Abstract
Idiopathic pulmonary fibrosis (IPF) lacks effective treatment. Pirfenidone has been used to treat IPF patients. N-acetylcysteine (NAC) exerts antioxidant and antifibrotic effects on IPF cases.
This study is a double-blind, modified placebo-controlled, randomized phase II trial of pirfenidone in Chinese IPF patients. We randomly assigned the enrolled Chinese IPF patients with mild to moderate impairment of pulmonary function to receive either oral pirfenidone (1800 mg per day) and NAC (1800 mg per day) or placebo and NAC (1800 mg per day) for 48 weeks. The primary endpoints were the changes in forced vital capacity (FVC) and walking distance and the lowest SPO2 during the 6-minute walk test (6MWT) at week 48. The key secondary endpoint was the progression-free survival time. This study is registered in ClinicalTrials.gov as number NCT01504334.
Eighty-six patients were screened, and 76 cases were enrolled (pirfenidone + NAC: 38; placebo + NAC: 38). The effect of pirfenidone treatment was significant at the 24th week, but this effect did not persist to the 48th week. At the 24th week, the mean decline in both FVC and ΔSPO2 (%) during the 6MWT in the pirfenidone group was lower than that in the control group (−0.08 ± 0.20 L vs −0.22 ± 0.29 L, P = 0.02 and −3.44% ± 4.51% vs −6.29% ± 6.06%, P = 0.03, respectively). However, there was no significant difference between these 2 groups at the 48th week (−0.15 ± 0.25 L vs −0.25 ± 0.28 L, P = 0.11 and −4.25% ± 7.27% vs −5.31% ± 5.49%, P = 0.51, respectively). The pirfenidone treatment group did not achieve the maximal distance difference on the 6MWT at either the 24th or the 48th week. But pirfenidone treatment prolonged the progression-free survival time in the IPF patients (hazard ratio = 1.88, 95% confidence interval: 1.092–3.242, P = 0.02). In the pirfenidone group, the adverse event (AE) rate (52.63%) was higher than that in the control group (26.3%, P = 0.03). Rash was more common in the pirfenidone group (39.5% vs 13.2%, P = 0.02).
Compared with placebo combined with high-dose NAC, pirfenidone combined with high-dose NAC prolonged the progression-free survival of Chinese IPF patients with mild to moderate impairment of pulmonary function. (ClinicalTrials.gov number, NCT01504334).
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal disease that lacks an effective treatment. The median survival time for IPF patients is just 3 years.1 A characteristic of the pathological manifestation of IPF is the presence of fibroblastic foci in the fibrotic lung. TGF-β is a central stimulator of collagen production during the pathogenesis of pulmonary fibrosis.2–4 Moreover, oxidative stress contributes to the pathogenesis of IPF and may coordinate with TGF-β during the progression of IPF.5–7
Pirfenidone (PFD), or 5-methyl-1-phenyl-2-[1H]-pyridone, is a novel agent developed for the treatment of IPF. PFD and its metabolites 5-hydroxypirfenidone (PFD-OH) and 5-carboxypirfenidone (PFD-COOH) exert antifibrotic and antioxidant effects.8–11 After several phase II and III clinical trials for PFD,12–15 PFD has been used for IPF patients in Japan, Europe, and India. And in the new treatment guideline for IPF, both PFD and nintedanib are conditionally recommended for the treatment for IPF patients.16N-acetylcysteine (NAC) also has antioxidant and antifibrotic effects, and high-dose NAC had been weakly recommended for IPF patients according to 2011 IPF guideline.1,17–20 But recent clinical trial has not showed the efficacy of high-dose NAC for IPF.21 In the new treatment guideline for IPF, NAC monotherapy is conditionally recommended against to use. But the ATS does recommend to keep patients on NAC if they are tolerating well.21 The evidence for or against the use of NAC is still not very clear.
Based on the results of these clinical trials and new treatment guideline for IPF, we performed a double-blind, modified placebo-controlled, randomized phase II trial of PFD in Chinese IPF patients. In our trial, the baseline treatment was high-dose NAC (600 mg 3 times daily), and PFD or placebo was added to the enrolled patients. To our knowledge, this may be the first registered clinical trial to use a PFD and NAC combination therapy for IPF.
METHODS PATIENTS
The diagnosis of IPF was in accordance with evidence-based guidelines for the diagnosis and management of IPF published in 2011.1 All patient high-resolution computed tomography (HRCT) images and lung-biopsy specimens were reviewed centrally (in our hospital) by 2 experienced radiologists and 2 experienced pathologists, respectively, to verify eligibility. The diagnosis for each enrolled case in our trial was approved based on a multidisciplinary discussion of experienced interstitial lung disease experts in respiratory, pathology, and radiology departments. The eligible patients were 18 to 75 years of age. Patients younger than 50-years old were required to exhibit a characteristic usual interstitial pneumonia (UIP) pattern based on a surgical lung biopsy. After the diagnosis of IPF was acquired, the patient was enrolled to this trial for further screening. In addition to the diagnosis of IPF, all enrolled cases met the following lung function criteria: percentage of predicted forced vital capacity (FVC) of at least 45%, percentage of predicted carbon monoxide diffusing capacity (DLCO) of at least 30%, and PaO2 of at least 50 mmHg when the patient is at rest and breathing room air.
The exclusion criteria were as follows:
aggravated dyspnea during the preceding 6 months;
currently in a period of acute exacerbation of IPF (AE-IPF);
fasting blood glucose level of more than 11.1 mmol/L
comorbid conditions including malignancy, bleeding tendency, severe hepatic dysfunction (alanine transaminase or aspartate transaminase level 2-fold above the upper limit of normal), or renal (serum creatinine level above the upper limit of normal) or cardiac disease (New York Heart Association grade 3–4);
use of immunosuppressants, antifibrotic drugs including interferon, d-penicillamine and colchicine, or oral corticosteroids at a dose ≥15 mg/day or the equivalent during the preceding 3 months;
current pregnancy or breastfeeding;
enrollment in another clinical trial during the preceding 1 month; or
denied by the researchers.
All enrolled patients provided written informed consent, and the protocol for our study was approved by the ethics committee of each center.
Study Design
The study was a double-blind, placebo-controlled, randomized, multicenter clinical trial that was conducted at 5 sites in northern China, including Beijing (3 centers), Tianjin (1 center), and Shenyang (1 center). The study was approved by the State Food and Drug Association of China (number 2010L03738). The trial is also registered in ClinicalTrials.gov (number NCT01504334).
Beijing Kawin Technology Share-Holding Co., Ltd. provided 200 mg PFD and matching placebo tablets, as well as NAC, for oral use. All patients were treated with 600 mg of NAC (Fluimucil, Zambon Group) 3 times daily as a baseline treatment. The patients were randomly assigned to the PFD or placebo (1:1) group using a modified permuted-block centralized dynamic randomization method with block sizes of 4. A dose titration schedule for PFD/placebo was arranged for all enrolled patients: the patients received oral tablets (PFD/placebo) at a dose of 200 mg 3 times daily for the first 7 days, 400 mg 3 times daily for the 2nd week, and 600 mg 3 times daily (target dose) for the 3rd week. The target dose was maintained in patients who tolerated it throughout the subsequent study period. If the patients did not tolerate 1800 mg of PFD daily, their treatment was modified to a reduced regimen according to the Standards for Classification of Serious Adverse Drug Reactions.13 It was suggested to administer PFD/placebo and NAC with food. The enrolled patients were advised to use sunscreen and/or long sleeves and hats during exposure to direct sunlight. All patients were asked to take a reliable method of contraception during the clinical trial when they and/or their partners were fertile.
The patients were observed in the clinic weekly for 2 weeks; after 2 weeks, they were asked to follow-up in the clinic at the 4th, 12th, 24th, 36th, and 48th weeks after they were enrolled. A complete blood count, urine analysis, the erythrocyte sedimentation rate (ESR), the C-reactive protein level, a blood chemistry panel (including hepatic and kidney function and lipid levels), an auto-antibody panel (including antinuclear antibodies, antiextractable nuclear antigen, antineutrophil cytoplasmic antibodies, rheumatoid factor [RF], and antiphospholipid antibodies) were tested. Complete blood count, urine analysis, and the blood chemistry panel were repeated on the 4th, 24th, and 48th weeks. All HRCT scans, pulmonary function tests (PFTs), 6-minute walk tests (6MWTs), analyses of arterial blood gas (ABG), electrocardiography, dyspnea, and St George's Respiratory Questionnaire (SGRQ) were performed in accordance with the predetermined protocol at baseline and at 24-week intervals.
The HRCT findings were graded on a scale of 1 to 6 based on the classification by Ichikado et al22 for areas with ground-glass attenuation, reticular changes, or honeycombing.
Efficacy Endpoints
The primary endpoints were defined as the change in FVC from baseline to week 48, and the change in the maximal distance on the 6MWT and the change in the lowest SPO2 during the 6MWT from baseline to week 48. The secondary endpoints were defined as the change in the result on the resting PFT, including the percentage predicted FVC, FEV1 and the percentage predicted FEV1, total lung capacity (TLC) and the percentage predicted TLC, DLCO and the percentage predicted DLCO, DLCO/VA from baseline to week 48; the change in ABGs including PaCO2, PaO2, and SaO2; episodes of AE-IPF;1 progression-free survival (PFS) duration, which was defined as the time until the first occurrence of any one of the following:15 a confirmed ≥10% decline in the percentage predicted FVC, a confirmed ≥15% decline in the percentage predicted DLCO (corrected based on the patient's actual hemoglobin levels), a confirmed progression of fibrosis defined by the HRCT fibrosis score, AE-IPF, or death; change in the dyspnea score; change in fibrosis according to HRCT; and change in the SGRQ score.
Safety was assessed based on clinical and laboratory evaluations at all visits and adverse events (AEs) recorded by investigators.
Statistical Analysis
Statistical analysis was performed using Statistical Analysis System (SAS) software version 9.2 (SAS Institute Inc., Cary, NC). The means and the 95% confidence intervals were calculated. Differences in the means were examined using 2-sided t-tests, and statistical significance was defined as P < 0.05. Baseline data were analyzed for each full analysis set (FAS). Efficiency variables were calculated for each FAS and per protocol set (PPS). Safety analyses were performed using each safety set (SS). Quantitative data regarding the clinic visits of the 2 groups were expressed as the means and standard deviations. Paired t-tests were employed to test the difference between the basic characteristics at baseline. Analysis of variance was performed to compare the parameters before and after treatment. Qualitative data were expressed as frequencies, and differences between the groups were examined using the χ2 test or a nonparametric test.
RESULTS
Enrolled Cases and Follow-Up
Eighty patients were screened from January 10, 2012 to July 6, 2012, and 76 cases were enrolled in this study (PFD: 38, placebo: 38) (Figure 1). Among the 38 cases in the PFD group, 34 cases were included in the PPS, and 38 cases were included in the FAS and the SS. Among the 38 cases in the control group, 37 cases were included in the PPS, and 38 cases were included in the FAS and the SS. Six cases withdrew from the study, including 3 cases that withdrew consent and 3 cases that had trial-associated AEs. Four cases died during the study, including 2 cases in the PFD group and 2 cases in the control group. Sixty-six cases completed the entire study.
FIGURE 1.
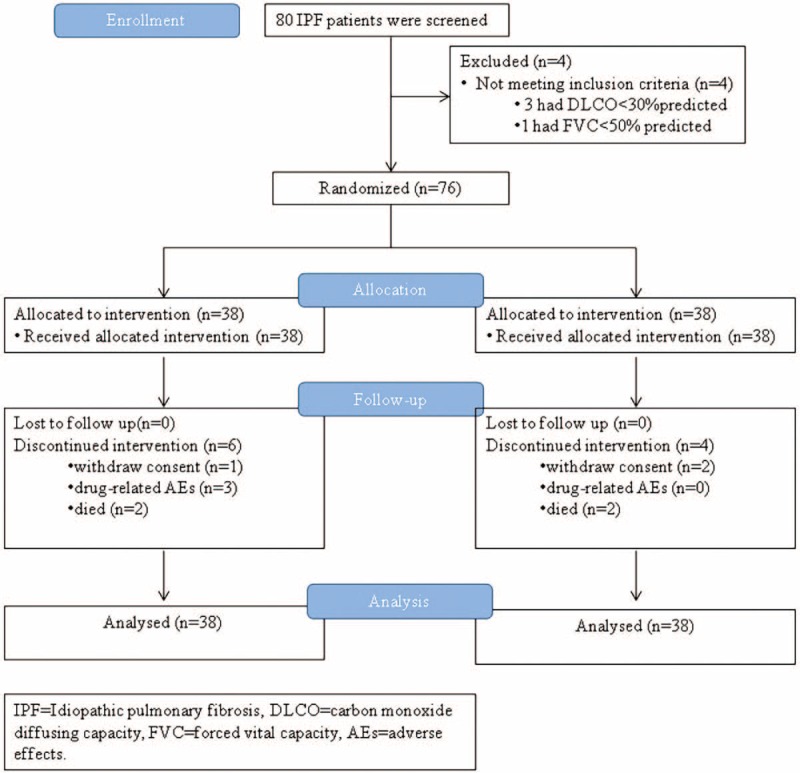
Enrollment and outcomes.
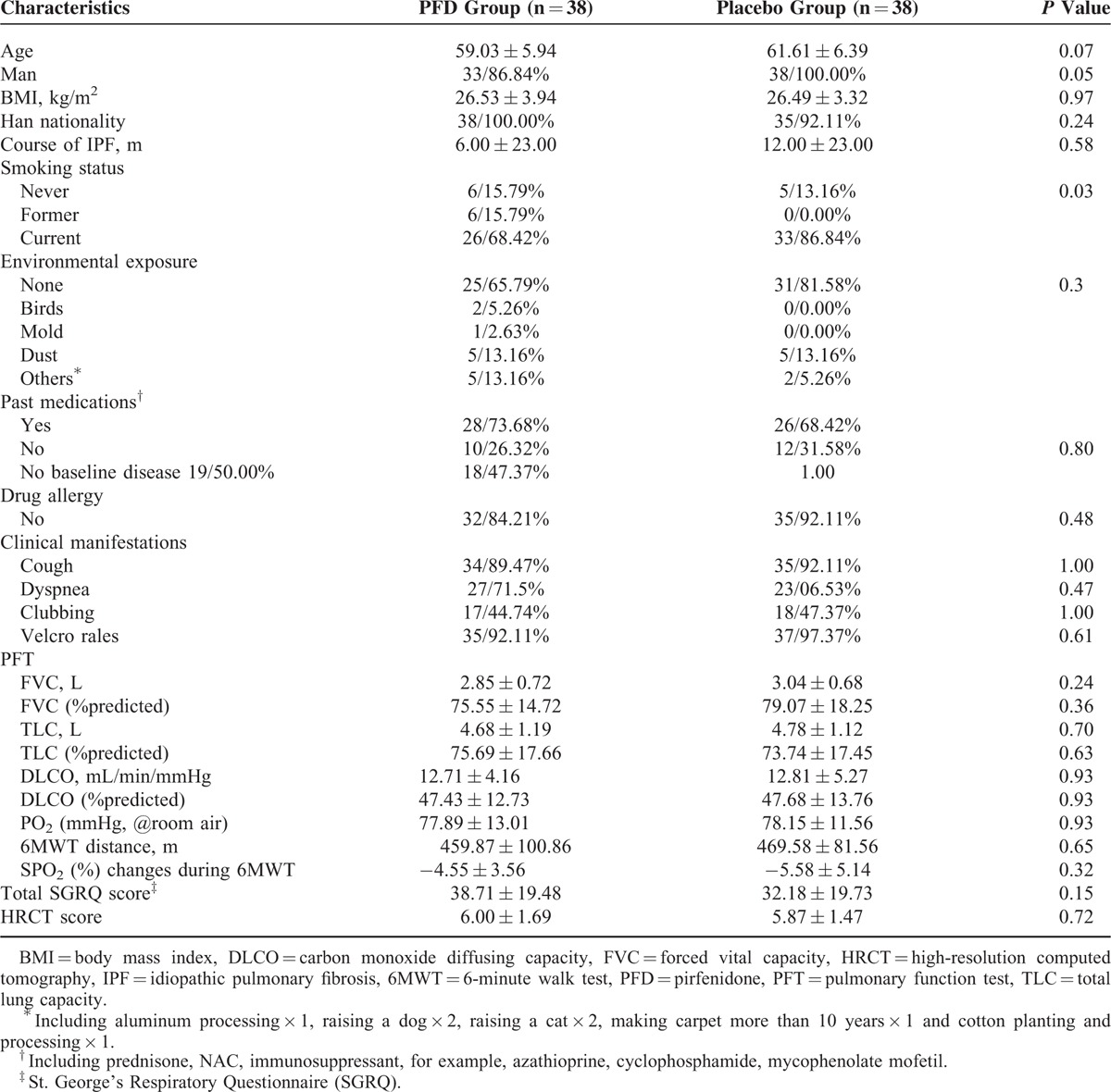
Among these 76 IPF cases, 56 cases were diagnosed with a definite UIP pattern according to HRCT, and the others were diagnosed with a probable UIP pattern. Four cases underwent a surgical lung biopsy and were found to exhibit a characteristic pathological UIP pattern. There were no significant differences in the baseline characteristics between the PFD group and the control group (Table 1). Most of the enrolled cases were male (86.8% in the PFD group and 100% in the control group, P = 0.05). On the baseline 6MWT, the maximal distance was 459.87 ± 100.86 m in the PFD group and 469.58 ± 81.56 m in the control group (P = 0.65), and the SPO2 (%) decreased by 4.55% ± 3.56% in the PFD group and by 5.58% ± 5.14% in the control group (P = 0.32). There were no significant differences in the baseline PFT indices, including FVC, TLC, and DLCO, between the PFD and control groups (Table 1).
TABLE 1.
Baseline Characteristic of the Enrolled Patients
Primary Efficacy Analysis
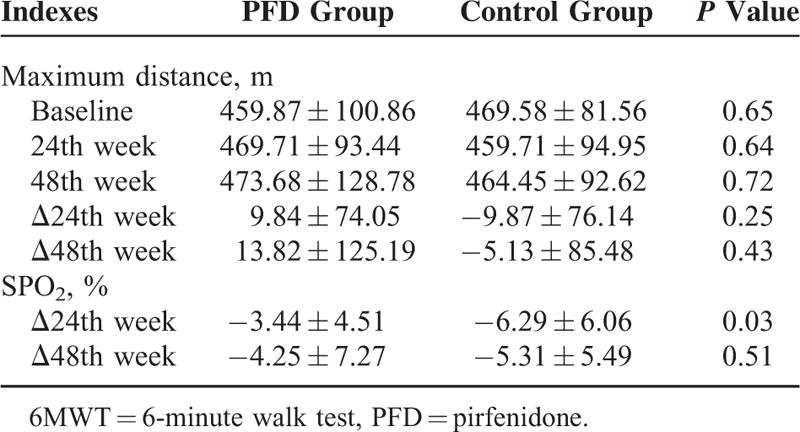
The effect of PFD treatment was significant at the 24th week, but this effect did not persist to the 48th week. At the 24th week, the mean decline in both FVC and ΔSPO2 (%) on the 6MWT in the PFD group was lower than that in the control group (−0.08 ± 0.20 L vs −0.22 ± 0.29 L, P = 0.02 and −3.44% ± 4.51% vs −6.29% ± 6.06%, P = 0.03, respectively). Although the mean decline in these indices tended to be smaller in the PFD group, there was no evident difference between these 2 groups at the 48th week (−0.15 ± 0.25 L vs −0.25 ± 0.28 L, P = 0.11 and −4.25% ± 7.27% vs −5.31% ± 5.49%, P = 0.51, respectively) (Figure 2A–D). PFD treatment did not result in a maximum distance difference on the 6MWT at either the 24th or the 48th week. However, there was a trend of a smaller decline in the PFD group (Table 2).
FIGURE 2.
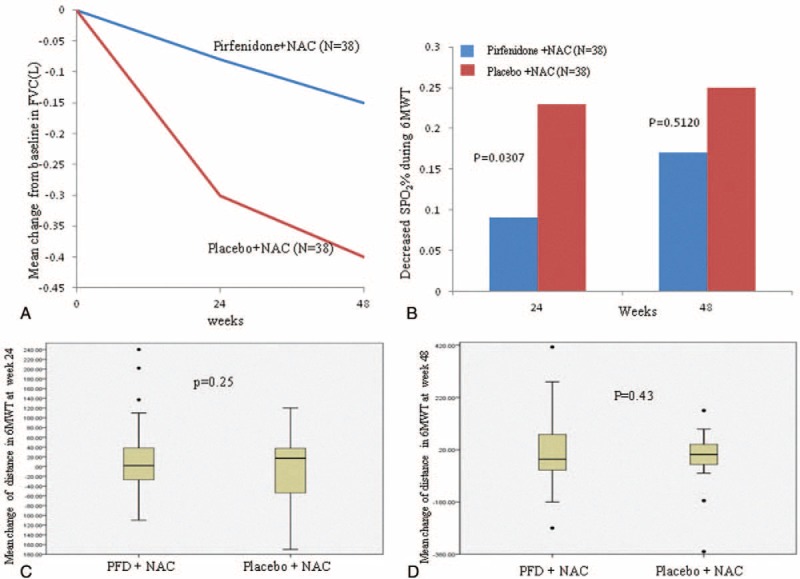
(A) Shows the mean change in FVC from baseline to the 24th week (P = 0.02) and the 48th week in the PFD and placebo groups (P = 0.11). (B) Shows the mean decline in ΔSPO2 (%) during the 6MWT. (C) Shows the mean change in the maximum walk distance on the 6MWT from baseline to the 24th week. (D) Shows the mean change in the maximum walk distance on the 6MWT from baseline to the 48th week. FVC = forced vital capacity, 6MWT = 6-minute walk test, PFD = pirfenidone.
TABLE 2.
Changes of Indexes in the 6MWT During the Study
Based on subgroup analysis, we found that 4 cases (3 cases in the PFD group and 1 case in the control group) had a significant AE-associated decline in the PFT results during the second 24 weeks. In the PFD group, 1 case suffered from an aggravated dry cough after a cold during the 28th week, and this event influenced the PFT procedure; 1 case caught an upper respiratory infection that was complicated by acute bronchiolitis on the 47th week and completed the end-of-treatment (EOT) follow-up on the 48th week; and 1 case suffered from AE-IPF on the 46th week and completed the EOT follow-up on the 48th week 3 days after discharge. In the control group, one case suffered from AE-IPF on the 44th week and completed the EOT follow-up on the 48th week, 8 days after discharge. If we excluded these 4 cases, there was a significant difference in FVC between the PFD group and the control group at both the 24th (−0.08 ± 0.20 vs −0.22 ± 0.29, 38 vs 38 cases, P = 0.018) and 48th weeks (−0.11 ± 0.25 vs −0.24 ± 0.28, 35 vs 37 cases, P = 0.048).
Secondary Efficacy Analysis
For the PFT indices, the mean decline in FVC% and DLCO at the 24th week in the PFD group was lower than that in the control group (0.68% ± 7.66% vs 6.11% ± 7.70%, P = 0.004 and 0.73% ± 9.36% vs 5.29% ± 9.62%, P = 0.048, respectively). Although the difference between the PFD group and the control group disappeared at the 48th week, there smaller declines were observed in the PFD group. There were no significant differences in the other PFT indices included as secondary endpoints between the 2 groups (Table 3).
TABLE 3.
Summary of the Secondary Endpoints
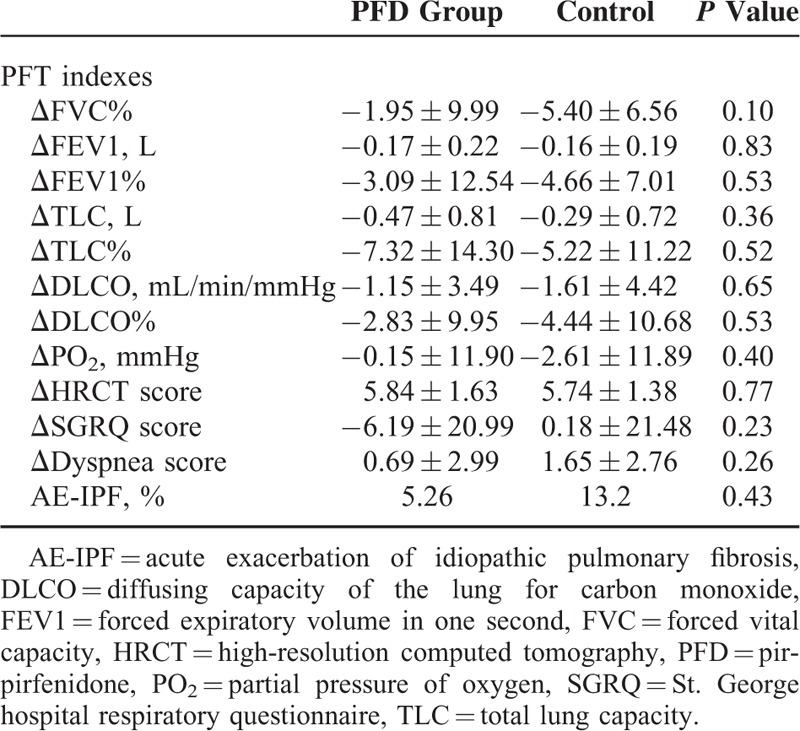
No significant differences were observed in the percent change in the ABG (PaCO2, PaO2, and SaO2) levels, the dyspnea score, the HRCT findings, the SGRQ score, or the number of AE-IPF episodes between the PFD and placebo groups (Table 3).
However, PFD treatment prolonged the PFS duration of the IPF patients (hazard ratio = 1.88, 95% confidence interval: 1.092–3.242, P = 0.02) (Figure 3).
FIGURE 3.
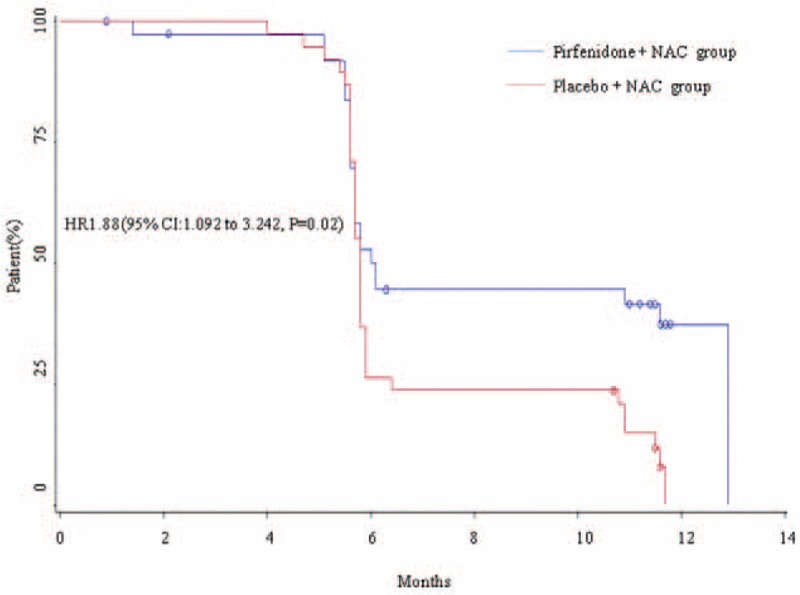
Kaplan–Meier curves for the duration of PFS in the PFD and placebo groups. PFD = pirfenidone, PFS = progression-free survival.
Adverse Effects
The AEs that occurred during the study period are summarized in Table 4. In the PFD group, the AE rate was 52.63% higher than in the control group (26.3%, P = 0.03). Rash was more common in the PFD group (39.5% vs 13.2%, P = 0.02). Rash, pruritus, and allergic dermatitis were the skin-related AEs. All of these events were mild to moderate in severity, reversible, and without clinical sequelae. There were no significant differences in the occurrence of other AEs, including gastrointestinal-related events, weight loss, back pain, and changes in hepatic function, between the groups (Table 4). Three cases withdrew from the study because of back pain (1 case), rash (1 case), or an elevation in the alanine aminotransferase and aspartate aminotransferase levels (1 case). After they stopped taking the trial medications, these AEs disappeared. No cases in the control group withdrew from the trial because of AEs.
TABLE 4.
Treatment-Emergent Adverse Effects
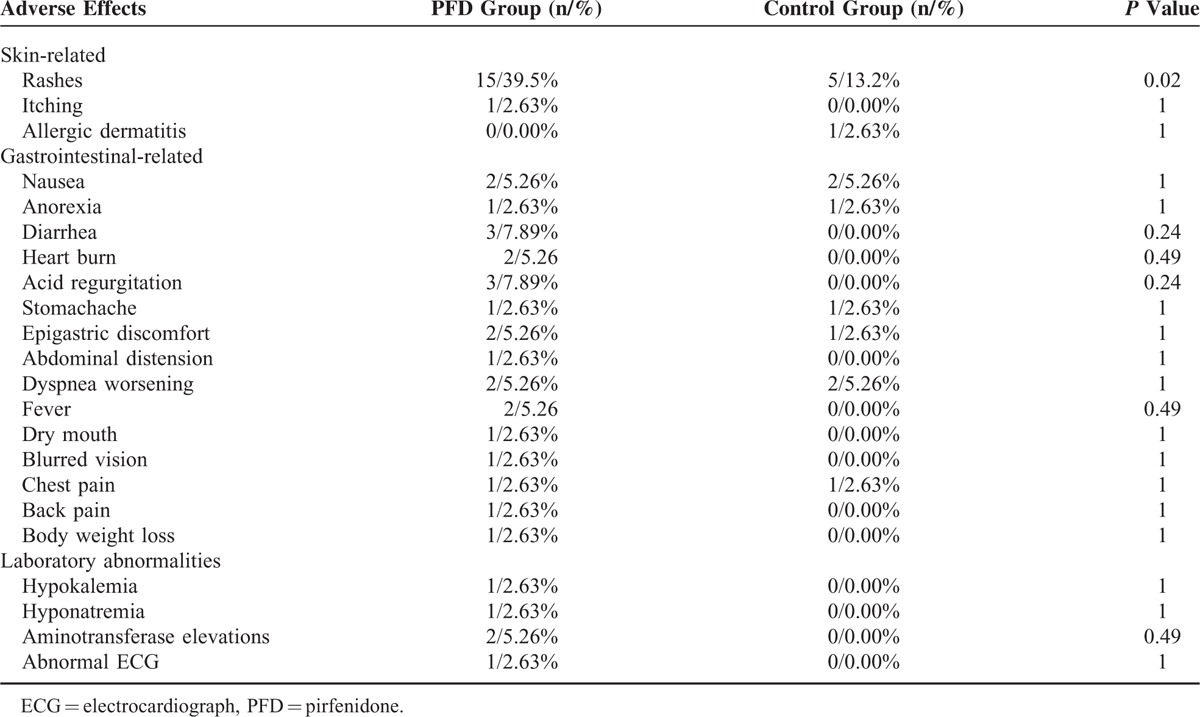
Serious AEs occurred in 4 cases (10.53%), 2 each in the PFD and control groups. In the PFD group, 2 cases (5.26%) died of AE-IPF, 1 case (2.63%) was admitted because of a respiratory infection, and 1 case (2.63%) was hospitalized for ischemic heart disease. In the control group, 2 cases (5.26%) died of AE-IPF, 1 case (2.63%) was hospitalized for dizziness, and 1 case (2.63%) was hospitalized for coronary artery disease.
DISCUSSION
PFD, a new and promising antifibrotic agent,8 has been approved due to its efficacy in the treatment of patients with mild to moderate IPF in Japan,13,14,23 the European Union,15,24 and the United States25 based on an open-label phase II study.12 It had never been used in a Chinese clinical trial of IPF cases. This double-blind, placebo-controlled, randomized phase II trial is the first study in China to evaluate the efficacy and safety of PFD in IPF patients. A summary of these 4 RCT studies is shown in Table 5.
TABLE 5.
Comparison of RCT Researches About Pirfenidone for IPF Cases
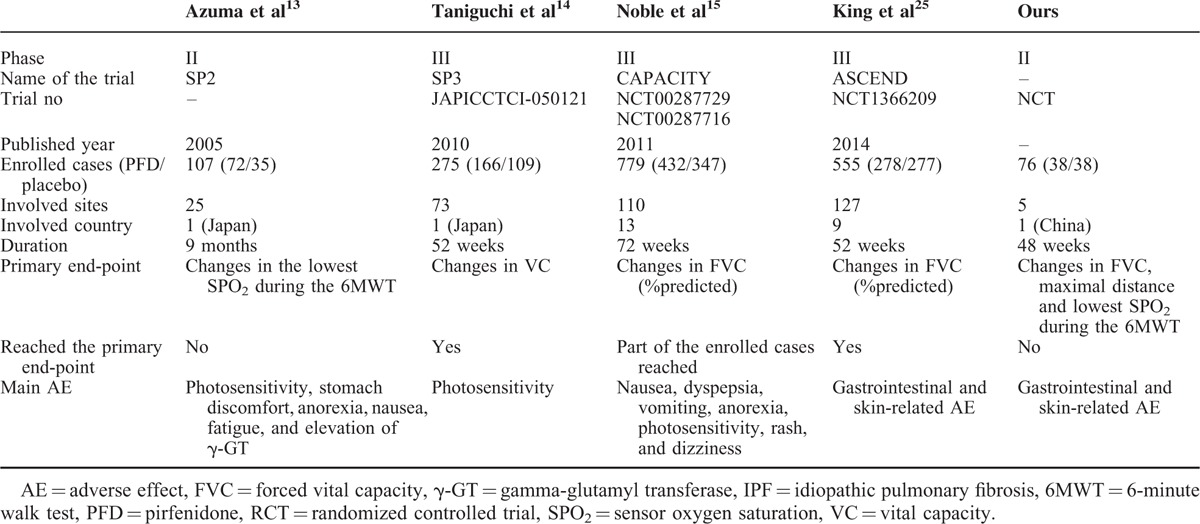
In our study, although no significant difference in the primary endpoints was observed between the PFD and control groups, significant PFD efficacy was shown based on the decline in FVC at the 24th week between these 2 groups. We also showed that PFD treatment prolonged the PFS duration of IPF patients. Somewhat similar results were observed in one of the CAPACITY studies (ie, study 006), in which no significant difference in the primary endpoint (ie, percentage predicted FVC at the 72nd week [the EOT point]) was shown between the PFD and control groups. However, a significant PFD treatment effect on the percentage-predicted FVC (the primary endpoint) was observed between week 12 and week 48.15 In contrast, the pooled data from the CAPACITY study supported a PFD effect on the percentage predicted FVC, the PFS time, and the distance on the 6MWT. Furthermore, this PFD effect on the percentage-predicted FVC and the PFS time was verified in the ASCEND study.25 We performed a further analysis of all of the data, and we found that 4 cases, 3 cases in the PFD group and 1 case in the control group, showed a substantial decline in the PFT parameters, including FVC, TLC, and DLCO, because of AEs within 4 weeks of the EOT timepoint. After we reevaluated the data excluding theses 4 cases, a significant PFD treatment effect on FVC was observed at both 24 and 48 weeks. Larger sample sizes or long-term similar study is expected to verify the results.
Treatment with 600 mg of NAC 3 times daily was used as the baseline treatment for both the PFD and control groups. Although a recent trial of NAC in IPF showed that high-dose NAC did not help to preserve FVC in IPF patients who had mild to moderate impairment in pulmonary function at baseline,21 some basic research has shown that NAC displays antioxidant activity, which helps suppress oxidative stress in pulmonary fibrosis,18,26 inhibits the epithelial-mesenchymal transition in alveoli,20 and inhibits TGF-β1 release into the lung.27 Although in the new treatment guideline conditional recommendation against the use of NAC monotherapy for IPF was suggested, the ATS committee still does recommend to keep patients on NAC if they are tolerating well.16 All that means is that the overall evidence for or against the use of NAC is still not very clear. We cannot totally exclude the NAC effect on IPF cases. As high-dose NAC was administered to both the PFD and control groups, NAC might have added noise to the results of our trial. In Oltmann study,28 12 cases (19%) used NAC during PFD treatment, and these combination therapy patients had a higher rate of disease progression than their patients with PFD monotherapy. However, the cases receiving NAC and PFD combination therapy exhibited worse baseline pulmonary function. Our clinical trial might be the first study to administer PFD and NAC combination therapy to IPF cases. Larger sample sizes and long-term studies are needed in China to confirm the efficacy of PFD and NAC on Chinese IPF patients.
Similar to 4 RCT studies,13–15,25 AEs occurred more frequently in the PFD group than in the control group, and skin- and gastrointestinal-related AEs were the primary AEs in the PFD group. Sixteen cases (42.11%) had skin-related AEs in the PFD group. All of these skin-related events were mild to moderate in severity, reversible, and without clinical sequelae. Only 1 case discontinued the trial because of a severe rash. Similar to previous PFD trials,13–15,25 elevation of the aminotransferase levels occurred more frequently in the PFD group. However, all these events in our study were mild to moderate in severity, reversible, and without clinical sequelae. The long-term safety evaluation of PFD showed that the adjusted incidence of aminotransferase level elevation was 1.7 per 100 person exposure years, and the elevations in the alanine aminotransferase or aspartate aminotransferase levels were more than 3-fold above the upper limit of normal (3 × ULN), which only occurred in 2.7% of patients.24 Based on an analysis of cases with a median PFD exposure duration of 2.6 years, Valeyre et al24 suggested that long-term treatment with PFD was safe and generally well tolerated.
To decrease drug-related AEs, the expert panel discussed the management of PFD-related AEs. They supplied detailed recommendations for the prevention and management of PFD-related AEs, including education, general approaches, and additional measures.29 During our trial, education was provided by physicians and the PFD dispenser at the same time as the patients were enrolled. A dose titration schedule, suggestion to administer the medications with food, and recommendation to avoid exposure to direct sunlight were provided to all patients.
There were some limitations to our trial. First, all of our enrolled cases were prescribed high-dose NAC as the baseline treatment, and there was no placebo-only group. Although recent clinical trials have shown that NAC has no significant benefit to the preservation of FVC in IPF patients with mild to moderate impairment in lung function,21 the interaction between NAC and PFD is unclear. Because the IFIGENIA study showed the role of high-dose NAC in the preservation of FVC and DLCO in IPF patients, we cannot exclude the influence of high-dose NAC on our results. Second, there was some selection bias in our study. Although our cases were from different provinces, all cases were from China, and almost all were Han Chinese (73 cases, 96.1%). All of our cases had limited function based on the PFT results, and all were able to finish the 6MWT. Third, because all of the cases were from China, only 76 cases were enrolled, which is a smaller sample size than the CAPACITY study15 and the ASCEND study.25
However, our study is the first randomized, double blind, modified-placebo controlled clinical trial examining the efficacy and safety of PFD combined with high-dose NAC in IPF patients with mild to moderate impairment of pulmonary function. This trial is also the first multicenter study investigating PFD treatment in Chinese IPF patients.
CONCLUSIONS
In conclusion, we found that PFD combined with high-dose NAC compared to placebo combined with high-dose NAC prolonged the PFS time in Chinese IPF patients with mild to moderate impairment of pulmonary function. The percent-changes in FVC and SPO2 on the 6MWT were significantly different at the 24th week, but these effects did not persist to the 48th week. Although AEs were common in the PFD group, they were mild to moderate in severity, reversible, and without clinical sequelae. Further studies including a larger sample size and a longer study duration are needed to confirm these encouraging results regarding the treatment of IPF patients with PFD.
Acknowledgments
The authors thank Beijing Kawin Technology Shareholding Co., Ltd. for providing the NAC, PFD, and matching placebo tablets that were used in this study. And the authors also thank all of the patients who volunteered to participate in this study.
Footnotes
Abbreviations: ABG = analyses of arterial blood gas, AE = adverse event, AE-IPF = acute exacerbation of IPF, DLCO = carbon monoxide diffusing capacity, EOT = end-of-treatment, ESR = erythrocyte sedimentation rate, FAS = full analysis set, FVC = forced vital capacity, HRCT = high-resolution computed tomography, IPF = idiopathic pulmonary fibrosis, 6MWT = 6-minute walk test, NAC = N-acetylcysteine, PFD = pirfenidone, PFS = progression-free survival, PFT = pulmonary function tests, PPS = per protocol set, RF = rheumatoid factor, SAS = Statistical Analysis System, SGRQ = St George's Respiratory Questionnaire, SS = safety set, UIP = usual interstitial pneumonia.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 1986; 83:4167–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez IE, Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 2012; 9:111–116. [DOI] [PubMed] [Google Scholar]
- 4.Tatler AL, Jenkins G. TGF-beta activation and lung fibrosis. Proc Am Thorac Soc 2012; 9:130–136. [DOI] [PubMed] [Google Scholar]
- 5.Iyer SS, Ramirez AM, Ritzenthaler JD, et al. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2009; 296:L37–L45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kliment CR, Oury TD. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 2010; 49:707–717. [DOI] [PubMed] [Google Scholar]
- 7.Cui Y, Robertson J, Maharaj S, et al. Oxidative stress contributes to the induction and persistence of TGF-beta1 induced pulmonary fibrosis. Int J Biochem Cell Biol 2011; 43:1122–1133. [DOI] [PubMed] [Google Scholar]
- 8.Carter NJ. Pirfenidone: in idiopathic pulmonary fibrosis. Drugs 2011; 71:1721–1732. [DOI] [PubMed] [Google Scholar]
- 9.Potts J, Yogaratnam D. Pirfenidone: a novel agent for the treatment of idiopathic pulmonary fibrosis. Ann Pharmacother 2013; 47:361–367. [DOI] [PubMed] [Google Scholar]
- 10.Maher TM. Pirfenidone in idiopathic pulmonary fibrosis. Drugs Today (Barc) 2010; 46:473–482. [DOI] [PubMed] [Google Scholar]
- 11.Togami K, Kanehira Y, Tada H. Possible involvement of pirfenidone metabolites in the antifibrotic action of a therapy for idiopathic pulmonary fibrosis. Biol Pharm Bull 2013; 36:1525–1527. [DOI] [PubMed] [Google Scholar]
- 12.Raghu G, Johnson WC, Lockhart D, et al. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med 1999; 159:1061–1069. [DOI] [PubMed] [Google Scholar]
- 13.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005; 171:1040–1047. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35:821–829. [DOI] [PubMed] [Google Scholar]
- 15.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377:1760–1769. [DOI] [PubMed] [Google Scholar]
- 16.Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015; 192:e3–e19. [DOI] [PubMed] [Google Scholar]
- 17.Behr J, Maier K, Degenkolb B, et al. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med 1997; 156:1897–1901. [DOI] [PubMed] [Google Scholar]
- 18.Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005; 353:2229–2242. [DOI] [PubMed] [Google Scholar]
- 19.Sugiura H, Ichikawa T, Liu X, et al. N-acetyl-L-cysteine inhibits TGF-beta1-induced profibrotic responses in fibroblasts. Pulm Pharmacol Ther 2009; 22:487–491. [DOI] [PubMed] [Google Scholar]
- 20.Felton VM, Borok Z, WillisF BC N-acetylcysteine inhibits alveolar epithelial-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol 2009; 297:L805–L812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez FJ, de Andrade JA, et al. Idiopathic Pulmonary Fibrosis Clinical Research Network. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370:2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichikado K, Johkoh T, Ikezoe J, et al. Acute interstitial pneumonia: high-resolution CT findings correlated with pathology. AJR Am J Roentgenol 1997; 168:333–338. [DOI] [PubMed] [Google Scholar]
- 23.Okuda R, Hagiwara E, Baba T, et al. Safety and efficacy of pirfenidone in idiopathic pulmonary fibrosis in clinical practice. Respir Med 2013; 107:1431–1437. [DOI] [PubMed] [Google Scholar]
- 24.Valeyre D, Albera C, Bradford WZ, et al. Comprehensive assessment of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis. Respirology 2014; 19:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King TJ, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370:2083–2092. [DOI] [PubMed] [Google Scholar]
- 26.Kinnula VL, Fattman CL, Tan RJ, et al. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med 2005; 172:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cu A, Ye Q, Sarria R, et al. N-acetylcysteine inhibits TNF-alpha, sTNFR, and TGF-beta1 release by alveolar macrophages in idiopathic pulmonary fibrosis in vitro. Sarcoidosis Vasc Diffuse Lung Dis 2009; 26:147–154. [PubMed] [Google Scholar]
- 28.Oltmanns U, Kahn N, Palmowski K, et al. Pirfenidone in idiopathic pulmonary fibrosis: real-life experience from a german tertiary referral center for interstitial lung diseases. Respiration 2014; 88:199–207. [DOI] [PubMed] [Google Scholar]
- 29.Costabel U, Bendstrup E, Cottin V, et al. Pirfenidone in idiopathic pulmonary fibrosis: expert panel discussion on the management of drug-related adverse events. Adv Ther 2014; 31:375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]


