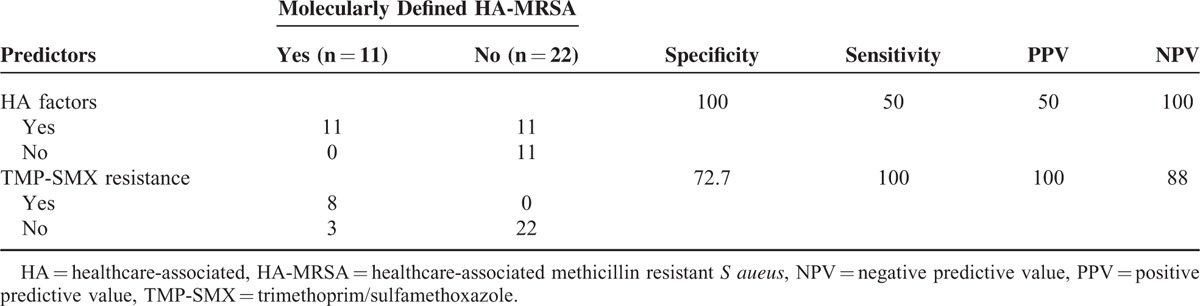
Validity of the Predictors for Molecularly Defined HA-MRSA
Considering the significant differences in the drug susceptibility to TMP-SMX between CA- and HA-MRSA, we tried to evaluate whether molecularly-defined HA strains could be predicted by the presence of HA factors and/or TMP-SMX resistance. Table 5 represents the results of validity analysis. As taken molecularly defined HA-MRSA as a gold standard, the sensitivity, specificity, positive predictive value, and negative predictive value of the presence of any HA factor were 100%, 50%, 50%, and 100%, respectively, and those of TMP-SMX resistance were 72.7%, 100%, 100%, and 88%, respectively.
TABLE 5.
Prediction of Molecularly Defined HA-MRSA by Healthcare-Associated Factors and TMP-SMX Resistance
DISCUSSION
This study extended our previous 10-year study,12 which specifically compared ocular CA- and HA-MRSA isolates in Taiwan, and replenished its insufficiency of genotyping, and antibiograms regarding fluoroquinolones. Our findings demonstrated that HA factors could distinguish patients with molecularly defined HA-MRSA ocular infections from those with molecularly defined CA-MRSA infections. In addition, TMP-SMX and fluoroquinolones revealed a considerably higher degree of activity against the CA-MRSA isolates than HA-MRSA isolates, regardless of the clinical or molecular definition.
Our study showed that the distribution of molecular characteristics for both CA- and HA-MRSA isolates was consistent with those reported from nonocular infections in Taiwan,25 which revealed CC59 with SCCmec IV/VT and CC239 with SCCmec III/IIIA were predominant in CA and HA clones, respectively. ST2952, a single locus variant of ST59, identified in this study was reported for the first time. In this study, ST45 with untypable SCCmec was not categorized into a molecular CA or HA group based on our definition, although this clone was reported to cause clinical HA infections in respiratory care wards in Taiwan.26 In addition, PFGE pattern F/SCCmec II/ST5/spa t002 was one of the major HA-MRSA clones in Taiwan since 2005.25
The sustained transmission of CA-MRSA clones into HA facilities has been a critical concern not only in Taiwan27 but also in other regions.28 This study indicated a higher prevalence rate of CA-MRSA by the molecular definition (66.7%) than that by the clinical definition (35.3%) because half of the patients with molecularly defined CA strains were associated with the HA factors. However, a strong correlation was still observed between the molecularly defined HA strains and the HA factors. An increasing proportion of MRSA isolates with SCCmec type IV in healthcare facilities was reported in the United States as well.29 These findings might implicate that molecularly defined community strains are transmitted to health care facilities. Continuous monitoring is warranted to determine whether the HA factors are sufficient to define and distinguish between CA- and HA-MRSA isolates.
In the present study, patients with clinically defined CA-MRSA infection exhibited a higher rate of lid disorder but lower rate of keratitis than did those with clinically defined HA-MRSA infection, which were consistent with our previous study.12 CA-MRSA has been reported to exhibit a predilection to cause nonvision-threatening infections10,12; therefore, patients with CA-MRSA infection could be primarily managed at outpatient clinics in our study. In addition to systemic factors, we also evaluated the local risk factors for ocular infections. It is not surprising that we found clinically defined HA-MRSA was associated with history of ocular surgery, particularly within 1 year, which was one of clinical criteria for HA-MRSA. Instead, molecularly defined HA-MRSA infection was associated with a history of ocular trauma; however, all the 4 patients had received surgical treatment and had been followed up at ophthalmology clinics for years before trauma, so they might be more exposed to HA-MRSA. Although we observed greater differences in clinical characteristics between clinically defined CA- and HA-MRSA than molecularly defined CA- and HA-MRSA, a study with a larger sample size is warranted to determine which of the 2 MRSA classifications are more suitable in predicting the clinical features and outcomes.
CA-MRSA isolates by both definitions in the present study exhibited high resistance (>80%) to clindamycin, which were different from those reported from the United States,4,14 but were comparable with those from Taiwan.12,30 In Taiwan, CA-MRSA also exhibited multidrug resistance. Susceptibility differed only for TMP-SMX; CA-MRSA isolates were significantly more susceptible than HA-MRSA isolates.
Two national surveys of ocular isolates conducted in the United States reported a resistance rate of >80% to fluoroquinolones for MRSA18,31; however, these studies did not provide any further classification of the MRSA isolates. Recently, a Chinese study by Hong et al reported that clinically defined CA-MRSA exhibited a significantly higher susceptibility to fluoroquinolones than HA-MRSA (61.1%–87% vs 32.9%–63.7%) 32 and a US study by Hesje et al reported that 37.5% of SCCmec type IV (molecularly defined CA-MRSA), but no SCCmec type II, (molecularly-defined HA-MRSA) isolates were susceptible to fluoroquinolones.14 In the present study, the rate of susceptibility to the 4 tested fluoroquinolones was significantly higher for CA-MRSA than for HA-MRSA, particularly by the molecular definition (all P < 0.001). All the isolates of CC 59, the most common CA-MRSA strains in Taiwan, were susceptible to fluoroquinolones, whereas >70% of the molecularly defined HA-MRSA isolates were resistant to fluoroquinolones. These findings suggested that distinguishing HA- from CA-MRSA isolates, particularly by genotyping, is crucial to guide the treatment of patients with MRSA ocular infection because CA-MRSA plays an essential role in ocular infections,12 and fluoroquinolones are the most popular empiric antibiotics prescribed by ophthalmologists. Unfortunately, the susceptibility of fluoroquinolones is not included in the recommended testing panel of antibiotics for S aureus proposed by the CLSI and thus is not performed routinely in some microbiological laboratories such as ours, not to mention genotyping, a time-consuming and clinician-unfriendly procedure.
To help the ophthalmologists to predict molecular characteristics of clinical MRSA isolates, we proposed 2 potential differential tools in the present study: one was HA criteria, related to the patient's epidemiologic characteristics; the other was the resistance of TMP-SMX, related to phenotype of the isolates. Both tools had a high negative predictive value and the latter had even a high positive predictive value. Simply speaking, we may prescribe fluoroquinolones to treat MRSA ocular infection without the concern of resistance when the patient has no HA factors. However, when one or more HA factors are identified, the possibility of fluoroquinolones resistance should be considered (50% resistance), particularly the isolates are resistant to TMP-SMX (100% resistance).
There are some limitations in the present study. Although we prospectively collected the specimens, we retrospectively reviewed the clinical data; some risk factor assessment might be incomplete. Relatively small sample size would affect the analysis of statistical significance, but a few differences between CA- and HA-MRSA were still observed. In addition, the in vitro susceptibility based on the serum systemic standards does not always correlate with clinical response because there are no susceptibility standards for topical therapies. As with different microbiological characteristics in different geographic areas, the findings of the present study should not be generalized to other regions or populations.
In conclusion, for ocular MRSA infections in Taiwan, CC59 with SCCmec IV/VT was the predominant CA clone, whereas CC239 with SCCmec III/IIIA was the predominant HA clone. Despite the strong correlation between the HA factors and the molecular HA strains, transmission of CA strains to healthcare facilities was observed. We also found a relatively high susceptibility of molecularly defined CA-MRSA strains to fluoroquinolones. HA factors as well as susceptibility to TMP-SMX could be used as predictive tools for molecular characteristics of MRSA strains. Accordingly, with the help of these tools, ophthalmologists can prescribe more appropriate antibiotic treatments and indirectly improve the prognosis of patients with MRSA ocular infection.
Acknowledgements
We thank Hsiao-Jung Tseng, MS in the Biostatistical Center for Clinical Research, Chang Gung Memorial Hospital, Taiwan for the assistance in statistical analyses, and Wen-Hsuan Chen, MS for the assistance in data analysis.
Footnotes
Abbreviations: CA = community-associated, HA = healthcare-associated, MLST = multilocus sequence type, MRSA = methicillin-resistant Staphylococcus aureus, MSSA = methicillin-sensitive Staphylococcus aureus, PFGE = pulsed-field gel electrophoresis, PVL = Panton–Valentine leukocidin, SCCmec = staphylococcal cassette chromosome mec, Spa = S. aureus protein A, TMP/SMX = trimethoprim/sulfamethoxazole.
The financial support to this study included the funding from National Science Council, Taiwan (NSC102-2314-B-182A-112-, NMRPG3C0401) and Chang Gung Memorial Hospital, Taiwan (CMRPG3C1901). The funding organization had no role in the design or conduct of this research.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998; 279:593–598. [DOI] [PubMed] [Google Scholar]
- 2.David Michael Z, Robert S Daum. Community-Associated Methicillin-Resistant Staphylococcus aureus:Epidemiology and Clinical Consequences of an Emerging Epidemic. Clin Microbiol Rev 2010; 23:616–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin Infect Dis 2005; 40:562–573. [DOI] [PubMed] [Google Scholar]
- 4.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003; 290:2976–2984. [DOI] [PubMed] [Google Scholar]
- 5.Hiramatsu K, Katayama Y, Yuzawa H, et al. Molecular genetics of methicillin-resistant Staphylococcus aureus. Int J Med Microbiol 2002; 292:67–74. [DOI] [PubMed] [Google Scholar]
- 6.Huang YC, Chen CJ. Community-associated meticillin-resistant Staphylococcus aureus in children in Taiwan, 2000 s. Int J Antimicrob Agents 2011; 38:2–8. [DOI] [PubMed] [Google Scholar]
- 7.Tristan A, Bes M, Meugnier H, et al. Global distribution of Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus, 2006. Emerg Infect Dis 2007; 13:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis Jan 2005; 1: 40:100–107. [DOI] [PubMed] [Google Scholar]
- 9.Otter JA, French GL. Community-associated meticillin-resistant Staphylococcus aureus: the case for a genotypic definition. J Hosp Infect Jul 2012; 81:143–148. [DOI] [PubMed] [Google Scholar]
- 10.Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2006; 104:322–345. [PMC free article] [PubMed] [Google Scholar]
- 11.Rutar T, Chambers HF, Crawford JB, et al. Ophthalmic manifestations of infections caused by the USA300 clone of community-associated methicillin-resistant Staphylococcus aureus. Ophthalmology 2006; 113:1455–1462. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao CH, Chuang CC, Tan HY, et al. Methicillin-resistant Staphylococcus aureus ocular infection: a 10-year hospital-based study. Ophthalmology 2012; 119:522–527. [DOI] [PubMed] [Google Scholar]
- 13.Amato M, Pershing S, Walvick M, et al. Trends in ophthalmic manifestations of methicillin-resistant Staphylococcus aureus (MRSA) in a northern California pediatric population,. J AAPOS 2013; 17:243–247. [DOI] [PubMed] [Google Scholar]
- 14.Hesje CK, Sanfilippo CM, Haas W, et al. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolated from the eye. Curr Eye Res 2011; 36:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan MA, Ahmad S, Banu N. Molecular characterisation of methicillin-resistant Staphylococcus aureus (MRSA) from keratitis patients: a microbiological analysis. Br J Ophthalmol Aug 2010; 94:994–998. [DOI] [PubMed] [Google Scholar]
- 16.Walvick MD, Amato M. Ophthalmic methicillin-resistant Staphylococcus aureus infections: sensitivity and resistance profiles of 234 isolates. J Community Health 2011; 36:1024–1026. [DOI] [PubMed] [Google Scholar]
- 17.McDonald M, Blondeau JM. Emerging antibiotic resistance in ocular infections and the role of fluoroquinolones. J Cataract Refract Surg 2010; 36:1588–1598. [DOI] [PubMed] [Google Scholar]
- 18.Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol Jun 2008; 145:951–958. [DOI] [PubMed] [Google Scholar]
- 19.Kondo Y, Ito T, Ma XX, et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother 2007; 51:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enright MC, Day NP, Davies CE, et al. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol Mar 2000; 38:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmsen D, Claus H, Witte W, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 2003; 41:5442–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis Nov 1999; 29:1128–1132. [DOI] [PubMed] [Google Scholar]
- 23.Huang YC, Ho CF, Chen CJ, et al. Comparative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin Microbiol Infect 2008; 14:1167–1172. [DOI] [PubMed] [Google Scholar]
- 24.Minnesota Department of Health. Community-Associated Methicillin-Resistant Staphylococcus aureus in Minnesota. Disease Control Newslett 2004; 32:61–72. [Google Scholar]
- 25.Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect 2014; 20:605–623. [DOI] [PubMed] [Google Scholar]
- 26.Lee YT, Lin DB, Wang WY, et al. First identification of methicillin-resistant Staphylococcus aureus MLST types ST5 and ST45 and SCCmec types IV and Vt by multiplex PCR during an outbreak in a respiratory care ward in central Taiwan. Diagn Microbiol Infect Dis 2011; 70:175–182. [DOI] [PubMed] [Google Scholar]
- 27.Huang YC, Su LH, Wu TL, et al. Changing molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates from a teaching hospital in Northern Taiwan. J Clin Microbiol 2006; 44:2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis Mar-Apr 2001; 7:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maree CL, Daum RS, Boyle-Vavra S, et al. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis 2007; 13:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CJ, Huang YC, Chiu CH, et al. Clinical features and genotyping analysis of community-acquired methicillin-resistant Staphylococcus aureus infections in Taiwanese children. Pediatr Infect Dis J 2005; 24:40–45. [DOI] [PubMed] [Google Scholar]
- 31.Haas W, Pillar CM, Torres M, et al. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol Oct 2011; 152:567–574.e563. [DOI] [PubMed] [Google Scholar]
- 32.Hong J, Cao W, Xu J, et al. Fluoroquinolones and ocular MRSA infections [letter]. Ophthalmology 2013; 120:218–219. [DOI] [PubMed] [Google Scholar]


