Abstract
This study aimed to investigate the impact of body mass index (BMI) on the short-term and long-term results of a large cohort of gastric cancer (GC) patients undergoing gastrectomy.
Recently, the “obesity paradox” has been proposed, referring to the paradoxically “better” outcomes of overweight and obese patients compared with nonoverweight patients. The associations between BMI and surgical outcomes among patients with GC remain controversial.
A single-institution cohort of 1249 GC patients undergoing gastrectomy between 2000 and 2010 were categorized to low-BMI (<18.49 kg/m2), normal-BMI (18.50–24.99 kg/m2), and high-BMI (≥25.00 kg/m2) groups. The postoperative complications were classified according to the Clavien-Dindo system, and their severity was assessed by using the Comprehensive Complication Index (CCI). The impact of BMI on the postoperative complications and overall survival was analyzed.
There were 908, 158, and 182 patients in the normal-BMI, low-BMI, and high-BMI groups, respectively. The overall morbidity in the high-BMI group (24.7%) was higher than that in either the low-BMI or the normal-BMI group (20.9% and 15.5%, respectively; P = 0.006), but the mean CCI in the low-BMI group was significantly higher (8.32 ± 19.97) than the mean CCI in the normal-BMI and high-BMI groups (3.76 ± 11.98 and 5.58 ± 13.07, respectively; P < 0.001). The Kaplan–Meier curve and the log-rank test demonstrated that the low-BMI group exhibited the worst survival outcomes compared with the normal-BMI group, whereas the high-BMI group exhibited the best survival outcomes (P < 0.001). In multivariate analysis, BMI was identified as an independent prognostic factor. In the stage-specific subgroup analysis, a low BMI was associated with poorer survival in the cases of stage III–IV diseases.
Low BMI was associated with more severe postoperative complications and poorer prognosis. Despite a higher risk of mild postoperative complications, the high-BMI patients exhibited paradoxically “superior” survival outcomes compared with the normal-BMI patients. These findings confirm the “obesity paradox” in GC patients undergoing gastrectomy.
INTRODUCTION
Gastric cancer (GC) is the fourth most common cancer worldwide and has become the third leading cause of cancer deaths.1 Despite the considerable effectiveness of surgical resections in conjunction with necessary adjuvant therapy, the prognosis varies greatly among GC patients with diverse baseline characteristics. Therefore, individualized treatment is required to improve the surgical outcomes in these patients.2 Recently, several studies have reported that the body mass index (BMI) may exhibit an impact on postoperative complications and could be associated with the long-term survival of GC patients.3–6 Thus, the impact of BMI on surgical outcomes in GC patients has attracted significant attention in the clinical setting.
In recent years, the surgical outcomes of patients in different BMI subgroups has become a widely discussed topic.7,8 Despite the evident link between obesity and surgical difficulties, the effects of BMI on both postoperative complications and long-term survival are disputed.3,9 The “obesity paradox” describes the paradoxically “superior” outcomes of overweight and obese patients compared with nonoverweight patients, which is in contrast to the commonly held belief that a high BMI is associated with an increased risk of death in the general population.10,11 To our knowledge, although the mechanisms underlying the positive effects of the BMI remain poorly understood, the “obesity paradox” has been addressed in numerous studies regarding surgical oncology.4,10
Therefore, based on a cohort of unselected, single-institution patients with GC, this study aimed to investigate the effects of BMI on both postoperative complications and long-term survival outcomes.
METHODS
Patient Selection
After the approval of the Biomedical Ethics Committee of West China Hospital, a consecutive group of patients with GC who underwent gastrectomy between January 2000 and December 2010 were retrospectively collected using the patients database of gastrointestinal surgery department of West China Hospital (Sichuan, China). The detailed methods for recruiting patients in the database have been previously described.12 Briefly, clinicopathological data were collected from the medical charts, including the demographic data, types of surgery, tumor location, pathological characteristics, TNM stage (according to the American Joint Committee on Cancer, AJCC 7th edition),13 and adverse events. Complaints of weight loss ≥10% in the 1 year before admission were recorded as a recent weight loss. The follow-up data were updated at 3-month intervals through outpatient visits, telephone calls, or office visits. The data were censored in November 2014. Cases with no records of BMI, complication, postoperative therapies or follow-up were excluded from this analysis. The personal information of the individuals was redacted during the analyses.
BMI Assessment and Classification
The height and weight of each patient were measured and recorded by trained staff at the time of hospitalization. The BMI was calculated as the weight (kg) divided by the square of the height (m) and was categorized according to the World Health Organization (WHO) classification, with cut-off points of <18.49 kg/m2 (underweight), 18.50–24.99 kg/m2 (normal weight), 25.00–29.99 kg/m2 (overweight), and ≥30 kg/m2 (obese).14 The patients in this cohort were assigned to the low-BMI group (underweight), normal-BMI group (normal weight), and high-BMI (overweight and obese) based on their respective BMI values.
Surgical Management
The surgical variables, including the resection pattern, extent of lymphadenectomy, operation time, blood loss, number of lymph nodes retrieved, postoperative complications, and postoperative hospital stay, were obtained. The pattern of resection was determined based on the tumor's location and the resection margins, but the BMI was not considered as a factor in the selection of the surgery. In principle, a distal gastrectomy was considered as the standard procedure for the low-third GC and was generally followed by a Billroth-I gastroduodenostomy, Billroth-II gastrojejunostomy, or a Roux-en-Y gastrojejunostomy according to the surgeons’ preference. A total gastrectomy with a Roux-en-Y esophagojejunostomy was performed for cases with middle and/or upper-third GC. For upper-third GC cases with no gross involvement of surrounding structures, a proximal gastrectomy with an esophagogastrostomy was performed. In accordance with the standards of the Japanese Gastric Cancer Association (JGCA) (Ver. 3), the lymphadenectomies were performed and classified as D1, D1+, or D2.15 The extent of the lymphadenectomy was mainly determined based on the preoperative staging, and macroscopic evaluation.
Assessment of Complications
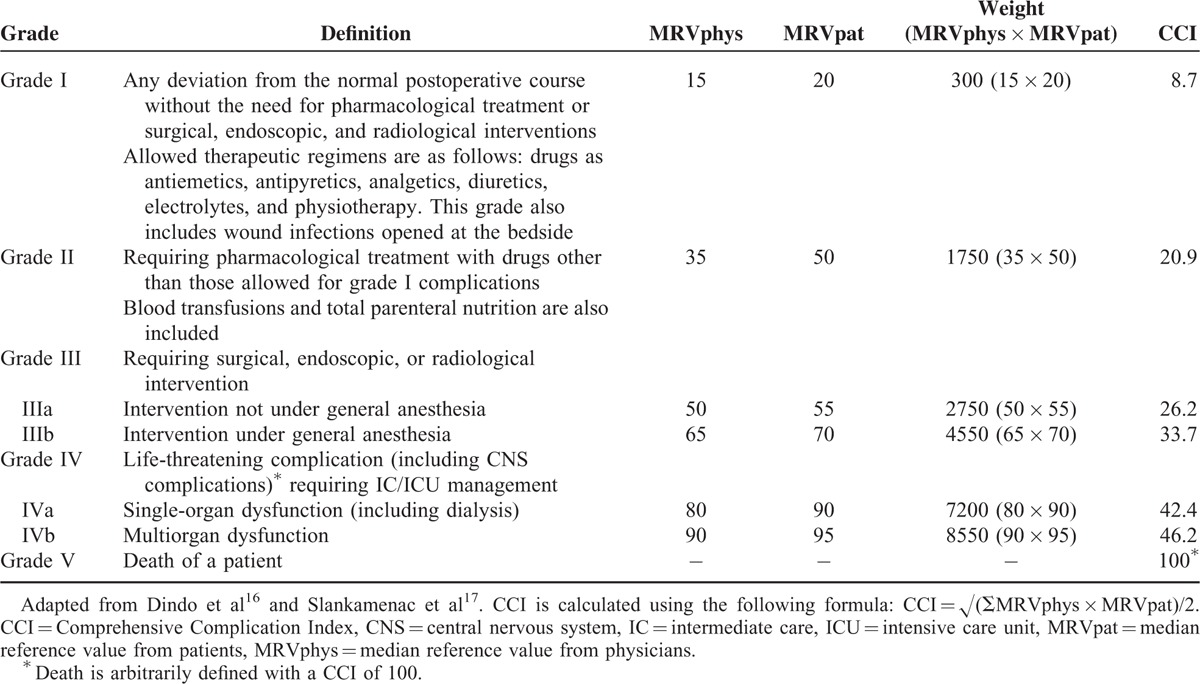
Early adverse events within 30 days after the surgery were recorded as postoperative complications (abdominal or systemic) and were further classified into 5 grades on the basis of the revised version of the Clavien-Dindo classification system with reference to the therapy.16 Additionally, the Comprehensive Complication Index (CCI), a numerical analogue scale from 0 to 100, was calculated to rank the severity of the combined complications in a single patient using the following formula, as previously described: CCI = √(∑MRVphys × MRVpat)/2 (Table 1).17
TABLE 1.
Clavien-Dindo Classification and CCI System
Statistics
The R software, version 3.1.2 (R Core Team, Austria), was used to complete all statistical analyses and graphics.18 The patients’ characteristics and clinicopathological variables were obtained and compared across the BMI groups. An 1-way ANOVA and Pearson χ2 test were used for the univariate analyses where appropriate. Post hoc tests, including Tukey honest significant difference method, were performed when an overall significant difference occurred among the groups. The methods for survival analysis have been previously described.12,19 Briefly, an event was defined as death from any cause, including postoperative death. The Kaplan–Meier method was used to analyze the survival rates. Cox proportional hazards model was used to analyze each single prognostic factor and the Efron method was used to determine the associations. The variables with a P-value of 0.05 or less in the univariate analysis were considered potential candidates for the final model of main effects and were evaluated in a stepwise Cox proportional hazards model. In multivariate analysis, the variables with a P-value >0.05 were discarded in a backward stepwise procedure for the selection of significant predictors. P-values less than 0.05 were considered statistically significant.
RESULTS
Clinicopathological Characteristics
From January 2000 to December 2010, a total of 1936 consecutive patients with GC who underwent gastrectomy were identified, of which 287 cases with no follow-up records, 156 cases with incomplete complication information, and 149 cases with no records of postoperative therapies were excluded. Additionally, 96 cases were also excluded due to incomplete BMI data (Supplementary Figure 1, http://links.lww.com/MD/A465). Finally, a total of 1248 patients were analyzed in this study. For the entire cohort, the mean BMI was 21.79 ± 3.04 kg/m2, with a range of 14.06 to 35.54 kg/m2. There were 908 patients (72.76%) in the normal-BMI group (18.50–24.99 kg/m2), 158 patients (12.66%) in the low-BMI group (<18.49 kg/m2), and 182 patients (14.58%) in the high-BMI group (BMI ≥25 kg/m2). The patients’ clinicopathological characteristics were summarized in Table 2. There was a significant difference in the mean number of lymph nodes harvested across the BMI groups (25.8 ± 13.3 vs. 24.3 ± 14.1 vs. 21.0 ± 14.0 in the low-, normal-, and high-BMI groups, respectively; P = 0.002). In particular, there was no statistical significance with respect to recent weight loss (>10%) across the BMI groups. More advanced diseases (stage III–IV) were more frequent in the lower BMI group. Additionally, among the low-BMI patients, the proportion of patients with advanced disease (stage III–IV) was greater among patients with recent weight loss (Fig. 1).
TABLE 2.
Baseline Characteristics of Patients According to BMI Groups
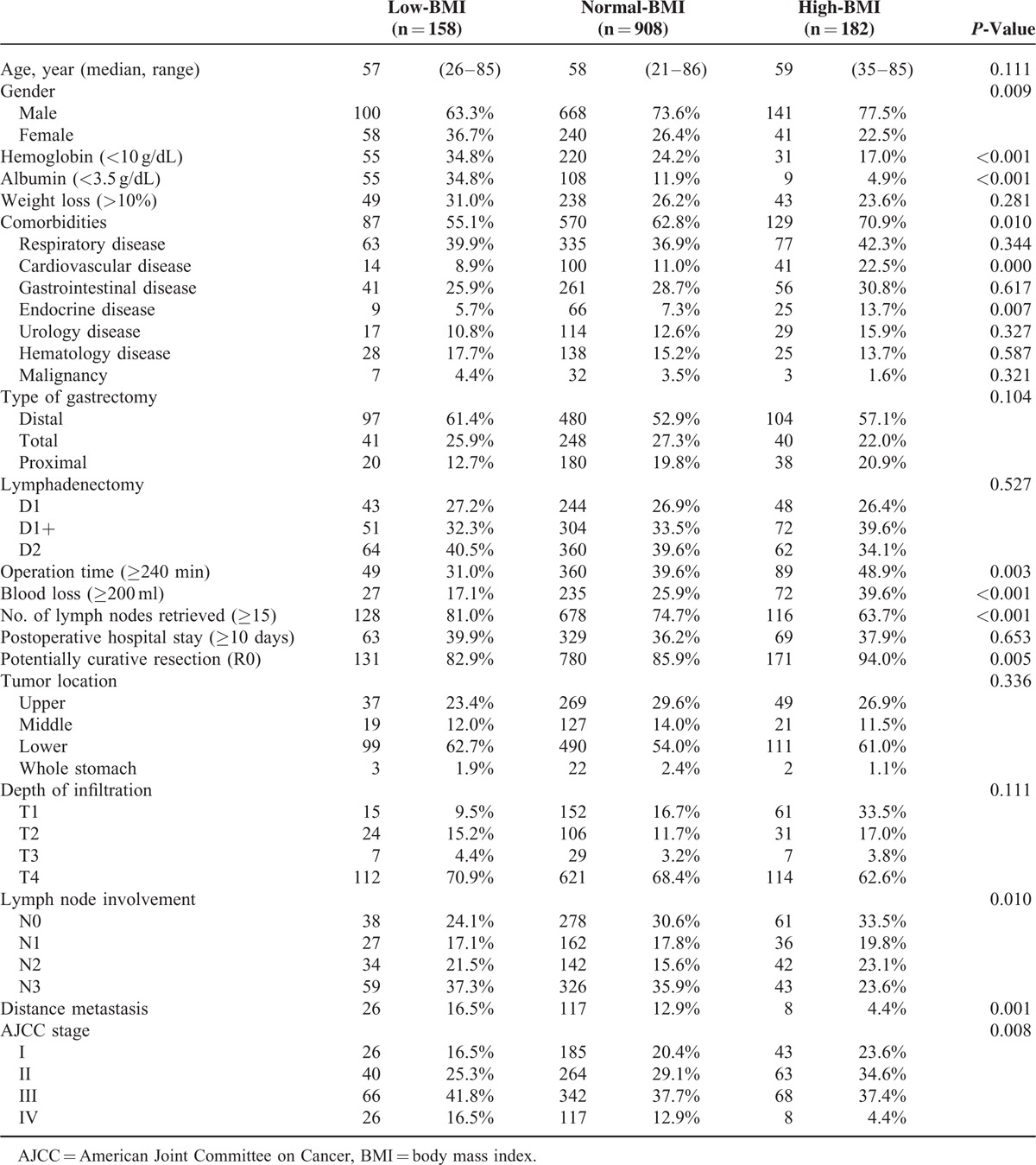
FIGURE 1.
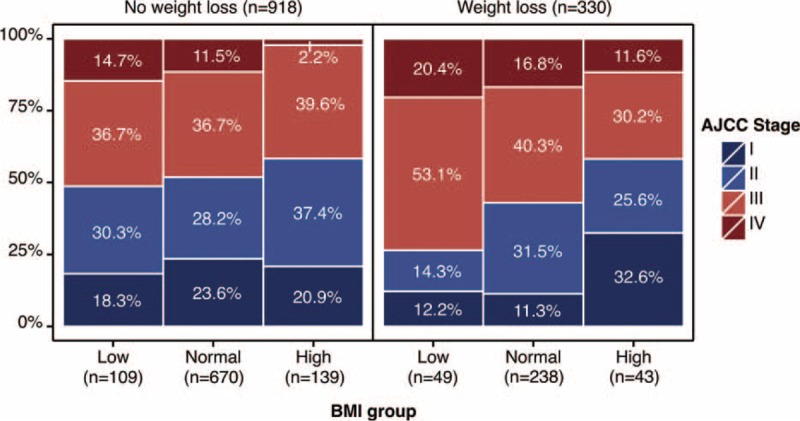
The distribution of the tumor stages according to the BMI group, stratified by the signs of recent weight loss.
Postoperative Complications
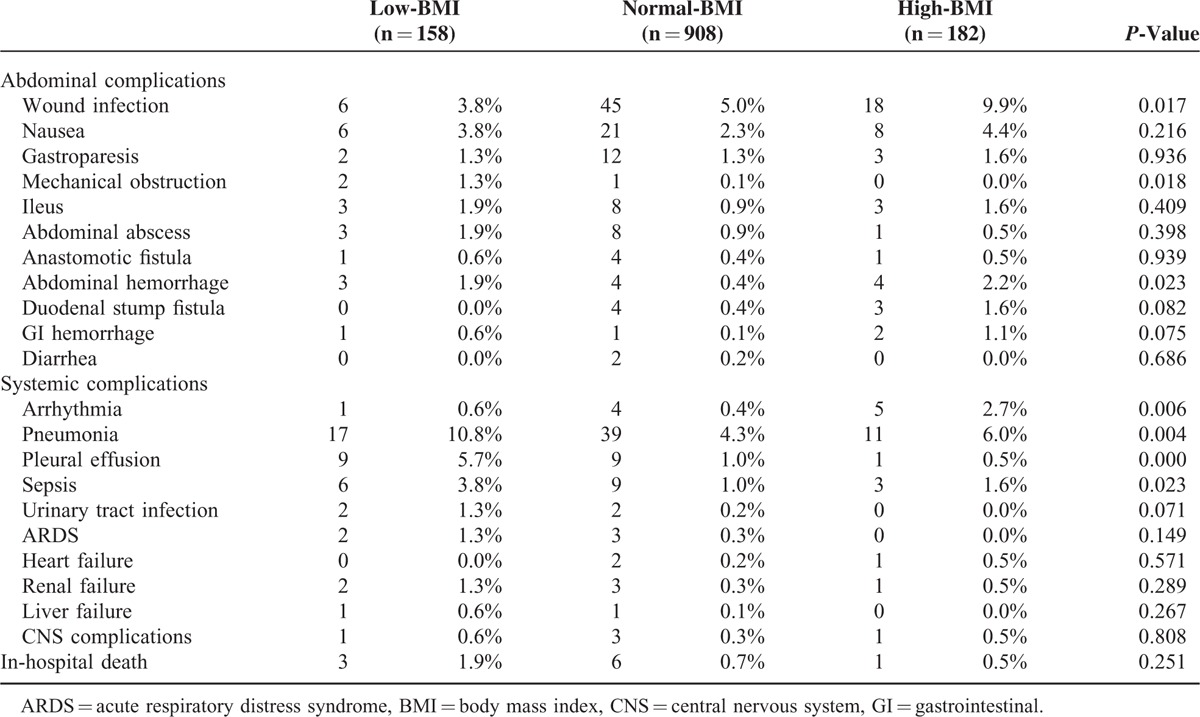
A total of 10 patients died within 30 days postoperatively: 3 (1.9%) in the low-BMI group, 6 (0.7%) in the normal-BMI group, and 1 (0.5%) in the high-BMI group (P = 0.251). The overall postoperative morbidity risk was significantly higher among the high-BMI patients (n = 45, 24.7%) and the low-BMI patients (n = 33, 20.9%) than among the normal-BMI group (n = 141, 15.5%, P = 0.006). As shown in Table 3, the patients in the high-BMI group were more likely to have wound infections (P = 0.017), abdominal hemorrhages (P = 0.023), and cardiac complications such as arrhythmias (P = 0.006), whereas the low-BMI patients had a higher rate of mechanical obstruction (P = 0.018), sepsis (P = 0.023), and pulmonary complications such as pneumonia (P = 0.004) and pleural effusion (P < 0.001).
TABLE 3.
Postoperative Complications After Gastrectomy
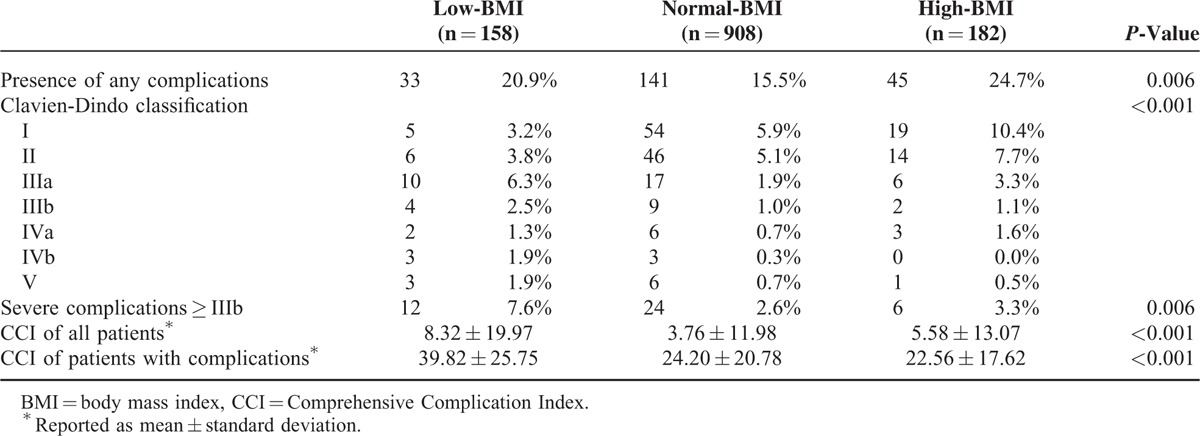
The classification of the severity of the complications among the BMI groups is presented in Table 4. In comparison with the normal-BMI (2.6%) and high-BMI groups (3.3%), more of the patients in the low-BMI group (7.6%) exhibited severe complications (≥IIIb) (P = 0.006). The mean CCI of all of the patients in the low-BMI group (8.32 ± 19.97) was higher than the mean CCI in both the normal-BMI (3.76 ± 11.98) and high-BMI groups (5.58 ± 13.07) (P < 0.001). In subgroups of the patients with complications, the CCI was highest in the low-BMI group, with a mean CCI of 39.82.
TABLE 4.
Effect of BMI Group on Postoperative Complications Using Clavien-Dindo Classification and CCI System
Long-Term Survival
The Kaplan–Meier survival curves for the normal-BMI, low-BMI, and high-BMI groups following gastrectomy are presented in Figure 2. The 5-year overall survival (OS) was 60.7% in the high-BMI group, 50.8% in the normal-BMI group, and 39.2% in the low-BMI group (P < 0.001). The 5-year OS of patients with stage III–IV disease in the high-BMI group was superior to that of the patients in the other two groups (17.6%, 27.3%, and 44.4% in the low-BMI, normal-BMI, and high-BM groups, respectively; P < 0.001). However, no significant survival differences were observed among the BMI groups in stage I–II patients (71.1%, 73.1%, and 72.4% in the low-BMI, normal-BMI, and high-BMI groups, respectively; P = 0.939) (Figure 2).
FIGURE 2.
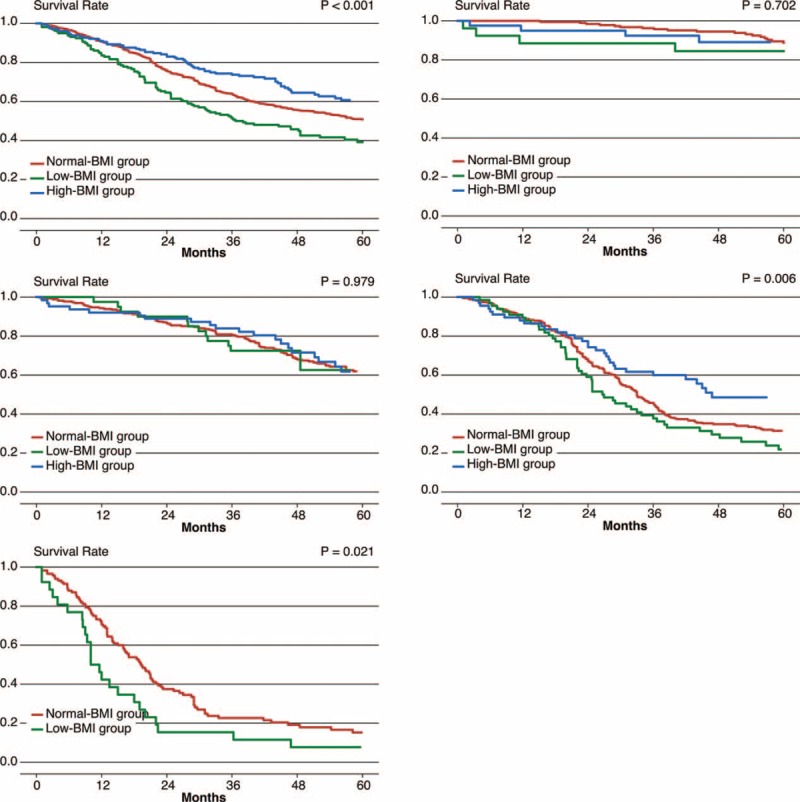
Kaplan–Meier survival analyses according to the BMI, stratified by the AJCC stages. (A) All stages; (B) stage I; (C) stage II; (D) stage III; (E) stage IV. ∗A small number of high-BMI stage IV patients were excluded from the corresponding subgroup analysis.
In the univariate analyses, the OS was associated with age, BMI group, hemoglobin (<10 g/dL), albumin (<3.5 g/dL), recent weight loss (>10%), severe complications (≥IIIb), type of gastrectomy, extent of lymphadenectomy, operation time (≥240 minutes), blood loss (≥200 ml), number of lymph nodes retrieved (≥15), postoperative hospital stay (≥10 days), potentially curative resection (R0), tumor location, T stage, N stage, and M stage. The variables with a P-value of 0.05 or less in the univariate analysis were pooled in a multivariate analysis. The multivariate analysis revealed that the age, BMI group, severe complications (≥IIIb), type of gastrectomy, number of lymph nodes retrieved (≥15), potentially curative resection (R0), T stage, N stage, and M stage were independent predictors of the OS (Table 5, Supplementary Table 1, http://links.lww.com/MD/A465). The hazard ratio (HR) in the high-BMI and low-BMI patients relative to those of normal-BMI patients were 0.764 (95% confidence interval (CI): 0.588–0.993, P = 0.044) and 1.380 (95% CI: 1.102–1.727, P = 0.005), respectively. Considering the different survival rates observed among the tumor stages, an additional Cox model was used to compare the effect of BMI on the mortality risk in different AJCC stages (Figure 3). Only patients with stage III and IV disease exhibited a strong survival difference; the low-BMI patients exhibited a higher mortality risk than the normal- and high-BMI patients.
TABLE 5.
Univariate and Multivariate Analysis of Prognostic Factors for Overall Survival
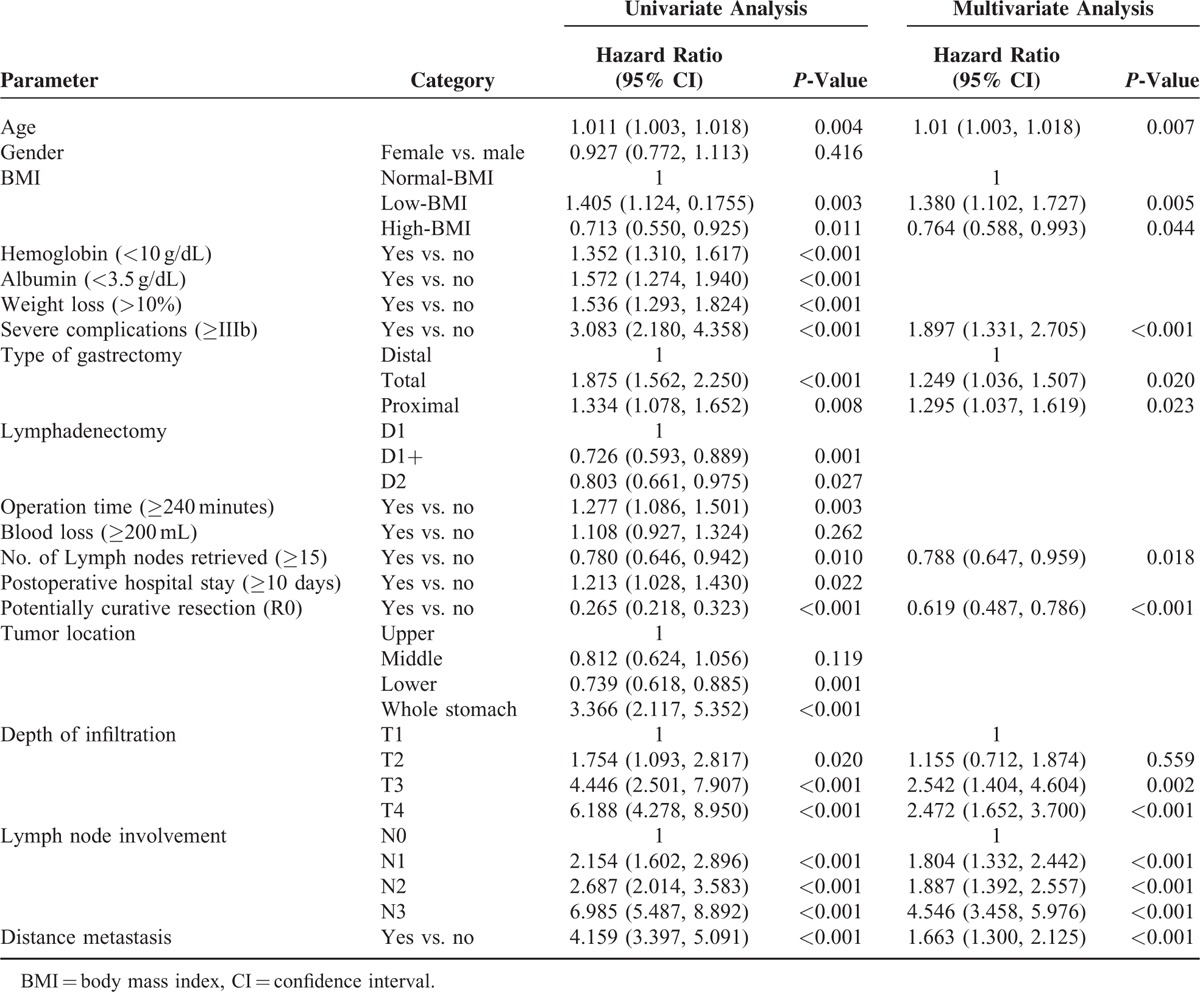
FIGURE 3.
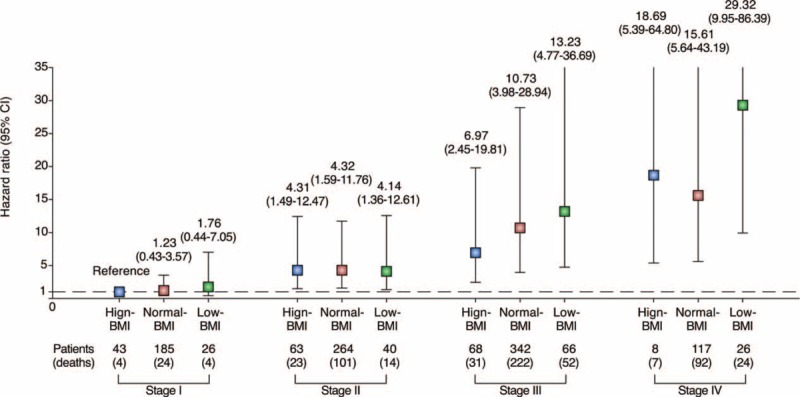
The effect of BMI on overall survival in different AJCC stages. The HR (boxes) and 95% CI (error bars represent values) were calculated after adjusting for age, severe complications (≥IIIb), number of lymph nodes retrieved (≥15), and potentially curative resection (R0). CI = confidence interval, HR = hazard ratio.
DISCUSSION
This study was conducted in an unselected and consecutive cohort of Asian patients diagnosed of GC at a single institution to assess the association between BMI and surgical outcomes following gastrectomy, with a focus on both the postoperative complications and long-term survival. Using the Clavien-Dindo classification and CCI system, our results revealed that the postoperative complications related to a high BMI primarily concern minor complications. On the contrary, patients in the low-BMI group suffered more serious complications compared with the other 2 groups, resulting in a higher postoperative mortality rate. Indeed, the BMI category was proven to be an independent prognostic factor in the multivariate Cox model, as the survival outcomes were gradually associated with the BMI categories. These findings, in conjunction with recent evidence that some classes of obesity can be considered “healthy,” provide some insight into the obesity paradox.20,21
Due to the simplicity of its measurement, BMI has become a widely used variable in clinical practice, classifying specific sets of comorbidities and differential clinicopathological characteristics.8 In essence, a low-BMI is typically accompanied by low albumin and hemoglobin levels, as we observed in this cohort, possibly due to poor nutritional status. This finding becomes particularly obvious in cancer patients because of the energy disturbance resulting from cachexia.22 On the contrary, a high BMI involves a serious change in the endocrine and metabolic responses, resulting in several specific diseases known collectively as metabolic syndrome.23 Additionally, excess adipose tissue may extend the operative time, cause more blood loss, impair the dissection of the lymph nodes and cause the entire operation to be more difficult.24 Thus, it is reasonable to hypothesize that all of these features may eventually result in poorer surgical outcomes for both low-BMI and high-BMI patients.
In previous studies that assessed the association between BMI and postoperative complications, the overall morbidity was represented by counting only the single most severe complication, rather than combining it with the other complications of the individuals.5,9,25,26 Thus, a confounding bias may have occurred, considering that the definitions and the interpretation of complications varies greatly among different medical centers. In our view, the overall morbidity is an essential outcome measurement; however, the effect of BMI on the severity of the complication is of more concern in general practice. The Clavien-Dindo classification and CCI system used in the present study provided an ideal method for yielding a standardized assessment of the burden and severity of the postoperative complication in each patient.16,17 Using this method, we stratified the postoperative complications by their severity with reference to the therapy, and we then incorporated all of the complications that occurred in a single patient, which allowed us to assess the effect of the BMI on the surgical complications from the perspective of their severity.
This study demonstrated a higher overall morbidity in the high-BMI group than in the low-BMI group, which is consistent with previous studies reporting a U-shaped association between BMI and the surgical outcome.4 The higher overall morbidity among the high-BMI patients primarily resulted from the high incidence of wound infections, which corresponded to nearly 40% of the patients with complications in the high-BMI group. Despite the surgical complexity caused by excessive subcutaneous fat,9 the high-BMI patients were more likely to exhibit insulin resistance and poor glycemic control, both of which have been confirmed as risk factors for slow wound recovery.27,28 Furthermore, the metabolic states of high-BMI patients may alter the volume of the distribution and clearance of drugs, impairing the effectiveness of standard antibiotic agents, which may also contribute to the relationship between a high BMI and wound infection.29 Thus, we suggest that the high-BMI patients were at greater risk for overall postoperative complications than the patients in the other BMI groups due to technical difficulties and metabolic disturbances.
However, in addition to the unequal distribution of morbidity across the BMI groups, we noted substantial differences within the severity of the complications across the BMI groups. Based on the CCI measurement, the patients in the low-BMI group were likelier to suffer complications that were more frequent and more severe compared with the high-BMI patients. The higher CCI of the postoperative complications in the low-BMI patients were primarily due to a combination of infections, including pneumonia, pleural effusion, and sepsis. This finding is not unexpected, given that malnutrition has been considered a major risk factor for morbidity and mortality due to infections.30,31 With a higher risk of advanced GC, the underweight patients were more likely to suffer from malnutrition secondary to impaired oral intake, cancer-related anorexia, and increased catabolic energy expenditure.22,32 These major aberrations in energy metabolism in turn impair the immunologic response.33 Based on these considerations, we believe that a more cautious therapeutic strategy, including nutritional supplementation and prophylactic antibiotics, should be recommended for underweight patients who require a gastrectomy to help prevent any severe adverse events.34
These have been several studies investigating the impact of BMI on the long-term outcomes of GC patients who underwent gastrectomy. A meta-analysis by Wu et al35 demonstrated that GC patients with a BMI ≥25 kg/m2 correlated with an increased surgical difficulty, complication, and poor long-term survival. In their analyses, the authors hypothesized that the excess accumulation of visceral fat might impair patient survival as a result of increased comorbidities and complications. However, a study from Asia found no significant difference in 5-year overall survival among patients with BMI <25, 25–30, and >30 kg/m2.36 In another study, Ejaz et al26 investigated 775 patients using a multi-institutional data set of US population and found that underweight patients with BMI <18.5 kg/m2 had a significantly decreased OS after gastrectomy while OS did not differ among normal-BMI and high-BMI patients. More recently, Wong et al37 examined 186 patients at a single institutional in United States and concluded that overall and disease-free survival were significantly associated with increased BMI. However, BMI did not associate with OS in multivariate analysis.
Based on a cohort of unselected, single-institution patients, our study demonstrated that the high-BMI GC patients exhibited a significantly prolonged OS compared with their normal counterparts while underweight patients tended to have a significantly decreased OS. This is contrast to the findings presented by Lin et al,36 but consistent with several studies in literature.3,6,26,37 The inconsistent results between these studies might be attributed to numerous factors including study population, selection criteria of patients, and sample size. Among all these factors, the selection criteria of patients are crucial due to the fact that our present study unselectively included GC patients with distant metastasis. One important factor in the association between BMI and prognosis is the tumor stage, wherein the risk of advanced cancers was nearly doubled in underweight patients compared with the high-BMI patients in this cohort. It is possible that underweight patients in the setting of advanced tumors may account for the higher risk of death in the low-BMI group. However, the BMI group remained an independent predictor of OS after controlling for a broad range of stage-related confounding variables, such as the depth of infiltration, lymph node involvement, distant metastasis, and potentially curative resection. Furthermore, taking into account both the BMI group and the cancer stage, we demonstrated that the survival of the patients with stage III–IV disease was significantly influenced by their BMI group, with the poorest prognosis in the low-BMI group and a better prognosis in both the normal-BMI and high-BMI groups. In contrast, the effect of BMI on survival was not large enough to be appreciable in the patients with stage I–II disease. These results suggest that only in the patients with advanced cancers does the prognosis differ greatly among the BMI groups. Thus, we suppose that the survival differences among the BMI groups are not entirely the result of the unequal distribution of the tumor stage, and the patients with a high BMI may be more able to withstand the cancer-induced consumption and stress compared with the low-BMI patients, supporting the “obesity paradox.”
The “obesity paradox” was firstly documented by Dr. Carl Lavie study regarding heart failure.38 In the subsequent studies, the positive effects of BMI have been addressed in patients with coronary disease, stroke, kidney disease, diabetes, and certain forms of cancer.20 In a study including over 2 million people, being overweight was associated with a significantly lower all-cause mortality, whereas grade 1 obesity was not associated with a higher mortality, suggesting that individuals with a BMI between 25 and 35 could be considered healthy rather than ill.21 This finding becomes particularly interesting considering that the majority of high-BMI patients in this cohort, who had a superior survival outcome compared with normal-, and low-BMI patients, were classified either as overweight or as class I obese. Although the mechanism underlying the positive effects of BMI are still not well understood, intensive researches on this topic have revealed that factors secreted by adipose tissue, such as adipokines, may play essential roles in the “obesity paradox” via the manipulation of both inflammation and immunity.39 Anyway, this study confirmed the existence of “obesity paradox” in GC patients who underwent gastrectomy, which may facilitate further study to reveal the potential molecular mechanism underlying this phenomenon.
Our study has some limitations. First, even with a cohort of more than 1000 patients, the sample size was still relatively small in subgroup analyses (eg, the tumor stages). This became particularly obvious when we sought statistically significant findings for the postoperative complications between the groups. Second, as this is a single-institution study based on an Asian cohort, the distribution of BMI was obviously left shifted compared to those in Western populations. The range of BMI included in this study may not characterize the high side of BMI distribution in Western population (ie, morbidly obesity). Thus, the results from this study need to be validated in Western countries before extrapolation to other surgeons or institutions. Another limitation of our study is that we did not have data for the postoperative BMI changes. Therefore, we cannot comment on the impact of surgery on changes in the BMI among patients with GC who underwent gastrectomy.
In conclusion, the present study suggests that low BMI may be associated both with more severe postoperative complications and poor prognosis among stage III–IV GC patients. Despite a higher risk of mild postoperative complications, high-BMI GC patients undergoing gastrectomy exhibit a paradoxically “superior” survival outcome compared with normal-BMI patients, confirming the existence of “obesity paradox.” With a distinct left-shifted distribution of BMI, Asian GC patients, particularly those with low BMI, should receive optimized management based on their BMI.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, ARDS = acute respiratory distress syndrome, BMI = body mass index, CCI = Comprehensive Complication Index, CI = confidence interval, CNS = central nervous system, GC = gastric cancer, GI = gastrointestinal, HR = hazard ratio, IC = intermediate care, ICU = intensive care unit, JGCA = Japanese Gastric Cancer Association, MRVpat = median reference value from patients, MRVphys = median reference value from physicians, WHO = World Health Organization.
Hai-Ning Chen and Xin-Zu Chen contributed equally to this work.
Domestic support from National Natural Science Foundation of China (no. 81071777 and 81372344).
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. 2013; Lyon, France: International Agency for Research on Cancer, Available from: http://globocan.iarc.fr. Accessed on August 14, 2014.. [Google Scholar]
- 2.Roukos DH, Kappas AM. Perspectives in the treatment of gastric cancer. Nat Clin Pract Oncol 2005; 2:98–107. [DOI] [PubMed] [Google Scholar]
- 3.Tokunaga M, Hiki N, Fukunaga T, et al. Better 5-year survival rate following curative gastrectomy in overweight patients. Ann Surg Oncol 2009; 16:3245–3251. [DOI] [PubMed] [Google Scholar]
- 4.Yasunaga H, Horiguchi H, Matsuda S, et al. Body mass index and outcomes following gastrointestinal cancer surgery in Japan. Br J Surg 2013; 100:1335–1343. [DOI] [PubMed] [Google Scholar]
- 5.Bickenbach KA, Denton B, Gonen M, et al. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann Surg Oncol 2013; 20:780–787. [DOI] [PubMed] [Google Scholar]
- 6.Kulig J, Sierzega M, Kolodziejczyk P, et al. Implications of overweight in gastric cancer: a multicenter study in a Western patient population. Eur J Surg Oncol 2010; 36:969–976. [DOI] [PubMed] [Google Scholar]
- 7.Dindo D, Muller MK, Weber M, et al. Obesity in general elective surgery. Lancet 2003; 361:2032–2035. [DOI] [PubMed] [Google Scholar]
- 8.Tao W, Lagergren J. Clinical management of obese patients with cancer. Nat Rev Clin Oncol 2013; 10:519–533. [DOI] [PubMed] [Google Scholar]
- 9.Tsujinaka T, Sasako M, Yamamoto S, et al. Influence of overweight on surgical complications for gastric cancer: results from a randomized control trial comparing D2 and extended para-aortic D3 lymphadenectomy (JCOG9501). Ann Surg Oncol 2007; 14:355–361. [DOI] [PubMed] [Google Scholar]
- 10.Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg 2009; 250:166–172. [DOI] [PubMed] [Google Scholar]
- 11.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006; 355:763–778. [DOI] [PubMed] [Google Scholar]
- 12.Chen HN, Chen XZ, Zhang WH, et al. Necessity of harvesting at least 25 lymph nodes in patients with stage N2-N3 resectable gastric cancer: a 10-year, single-institution cohort study. Medicine (Baltimore) 2015; 94:e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephen B, Edge DRB, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag; 2010. [Google Scholar]
- 14.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163. [DOI] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013; 258:1–7. [DOI] [PubMed] [Google Scholar]
- 18.Core R, Team R. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 19.Bruin J. Survival Analysis with Stata. UCLA: Statistical Consulting Group. Available from: http://www.ats.ucla.edu/STAT/stata/seminars/stata_survival/. Accessed on August 14, 2014. Los Angeles. [Google Scholar]
- 20.Lavie CJ, De Schutter A, Milani RV. Healthy obese versus unhealthy lean: the obesity paradox. Nat Rev Endocrinol 2015; 11:55–62. [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. Cancer J Clin 2002; 52:72–91. [DOI] [PubMed] [Google Scholar]
- 23.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444:881–887. [DOI] [PubMed] [Google Scholar]
- 24.Eom BW, Joo J, Yoon HM, et al. A body shape index has a good correlation with postoperative complications in gastric cancer surgery. Ann Surg Oncol 2014; 21:1115–1122. [DOI] [PubMed] [Google Scholar]
- 25.Ojima T, Iwahashi M, Nakamori M, et al. Influence of overweight on patients with gastric cancer after undergoing curative gastrectomy: an analysis of 689 consecutive cases managed by a single center. Arch Surg 2009; 144:351–358.discussion 8. [DOI] [PubMed] [Google Scholar]
- 26.Ejaz A, Spolverato G, Kim Y, et al. Impact of body mass index on perioperative outcomes and survival after resection for gastric cancer. J Surg Res 2015; 195:74–82. [DOI] [PubMed] [Google Scholar]
- 27.Sørensen LT, Hemmingsen U, Kallehave F, et al. Risk factors for tissue and wound complications in gastrointestinal surgery. Ann Surg 2005; 241:654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007; 117:1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 2010; 49:71–87. [DOI] [PubMed] [Google Scholar]
- 30.Palma S, Cosano A, Mariscal M, et al. Cholesterol and serum albumin as risk factors for death in patients undergoing general surgery. Br J Surg 2007; 94:369–375. [DOI] [PubMed] [Google Scholar]
- 31.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 2007; 4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argiles JM, Busquets S, Stemmler B, et al. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014; 14:754–762. [DOI] [PubMed] [Google Scholar]
- 33.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 2005; 5:844–852. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin C, Weekes CE. Dietary advice with or without oral nutritional supplements for disease-related malnutrition in adults. Cochrane Database Syst Rev 2011; CD002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu XS, Wu WG, Li ML, et al. Impact of being overweight on the surgical outcomes of patients with gastric cancer: a meta-analysis. World J Gastroenterol 2013; 19:4596–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin YS, Huang KH, Lan YT, et al. Impact of body mass index on postoperative outcome of advanced gastric cancer after curative surgery. J Gastrointest Surg 2013; 17:1382–1391. [DOI] [PubMed] [Google Scholar]
- 37.Wong J, Rahman S, Saeed N, et al. Effect of body mass index in patients undergoing resection for gastric cancer: a single center US experience. J Gastrointest Surg 2014; 18:505–511. [DOI] [PubMed] [Google Scholar]
- 38.Lavie CJ, Osman AF, Milani RV, et al. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol 2003; 91:891–894. [DOI] [PubMed] [Google Scholar]
- 39.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6:772–783. [DOI] [PubMed] [Google Scholar]


