Abstract
Albuminuria and periodontitis are both commonly associated with systemic inflammation. However, the association between urinary albumin excretion (UAE) and periodontitis in patients with type 2 diabetes has not been fully investigated. This study aimed to investigate the association between UAE and periodontitis in Korean adults with type 2 diabetes.
This study performed a cross-sectional analysis and used hierarchical multivariable logistic regression analysis models. Data from the 2012 Korean National Health and Nutrition Examination Survey were analyzed. A total of 547 patients, with type 2 diabetes without renal impairment, were included in this study. UAE was assessed using the urinary albumin to creatinine ratio (UACR). A community periodontal index greater than or equal to code 3 was used to define periodontitis.
The risk of periodontitis tended to increase as UACR increased even after adjustment for potential confounders (P for trend in the odds ratios = 0.05 in model 1; 0.02 in model 2; and 0.01 in model 3). In a subgroup analysis, the prevalence of periodontitis was significantly higher in the patients with albuminuria (UACR >30 mg/g) than in those without albuminuria among patients younger than 65 years (P = 0.03), those with newly diagnosed diabetes (P = 0.04), or those without obesity (P = .04).
UAE was positively associated with the risk of periodontitis in Korean adults with type 2 diabetes. In the patients who were younger, were newly diagnosed with diabetes, or had normal body mass index, individuals with albuminuria were more likely to have a higher prevalence of periodontitis. Early identification of periodontitis may be helpful in Korean diabetic adults with increased UAE.
INTRODUCTION
Albuminuria is already known to be an early marker for diabetic nephropathy. Furthermore, it has been reported to be independently associated with an increased risk of future cardiovascular morbidity and mortality in patients with diabetes or hypertension and even in the general population.1–4 It is a potential indicator of general vascular damage reflecting subclinical atherosclerosis.5,6
Periodontitis is a common inflammatory disease of the periodontal tissue and is reported to affect almost half of the general population worldwide.7 In diabetic patients, the risk of periodontitis is greater and the disease course leading to tooth loss may be more severe.8,9 Recently, periodontitis has been associated with atherosclerotic vascular disease including diabetes, coronary heart disease, and stroke.10,11 Furthermore, it is reported to be predictive of cardiovascular disease (CVD) risk and deaths from renal disease and CVD in people with type 2 diabetes.12,13 Chronic inflammatory processes of periodontitis seem to play a role in these associations.14 Therefore, it is important to determine factors that are associated with periodontitis.
Both albuminuria and periodontitis appear to be related with systemic inflammation, but there is limited evidence for the association between them. A few previous studies showed the association between severe periodontitis and renal alterations.15,16 However, the association between periodontitis and urinary albumin excretion (UAE) indicating early renal changes in patients with type 2 diabetes has yet to be fully investigated. For these reasons, the present study aimed to investigate the association between UAE and periodontitis in Korean adults with type 2 diabetes using nationally representative data.
METHODS
Survey and Subjects
This population-based, cross-sectional study analyzed data from the 2012 Korea National Health and Nutrition Examination Survey. The Korea National Health and Nutrition Examination Survey is a nationwide survey of noninstitutionalized civilians, and since 1998, has been conducted by the Division of Chronic Disease Surveillance of the Korea Centers for Disease Control and Prevention and the Korean Ministry of Health and Welfare. The survey was designed to assess national health and nutritional status and consists of a health interview, nutritional assessment, and health examination. Participants were selected from sampled household units based on population and the housing census from the 2005 National Census Registry in Korea by using a stratified, multistage, and probability-based sampling design with proportional allocation.
Of 8058 participants sampled, 1765 individuals were aged <19 years, 1365 had missing data, 4325 did not have type 2 diabetes, and 56 with estimated glomerular filtration rate (GFR) <60 mL/min/1.73 m2 were excluded. Data for 547 diabetic adults (266 men and 281 women) were used for the analyses. All participants signed an informed consent form and the institutional review board of the Korea Centers for Disease Control and Prevention approved the study protocol.
Lifestyle and Socioeconomic Variables
All patients were asked about their smoking status, alcohol consumption, physical activity, educational level, and monthly household income. Based on their answers to the self-reported questionnaire, patients were classified as nonsmokers or ever-smokers. Ever-smokers were defined as patients who had smoked at least 5 packs of cigarettes in their whole lives. Patients were categorized as nondrinkers, light to moderate drinkers (1–30 g/day), or heavy drinkers (>30 g/day) on the basis of their average alcohol intake per day in the month before the interview.17 Depending on the International Physical Activity Questionnaire short form modified for Korea,18 patients were considered regular physical exercisers if they exercised moderately more than 5 times per week for >30 minutes per session or exercised vigorously more than 3 times per week for >20 minutes per session. Educational level was categorized into 2 groups according to number of years of schooling: >12 years (high school graduate or some high school) or less. Monthly household income level was divided into the lower 25 percentile of the total patients or higher.
Anthropometric and Biochemical Measurements
Anthropometric and biochemical measurements were performed by trained staff members. Waist circumference was measured at the midpoint between the lower border of the rib cage and the iliac crest in a standing position. The height (cm) and weight (kg) were measured to the nearest 0.1 cm and 0.1 kg, respectively, with patients wearing light clothing without shoes. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). A BMI ≥25 kg/m2 was defined as the presence of obesity.19,20 Blood pressure (BP) was measured 3 times with 5-minute intervals on the right arm in the seated position using a mercury sphygmomanometer (Baumanometer, WA Baum Co., Copiague, NY), and the mean value of the 2nd and 3rd measurements was used in the analysis.
Blood and urine samples were obtained after fasting for at least 8 hours. They were appropriately processed and immediately refrigerated and transported in cold storage to the Central Testing Institute in Seoul Korea. Serum levels of total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol, and fasting plasma glucose (FPG) were measured using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) by enzymatic methods using commercially available kits (Daiichi, Tokyo, Japan). Glycated hemoglobin (HbA1c) level was measured using an HLC-723G7 (Tosoh, Japan) by high performance liquid chromatography. Type 2 diabetes was defined by an FPG level ≥126 mg/dL, HbA1c level ≥6.5%, treatment with insulin or oral hypoglycemic agents, or diagnosis by a physician.21 Additionally, newly diagnosed diabetes was defined by an FPG level ≥126 mg/dL or HbA1c level ≥6.5%. The level of low-density lipoprotein cholesterol was calculated using Friedewald formula in patients with a TG level <400 mg/dL,22 but it was measured directly using commercially available kits (Cholestest LDL, Sekisui Medical, Tokyo, Japan) in subjects with a TG level ≥400 mg/dL. White blood cell (WBC) counts were measured using a Sysmex XE-2100D (Sysmex, Kobe, Japan) by laser flow cytometry. Serum and urine creatinine levels were measured using a Hitachi Automatic Analyzer 7600 by kinetic colorimetry. Urinary albumin level was measured using the same tool by a turbidimetric assay. Albuminuria was defined as a urinary albumin to creatinine ratio (UACR) of ≥30 mg/g.23 The estimated GFR was calculated by using the formula referred to in the Modification of Diet in Renal Disease study.24
Oral Health Behaviors and Periodontitis
The daily frequency of tooth brushing and use of secondary oral products were recorded as oral health behaviors. Secondary oral products included dental floss, mouthwash, an interdental brush, and an electric toothbrush.
Trained and calibrated dentists examined the periodontal status of the patients. The World Health Organization (WHO) community periodontal index (CPI) was used to evaluate periodontitis. Periodontitis was diagnosed when a CPI was greater than or equal to code 3.25–27 The code 3 represents that there was 1 or more site of >3.5 mm pocket in the index teeth. The numbers of index teeth were 2, 3, 8, 14, 15, 18, 19, 24, 30, and 31 according to the Universal Numbering System adopted by the American Dental Association. The mouth was divided into sextants and a sextant was examined using a CPI probe with a 0.5 mm ball tip that met the WHO guideline. A sextant was examined only if there at least 2 teeth present that were not planned for extraction. If index teeth satisfied for examination were not present in a sextant, the highest score from all remaining teeth examined was selected as the score for that sextant.
Statistical Analyses
All data are presented as mean ± standard error (SE) or percentage (SE). A chi-square test for categorical variables or an independent t-test for continuous variables was performed to assess the differences in characteristics according to the presence of periodontitis or range of UACR. A hierarchical multivariable logistic regression analysis was used to evaluate the risk of periodontitis in relation to UAE, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated after adjusting for potential confounders. Model 1 was adjusted for age, sex, and duration of diabetes. Variables adjusted in model 1 plus BMI, smoking status, alcohol consumption, physical activity, education level, and household income level were adjusted in model 2. Model 3 was adjusted for the variables adjusted in model 2 plus daily frequency of tooth brushing, use of secondary oral products, systolic BP, TG, and WBC count. Statistical analyses were performed by using the survey procedure of SAS (Version 9.2, SAS Institute, Cary, NC) to account for the complex sampling design. Two-sided P values of <0.05 were considered an indicator of statistical significance.
RESULTS
A total of 547 patients with type 2 diabetes, but without renal impairment, were included in this study. Table 1 shows baseline clinical characteristics of the patients according to the presence of periodontitis. Patients with periodontitis were more likely to be ever-smokers and to have a lower educational level. The daily frequency of tooth brushing was similar between 2 groups, but the rate of secondary oral products use was higher in patients without periodontitis. Duration of diabetes, proportion of medical treatment for diabetes, prevalence of newly diagnosed diabetes or hypertension was not significantly different between the patients with and without periodontitis. The cardiometabolic markers including BMI, waist circumference, systolic and diastolic BP, FPG, HbA1c, total cholesterol, TG, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and WBC count did not differ significantly between the groups. The mean UACR was 15.4 mg/g in patients with periodontitis and the value was 11.0 mg/g in patients without periodontitis (P = 0.02). The prevalence of albuminuria (defined as UACR >30 mg/g) was significantly higher in patients with periodontitis (P = 0.02). Figure 1 shows the prevalence of periodontitis according to UACR. The periodontitis prevalence increased with increasing UACR (P for trend <0.001).
TABLE 1.
Baseline Clinical Characteristics of Study Subjects According to the Presence of Periodontitis
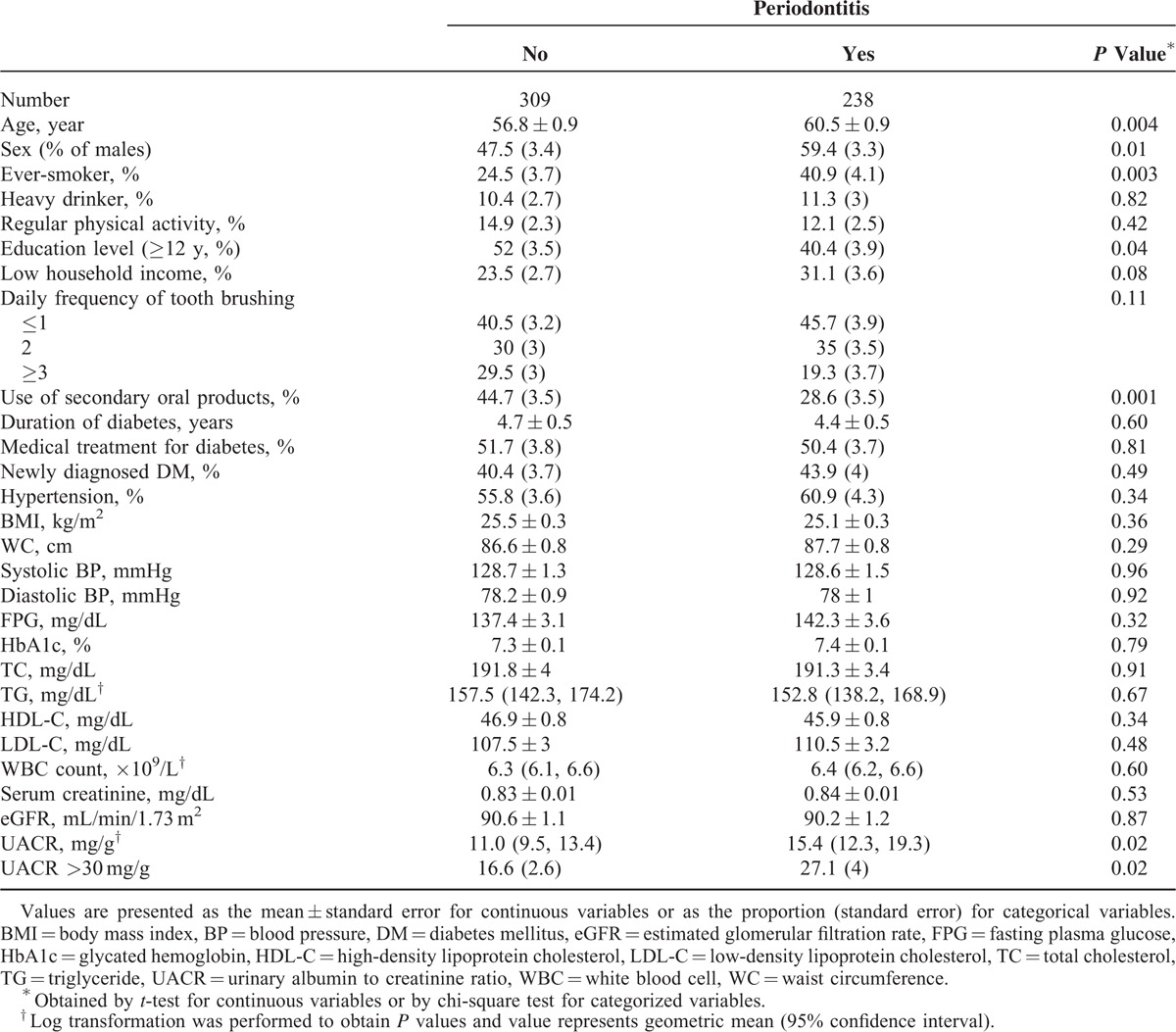
FIGURE 1.
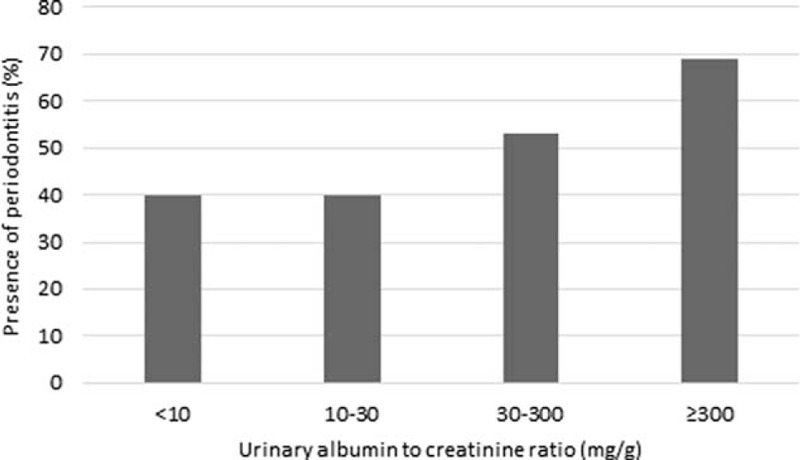
Prevalence of periodontitis according to urinary albumin to creatinine ratio (P for trend <0.001).
Table 2 shows the risk of periodontitis according to UAE. After adjustment for age, sex, and duration of diabetes (model 1), the OR (95% CI) of periodontitis was 0.95 (0.55–1.64) in patients with 10 ≤UACR <30 mg/g, 1.59 (0.89–2.82) in those with 30 ≤UACR <300 mg/g, and 3.25 (1.21–8.71) in those with UACR ≥300 mg/g and the ORs show an increasing trend with a borderline significance as the UACR values increased (P for trend = 0.05). After additional adjustment for BMI, smoking status, alcohol consumption, physical activity, education level, and household income level (model 2), the OR (95% CI) of periodontitis was 1.22 (0.65–2.30) in patients with 10 ≤UACR <30 mg/g, 1.87 (0.97–3.58) in those with 30 ≤UACR <300 mg/g, and 4.56 (1.21–17.14) in those with UACR ≥300 mg/g and the ORs tended to increase as the UACR increased (P for trend = 0.02). These associations persisted in the analyses after further adjustment for daily frequency of tooth brushing, use of secondary oral products, systolic BP, TG, and WBC count (model 3). The OR (95% CI) of periodontitis was 1.35 (0.69–2.65) in patients with 10 ≤UACR <30 mg/g, 2.11 (1.05–4.25) in those with 30 ≤UACR <300 mg/g, and 4.09 (1.14–14.73) in those with UACR≥300 mg/g and the ORs show an increasing trend as the UACR increased (P for trend = 0.01).
TABLE 2.
Odds Ratios for Periodontitis According to Urinary Albumin Excretion
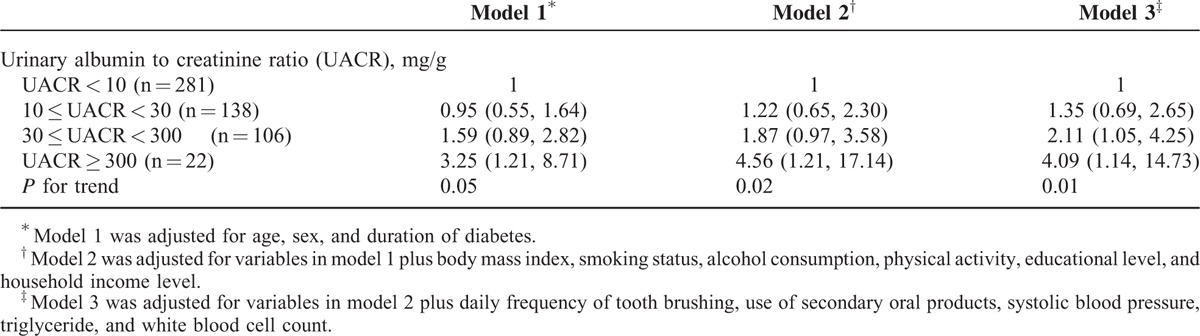
Figure 2 shows the association between UACR and the prevalence of periodontitis in subgroups based on age, time of diagnosis of diabetes, and BMI. The prevalence was significantly higher in the patients with albuminuria (UACR >30 mg/g) than those without albuminuria in patients younger than 65 years (P = 0.03), those with newly diagnosed diabetes (P = 0.04), or those without obesity (P = 0.04). The prevalence did not differ in the other subgroups.
FIGURE 2.
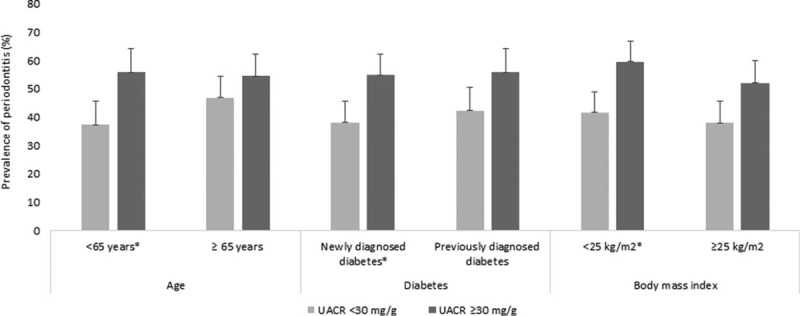
Association between urinary albumin to creatinine ratio (mg/g) and the prevalence of periodontitis in subgroups based on age, time of diagnosis of diabetes, and body mass index (∗P < 0.05).
DISCUSSION
In the present study, the risk of periodontitis was positively associated with UAE in Korean adults with type 2 diabetes. This association between UACR and periodontitis was independent of various potential-confounding factors including age, sex, duration of diabetes, BMI, smoking status, alcohol consumption, physical activity, educational and income levels, systolic BP, TG, WBC count, daily frequency of tooth brushing, and use of secondary oral products. A subgroup analysis revealed that in the younger patients, those with newly diagnosed diabetes, and those without obesity, the prevalence of periodontitis was significantly higher in the patients with albuminuria than in those without albuminuria.
Periodontitis has increasingly been reported to be associated with chronic kidney disease (CKD).28,29 Both periodontitis and CKD pertain to chronic, low-grade inflammation. The measures of periodontal disease were demonstrated to be associated with marked leukocytosis in patients with CKD.30 Persistent inflammation caused by periodontitis may lead to endothelial dysfunction and contribute to the development of kidney disease.31 Although the exact mechanism of the association between periodontitis and UAE in diabetic patients without renal impairment that is shown in this study has not been fully elucidated, a chronic inflammatory process seems to be the link. Albuminuria is known to indicate systemic inflammation and vascular endothelial dysfunction, and to be associated with the risk of CKD or CVD.32 Periodontitis has been recognized as a cause of low-grade chronic inflammation, which results in the production of proinflammatory cytokines and promotes systemic inflammation.33 Inflammation caused by periodontitis is also associated with atheroma or abnormal lipid metabolism resulting in CVD.34 Inversely, previous studies have shown that the treatment for periodontitis lowers the levels of inflammatory markers.35,36 As such, both UAE and periodontitis are commonly related to persistent inflammation that may lead to vascular endothelial dysfunction. They may also be interrelated bidirectionally and deleteriously affect each other. In addition, the reason for the positive correlation between UAE and periodontitis might be that both are associated with metabolic syndrome or insulin resistance-related characteristics.37–39 However, further study is needed to confirm this association because the cross-sectional design of this study cannot directly explain it.
Periodontitis is well known to be not only a local phenomenon but also connected with systemic diseases, including diabetes. Although periodontitis is recognized as a multifactorial disease, the risk factors of periodontitis appear to be controversial. Furthermore, the causative factors of periodontitis in diabetic patients remain to be elucidated.40 To our knowledge, no study has assessed the relationship between UAE and periodontitis in diabetic patients without renal impairment. A few studies investigated the association between renal changes and periodontitis in diabetic patients. A study of nonobese Japanese patients with type 2 diabetes showed that the IgG titier for Porphyromonas gingivalis was correlated with UACR. However, this study did not consider potential confounders.15 A study among American Indians with type 2 diabetes revealed that periodontitis predicts the development of overt nephropathy and end-stage renal disease.16 The findings of these previous studies appear to be partly consistent with our findings, but the previous studies did not consider confounders or assessed late-stage renal disease. This is the first study to investigate the role of UAE as a predictor of periodontitis only in diabetic patients without definite renal impairment with consideration of potential confounders possibly related to both UAE and periodontitis.
It is interesting that in the present study, a subgroup analysis revealed that in younger patients, those with newly diagnosed diabetes, or those without obesity, the prevalence of periodontitis was higher in patients with albuminuria than in those without albuminuria. Aging, longer duration of diabetes, and obesity can be characterized by chronic low-grade inflammation.41 Among the diabetic patients included in this study, younger patients, those with newly diagnosed diabetes, or those without obesity were expected to have milder inflammatory responses than the other groups. Thus, additional findings from subgroup analyses may support the main findings of this study, that is, the difference in periodontitis prevalence between patients with albuminuria and those without albuminuria was more distinct in these subgroups. UAE in type 2 diabetic patients without renal impairment may reflect early renal changes in patients with a relatively early stage of diabetes without severe complications. Concurrently, a previous animal study that used Zucker fatty rats (a well-characterized model of prediabetes) reported that periodontitis was associated with development of early renal changes.42 Therefore, the findings of this study may be meaningful in that this study investigated the association between periodontitis and UAE reflecting early renal change exclusively in type 2 diabetic patients without renal impairment. Our findings suggest that UAE is positively associated with periodontitis in patients with relatively early-stage diabetes as opposed to the findings shown in previous studies that assessed the association between periodontitis and overt nephropathy. These findings also suggest that UAE might be an independent and more relevant surrogate of periodontitis than GFR in diabetic patients without renal impairment.
The present study has several limitations. First, the cross-sectional design cannot explain the causal relationship between UAE and periodontitis. Second, the value of UACR assessed from single-void random urine samples could be incorrect. Third, the specific types of antihypertensive agents possibly related with UAE or renal function could not be evaluated from data based on self-reported questionnaires. Despite these limitations, this study provides epidemiological evidence for an association between UAE and periodontitis in type 2 diabetes by using nationally representative data reflecting a single ethnicity. This study was conducted exclusively with type 2 diabetic patients without definite renal impairment, and potential confounders related to UAE or periodontitis were considered in the analysis.
In conclusion, UAE was positively associated with the risk of periodontitis in Korean adults with type 2 diabetes without definite renal impairment. In a subgroup analysis, among the younger patients, those who were newly diagnosed with diabetes, or those who had a normal BMI, the patients with albuminuria were more likely to have periodontitis. Therefore, early identification of periodontitis as a surrogate marker of adverse renal and cardiovascular outcomes may be helpful in Korean diabetic adults with increased UAE.
A longitudinal study or an intervention trial is necessary to ascertain the association.
Acknowledgments
The authors thank the Korea Centers for Disease Control and Prevention for providing the data.
Footnotes
Abbreviations: BMI = body mass index, BP = blood pressure, CI = confidence interval, CKD = chronic kidney disease, CPI = community periodontal index, CVD = cardiovascular disease, FPG = fasting plasma glucose, GFR = glomerular filtration rate, HbA1c = glycated hemoglobin, OR = odds ratio, TG = triglyceride, UACR = urinary albumin to creatinine ratio, UAE = urinary albumin excretion, WBC = white blood cell.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426. [DOI] [PubMed] [Google Scholar]
- 2.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med 1997; 157:1413–1418. [PubMed] [Google Scholar]
- 3.Rosa TT, Palatini P. Clinical value of microalbuminuria in hypertension. J Hypertens 2000; 18:645–654. [DOI] [PubMed] [Google Scholar]
- 4.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782. [DOI] [PubMed] [Google Scholar]
- 5.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, et al. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226. [DOI] [PubMed] [Google Scholar]
- 6.Feldt-Rasmussen B. Microalbuminuria endothelial dysfunction and cardiovascular risk. Diabetes Metab 2000; 26:S64–S66. [PubMed] [Google Scholar]
- 7.Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012; 91:914–920. [DOI] [PubMed] [Google Scholar]
- 8.Shlossman M, Knowler WC, Pettitt DJ, et al. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc 1990; 121:532–536. [DOI] [PubMed] [Google Scholar]
- 9.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol 2001; 6:99–112. [DOI] [PubMed] [Google Scholar]
- 10.Choi YH, McKeown RE, Mayer-Davis EJ, et al. Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care 2011; 34:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janket SJ, Baird AE, Chuang SK, et al. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95:559–569. [DOI] [PubMed] [Google Scholar]
- 12.Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol 2005; 76:2089–2100. [DOI] [PubMed] [Google Scholar]
- 13.Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care 2005; 28:27–32. [DOI] [PubMed] [Google Scholar]
- 14.Southerland JH, Taylor GW, Moss K, et al. Commonality in chronic inflammatory diseases: periodontitis, diabetes, and coronary artery disease. Periodontol 20002006; 40:130–143. [DOI] [PubMed] [Google Scholar]
- 15.Kuroe A, Taniguchi A, Sekiguchi A, et al. Prevalence of periodontal bacterial infection in non-obese Japanese type 2 diabetic patients: relationship with C-reactive protein and albuminuria. Horm Metab Res 2004; 36:116–118. [DOI] [PubMed] [Google Scholar]
- 16.Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care 2007; 30:306–311. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol 2002; 37:409–415. [DOI] [PubMed] [Google Scholar]
- 18.Oh JY, Yang YJ, Kim BS, et al. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J Korean Acad Fam Med 2007; 28:532–541. [Google Scholar]
- 19.Weisell RC. Body mass index as an indicator of obesity. Asia Pac J Clin Nutr 2002; 11:S681–S684. [Google Scholar]
- 20.Oh SW, Shin SA, Yun YH, et al. Cut-off point of BMI and obesity-related comorbidities and mortality in middle-aged Koreans. Obes Res 2004; 12:2031–2040. [DOI] [PubMed] [Google Scholar]
- 21.Americal Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37 Suppl 1:S81–S90. [DOI] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499–502. [PubMed] [Google Scholar]
- 23.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158:825–830. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145:247–254. [DOI] [PubMed] [Google Scholar]
- 25.Kingman A, Susin C, Albandar JM. Effect of partial recording protocols on severity estimates of periodontal disease. J Clin Periodontol 2008; 35:659–667. [DOI] [PubMed] [Google Scholar]
- 26.Lee JB, Yi HY, Bae KH. The association between periodontitis and dyslipidemia based on the Fourth Korea National Health and Nutrition Examination Survey. J Clin Periodontol 2013; 40:437–442. [DOI] [PubMed] [Google Scholar]
- 27.Park JB, Han K, Park YG, et al. Association between alcohol consumption and periodontal disease: the 2008 to 2010 Korea National Health and Nutrition Examination Survey. J Periodontol 2014; 85:1521–1528. [DOI] [PubMed] [Google Scholar]
- 28.Kshirsagar AV, Moss KL, Elter JR, et al. Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 2005; 45:650–657. [DOI] [PubMed] [Google Scholar]
- 29.Fisher MA, Borgnakke WS, Taylor GW. Periodontal disease as a risk marker in coronary heart disease and chronic kidney disease. Curr Opin Nephrol Hypertens 2010; 19:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salimi S, Ng N, Seliger SL, et al. Periodontal disease renal dysfuncton and heightened leukocytosis. Nephron Clin Pract 2014; 128:107–114. [DOI] [PubMed] [Google Scholar]
- 31.Fisher MA, Taylor GW, West BT, et al. Bidirectional relationship between chronic kidney and periodontal disease: a study using structural equation modeling. Kidney Int 2011; 79:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA 2015; 313:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res 1993; 28:500–510. [DOI] [PubMed] [Google Scholar]
- 34.Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol 2013; 40 Suppl 14:S51–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang F, Wu B, Qu Q, et al. The clinical response and systemic effects of non-surgical periodontal therapy in end-stage renal disease patients: a 6-month randomized controlled clinical trial. J Clin Periodontol 2015; 42:537–546. [DOI] [PubMed] [Google Scholar]
- 36.Tawfig A. Effects of non-surgical periodontal therapy on serum lipids and C-reactive protein among hyperlipidemic patients with chronic periodontitis. J Int Soc Prev Community Dent 2015; 5:S49–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palaniappan L, Carnethon M, Fortmann SP. Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens 2003; 16:952–958. [DOI] [PubMed] [Google Scholar]
- 38.Sheng CS, Hu BC, Fan WX, et al. Microalbuminuria in relation to the metabolic syndrome and its components in a Chinese population. Diabetol Metab Syndr 2011; 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nibali L, Tatarakis N, Needleman I, et al. Clinical review: association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab 2013; 98:913–920. [DOI] [PubMed] [Google Scholar]
- 40.AlJehani YA. Risk factors of periodontal disease: review of the literature. Int J Dent 2014; 2014:182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Ellis A, Crowe K, Lawrence J. Obesity-related inflammation: implications for older adults. J Nutr Gerontol Geriatr 2013; 32:263–290. [DOI] [PubMed] [Google Scholar]
- 42.Pontes Andersen CC, Holmstrup P, Buschard K, et al. Renal alterations in prediabetic rats with periodontitis. J Periodontol 2008; 79:684–690. [DOI] [PubMed] [Google Scholar]


