Significance
The mammalian reproductive system is critically dependent upon pulsatile gonadotropin hormone release driven by a small population of gonadotropin-releasing hormone neurons in the brain. However, the mechanisms underlying the episodic activation of the gonadotropin-releasing hormone neurons to drive pulsatile hormone release are unknown. We report here that, using optogenetics in vivo, the synchronous activation of a population of kisspeptin neurons located in the hypothalamic arcuate nucleus is remarkably potent at generating pulsatile gonadotropin secretion. This system exhibits major sex differences and is modulated by the gonadal steroid environment of the organism. These results provide an important insight into the brain mechanism underlying gonadotropin pulsatility in mammalian reproductive biology.
Keywords: GnRH, kisspeptin, optogenetics, arcuate nucleus, gonadal steroids
Abstract
Normal reproductive functioning in mammals depends upon gonadotropin-releasing hormone (GnRH) neurons generating a pulsatile pattern of gonadotropin secretion. The neural mechanism underlying the episodic release of GnRH is not known, although recent studies have suggested that the kisspeptin neurons located in the arcuate nucleus (ARN) may be involved. In the present experiments we expressed channelrhodopsin (ChR2) in the ARN kisspeptin population to test directly whether synchronous activation of these neurons would generate pulsatile luteinizing hormone (LH) secretion in vivo. Characterization studies showed that this strategy targeted ChR2 to 70% of all ARN kisspeptin neurons and that, in vitro, these neurons were activated by 473-nm blue light with high fidelity up to 30 Hz. In vivo, the optogenetic activation of ARN kisspeptin neurons at 10 and 20 Hz evoked high amplitude, pulse-like increments in LH secretion in anesthetized male mice. Stimulation at 10 Hz for 2 min was sufficient to generate repetitive LH pulses. In diestrous female mice, only 20-Hz activation generated significant increments in LH secretion. In ovariectomized mice, 5-, 10-, and 20-Hz activation of ARN kisspeptin neurons were all found to evoke LH pulses. Part of the sex difference, but not the gonadal steroid dependence, resulted from differential pituitary sensitivity to GnRH. Experiments in kisspeptin receptor-null mice, showed that kisspeptin was the critical neuropeptide underlying the ability of ARN kisspeptin neurons to generate LH pulses. Together these data demonstrate that synchronized activation of the ARN kisspeptin neuronal population generates pulses of LH.
Reproduction is critically dependent upon pulsatile patterns of luteinizing hormone (LH) secretion driven by the episodic release of gonadotropin-releasing hormone (GnRH) into the pituitary portal vasculature (1–3). How the scattered population of GnRH neurons within the basal forebrain of mammals is able to generate an episodic pattern of GnRH release remains unknown. Early observations from immortalized GnRH-secreting cell lines indicated that periodic secretion was an intrinsic property of the GnRH neurons themselves (4, 5). However, it seems increasingly unlikely that this is the principal mechanism underling the episodic secretion of GnRH in adult mammals (6). As such, attention has shifted to the elucidation of an extrinsic “pulse generator” within the GnRH neuronal network that entrains GnRH neurons to release GnRH in an episodic manner (6–9).
Based upon early lesioning and deafferentation studies, it was suggested that the GnRH pulse generator may exist in the mediobasal hypothalamus (10–12). Within this region, particular attention has been focused on the arcuate nucleus (ARN) (13, 14), where studies have also recorded multiunit activity that correlates with pulsatile LH secretion in a variety of mammals (15–18). Although the identity of the neural elements giving rise to multiunit activity are unknown, it has been suggested that they may represent the activity of kisspeptin neurons within the ARN (19). These cells, also known as KNDy neurons—because they coexpress a range of neurotransmitters, including kisspeptin, neurokinin B, dynorphin, and glutamate—are thought to represent an afferent input within the GnRH neuronal network involved in several aspects of fertility control (20–22). In particular, speculation that these cells may generate synchronized oscillatory patterns of activity through shared excitatory and inhibitory inputs has raised the possibility that they may play a role in GnRH pulse generation (19, 20, 23, 24).
In the present study we have tested directly whether the selective and synchronous activation of KNDy neurons in vivo can generate pulsatile LH secretion in mice. We demonstrate that the synchronous activation of ARN KNDy neurons at ≥10 Hz is remarkably effective at generating repetitive pulses of LH secretion. The efficacy of ARN kisspeptin neurons to generate LH pulses is sexually differentiated and modulated by the gonadal steroid milieu. We also show that, among the various neurotransmitters used by KNDy neurons, the activation of LH pulses results from kisspeptin activation of the kisspeptin receptor GPR54.
Results
Adeno-Associated Virus Transfection of ARN Kisspeptin Neurons with Channelrhodopsin.
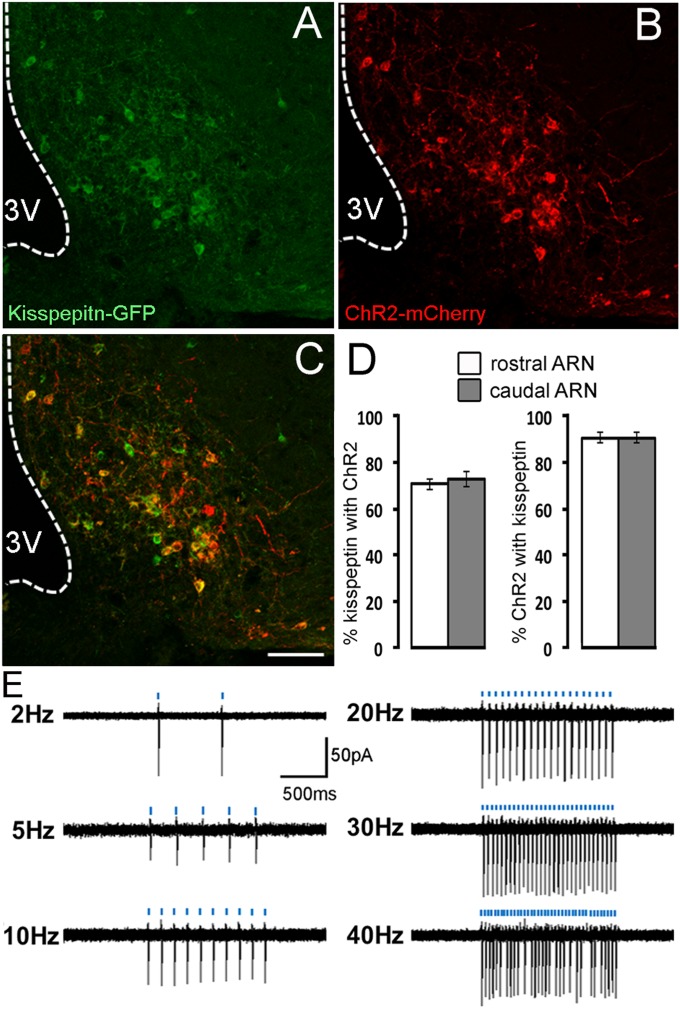
Because ARN kisspeptin cell bodies are difficult to visualize in mice, we performed experiments evaluating the efficiency of adeno-associated virus (AAV) transfection in Kiss1-IRES-Cre+/−;Rosa26-CAG-τGFP+/− mice in which kisspeptin neurons are tagged with GFP. Channelrhodopsin-2 (ChR2) fused with mCherry was targeted to ARN kisspeptin neurons by injecting a Cre-dependent AAV [AAV9-EF1-dflox-hChR2-(H134R)-mCherry-WPRE-hGH] bilaterally into the ARN of Kiss1-IRES-Cre mice. Three weeks after AAV injections, dual-label immunofluorescence studies showed that GFP (kisspeptin) neurons throughout the rostro-caudal extent of the ARN were transfected with mCherry (ChR2) (Fig. 1 A–C). Whereas GFP was typically restricted to the cell body (Fig. 1A), mCherry was often observed in processes in addition to the cell bodies (Fig. 1B). Just over 70% of kisspeptin neurons throughout the ARN (Fig. 1 A–C and Fig. S1 A–C) expressed ChR2 and these cells accounted for 91% of all ChR2 neurons (n = 5) (Fig. 1D and Fig. S1D). See Table S1 for the number of GFP, mCherry, and dual-labeled cells and their percentages in the rostral-, middle-, and caudal parts of the ARN. Cells with mCherry but no GFP were usually observed in the lateral margins of the ARN. Control AAV injections performed in wild-type C57BL/6 mice (n = 3) resulted in no mCherry expression confirming the Cre-dependence of the viral vector. These results show that AAVs can be used to target the majority of ARN kisspeptin neurons in a selective manner.
Fig. 1.
Characterization of ARN kisspeptin neurons transfected with ChR2. (A–C) Photomicrographs showing (A) kisspeptin-GFP, (B) ChR2-mCherry, and (C) merged immunofluorescence in the ARN. 3V, third ventricle. (Scale bar, 100μm.) (D) Histograms showing the mean (± SEM; n = 5) percentage of rostral and caudal ARN kisspeptin neurons expressing ChR2 and percentage of ChR2 expressing neurons expressing kisspeptin. (E) Representative cell-attached, voltage-clamp recordings of ChR2-expressing kisspeptin neurons in the acute brain slice preparation activated with 5-ms blue light pulses (indicated by blue bars) given at 2, 5, 10, 20, 30, and 40 Hz over 1 s.
Fig. S1.
Photomicrographs showing GFP (kisspeptin) immunoreactivity, mCherry (ChR2), and overlay in the caudal ARN. (Scale bar, 100 μM.)
Table S1.
The number of GFP-, mCherry-, and dual-expressing cells and their percentages in rostral, middle, and caudal parts of the ARN
| Location | No. of GFP | No. of mCherry | No. of GFP and mCherry | % GFP expressing mCherry | % mCherry expressing GFP |
| Rostral | 22 ± 2 | 17 ± 1 | 16 ± 1 | 71 ± 2 | 92 ± 2 |
| Middle | 26 ± 3 | 20 ± 1 | 18 ± 2 | 70 ± 3 | 86 ± 3 |
| Caudal | 32 ± 3 | 26 ± 3 | 25 ± 3 | 75 ± 3 | 94 ± 1 |
Values show numbers of cells counted per coronal section. n = 14 sections analyzed from five animals.
Optogenetic Activation of ARN Kisspeptin Neurons in Vitro.
To assess the ability of transfected ChR2 to control the firing of kisspeptin neurons, coronal brain slices were prepared from AAV-injected Kiss1-IRES-Cre male mice (3 wk postinjection) and cell-attached recordings made from mCherry-expressing ARN kisspeptin neurons. Laser pulses (473 nm, 5 ms) were delivered at 1, 2, 5, 10, 20, 30, or 40 Hz for 1 s in a repetitive manner once every 10 s over a period of 1 min. Kisspeptin neurons exhibited action potentials in response to blue light activation with high spike fidelity (Fig. 1E). Laser pulses at 1, 2, and 5 Hz induced action potentials with 100% fidelity, and frequencies of 10, 20, 30, and 40 Hz generated 97 ± 3% (n = 7), 91 ± 9% (n = 8), 80 ± 10% (n = 7), and 70 ± 14% (n = 7) fidelity, respectively (four mice) (Fig. 1E). Although kisspeptin neurons did not follow every light stimulation at higher frequencies (Fig. 1E), the overall mean fidelity rate was not different up to 30 Hz, with only 40 Hz generating significantly reduced firing fidelity (P < 0.05, one-way repeated-measures ANOVA). These data show that ARN kisspeptin neurons can faithfully follow blue light activation up to moderately high stimulation frequencies.
Optogenetic Activation of ARN Kisspeptin Neurons in Vivo.
We next examined whether activation of ARN kisspeptin neurons in vivo could alter LH secretion in anesthetized mice and whether this might be different in males and females or altered by ovariectomy. Prior studies in the laboratory have found that endogenous LH pulsatility is blocked in the isoflurane-anesthetized mouse (25).
Effects on LH secretion of activating ARN kisspeptin neurons at different frequencies in male mice.
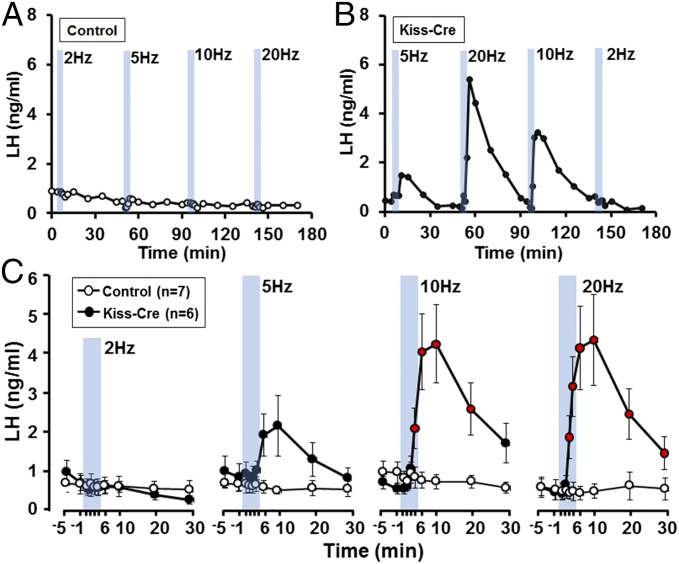
Anesthetized AAV-injected Kiss1-IRES-Cre male mice were implanted with a 100-μm-diameter optic fiber in the ARN and the effects of blue light activation for 5-min periods at 2, 5, 10, and 20 Hz tested. The different stimulations (2, 5, 10, and 20 Hz) were applied in a random order to each mouse and randomly distributed so that no animal received the same stimulation twice. In control mice (AAV-injected Kiss1-IRES-Cre−/− and sham-injected Kiss1-IRES-Cre+/− mice), fiber optic activation in the ARN had no effect on LH secretion (n = 7) (Fig. 2 A and C). In AAV-injected Kiss1-IRES-Cre+/− mice (n = 6), stimulation for 5 min at 2 and 5 Hz had no significant effect upon LH secretion (Fig. 2 B and C). However, 10- and 20-Hz stimulation generated significant pulse-like increments in LH release (Fig. 2 B and C) (one-way repeated-measures ANOVA with Dunnett’s post hoc test) with peak levels of evoked LH not being different between the 10- and 20-Hz stimulations (Fig. 2C).
Fig. 2.
Effects of optogenetic activation of ARN kisspeptin neurons on LH secretion in male mice. (A and B) Representative examples showing LH secretion in response to blue light activation of ARN kisspeptin neurons (indicated by blue bars, 5-min each) at different frequencies in control and Kiss-Cre male mice. (C) Summary graphs showing mean ± SEM changes in LH secretion in response to 2-, 5-, 10-, and 20-Hz activation of ARN kisspeptin neurons in control male (open circle, n = 7) and Kiss-Cre male (closed circle, n = 6) mice. Circles in red indicate LH levels significantly elevated compared with prestimulation values for each frequency (P < 0.05; repeated-measures ANOVA with Dunnett’s post hoc test).
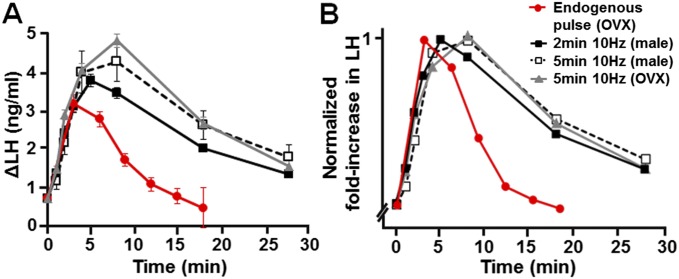
Increases in LH were pulse-like, being rapid in onset over the first ∼2 min of blue light activation and then decaying slowly over the next 20 min. Compared with endogenous LH pulses, 10-Hz stimulation generated a pulse with a similar initial profile but higher peak amplitude and longer decay (Fig. S2A). When evoked and endogenous LH pulses were normalized to peak amplitude, the profiles were the same with the exception of the delayed pulse decay (Fig. S2B). This prolonged decay was also observed to a lesser extent following optogenetic activation of GnRH neurons in anesthetized mice (25). This may result from blood flow differences between conscious and anesthetized mice or other factors, such as the duration of kisspeptin release following optogenetic stimulation in the present experiments. Taking these data together, this study demonstrates that ≥10-Hz activation of ARN kisspeptin neurons is remarkably effective at evoking pulse-like LH secretion in male mice.
Fig. S2.
Comparison of the mean optogenetic-evoked pulse-like increases in LH secretion with endogenous LH pulses (red). (A) Dynamics of evoked increments in LH secretion following 10-Hz activation of ARN kisspeptin neurons for 2-min (black, male) or 5-min (open square, male; gray, OVX female) intervals compared with endogenous LH pulses [red; data taken from Campos and Herbison (25)]. (B) Dynamics of evoked and endogenous LH pulses after normalizing values to the peak LH responses and start time of the pulses.
Effects on LH secretion of activating ARN kisspeptin neurons at different frequencies in intact and ovariectomized female mice.
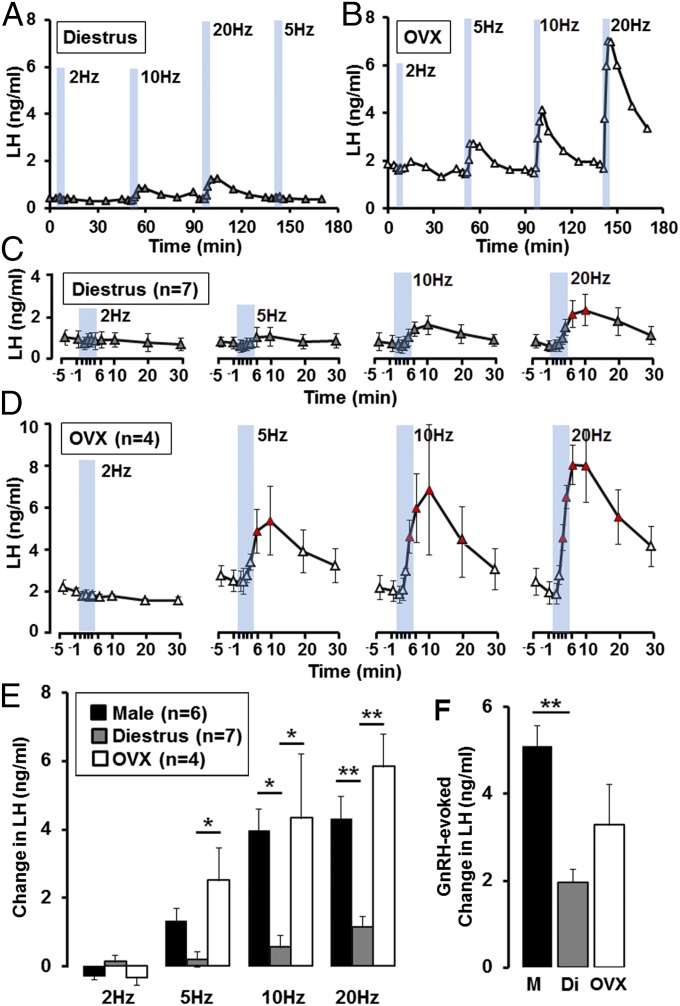
We repeated the exact same experiment undertaken in males on intact diestrous mice and also in ovariectomized (OVX) females to examine any impact that gonadal steroids may have upon the ability of kisspeptin neurons to activate LH secretion. Surprisingly, optogenetic stimulation of the ARN in AAV-injected Kiss1-IRES-Cre+/− diestrous mice (n = 7) was much less effective in evoking LH secretion compared with males (Fig. 3A). Although a trend for increased LH levels was observed following 10-Hz activation, only 20 Hz induced a significant elevation in LH release (P < 0.05; one-way repeated-measures ANOVA with Dunnett’s post hoc test) (Fig. 3C). In contrast, ARN activation in AAV-injected Kiss1-IRES-Cre+/− OVX mice (n = 4) generated significant, large amplitude, pulse-like increases in LH secretion following optogenetic stimulation with frequencies even as low as 5 Hz (Fig. 3 B and D) (one-way repeated-measures ANOVA with Dunnett’s post hoc test). The number of ChR2-expressing kisspeptin neurons in the ARN, at the level of the optic fiber, was not different between diestrous and OVX mice (20 ± 3 vs. 21 ± 2 cells per hemisection, respectively). The LH increments in OVX mice exhibited the same pulse profile as those found in male mice (Fig. S2 A and B).
Fig. 3.
Effects of optogenetic activation of ARN kisspeptin neurons on LH secretion in female mice. (A and B) Representative examples showing LH secretion in response to blue light activation of kisspeptin neurons (indicated by blue bars, 5-min each) at frequencies of 2, 5, 10, and 20 Hz, in (A) diestrous and (B) OVX female Kiss1-IRES-Cre mice. (C and D) Summary graphs showing mean ± SEM changes in LH secretion in response to 2-, 5-, 10-, and 20-Hz activation of ARN kisspeptin neurons in diestrous (n = 7) and OVX (n = 4) mice. Triangles in red indicate LH levels significantly elevated compared with prestimulation values for each frequency (P < 0.05; one-way repeated-measures ANOVA with Dunnett’s post hoc test). (E) Summary histograms showing mean + SEM increases in LH secretion evoked by 2, 5, 10, and 20 Hz of 5-min activation of ARN kisspeptin neurons in male (black), diestrous (gray), and OVX (white) mice. *P < 0.05, **P < 0.01, two-way ANOVA with Bonferroni’s post hoc test. (F) Histogram showing mean + SEM increase in LH evoked by exogenous subcutaneous GnRH in male (black), diestrous (gray), and OVX (white) mice. **P < 0.01, one-way ANOVA with Bonferroni’s post hoc test.
To make comparisons between intact male, female, and OVX mice, we determined the change in LH secretion evoked by optogenetic activation over the first 10 min at the different frequencies for each group (Fig. 3E). This showed that the magnitude of the LH responses in male and OVX female mice to 10 and 20 Hz were equivalent but approximately fourfold greater than that observed in intact diestrous females (P < 0.05 or 0.001, two-way ANOVA with Bonferroni’s post hoc test) (Fig. 3E). The response of OVX females to 5-Hz activation was also significantly increased compared with diestrous mice (P < 0.05) (Fig. 3E).
Differences in LH responses to ARN kisspeptin activation may result from differential sensitivity of the pituitary gland to GnRH in male, female, and OVX mice. We tested this by administering GnRH (200 ng/kg in 100-µL saline, subcutaneously) at the end of each experiment to assess pituitary responsiveness to GnRH. The increase in LH evoked by GnRH in male mice (5.1 ng/mL, n = 6) was significantly larger (P < 0.01, one-way ANOVA with Bonferroni’s post hoc test) (Fig. 3F) than diestrous mice (1.9 ng/mL, n = 7), whereas LH levels in OVX mice (3.3 ng/mL, n = 4) were not significantly different to intact males or females (Fig. 3F). This finding indicates that part of the sex difference results from sexually differentiated pituitary responses to GnRH.
Effects on LH secretion of activation of ARN kisspeptin neurons in Gpr54-null mice.
The potent activating effects of ARN kisspeptin neurons on LH secretion may theoretically come about from the release of kisspeptin, neurokinin B, and/or glutamate (26, 27). To examine the importance of kisspeptin as opposed to these other neurotransmitters in LH pulse activation, we performed the 2-, 5-, 10-, and 20-Hz experiments in AAV-injected Kiss1-IRES-Cre; Gpr54−/− male mice (n = 5). Optogenetic activation of ARN kisspeptin neurons was unable to stimulate LH release at any frequency in these mice (−0.22 ± 0.22, −0.23 ± 0.11, −0.06 ± 0.07, and −0.05 ± 0.13 ng/mL in 2, 5, 10, and 20 Hz, respectively) (Fig. 4A), whereas control AAV-injected Kiss1-IRES-Cre; Gpr54+/− male mice (n = 2) investigated at the same time showed the normal activation of LH secretion (Fig. 4B). Pituitary stimulation with exogenous GnRH evoked an ∼twofold increase in LH (before 0.56 ng/mL, after 0.91 ng/mL; P < 0.05, paired t test) in Kiss1-IRES-Cre; Gpr54−/− mice (n = 5) compared with an ∼fourfold increase in Kiss1-IRES-Cre; Gpr54+/− mice (before 0.86 ng/mL, after 3.25 ng/mL).
Fig. 4.
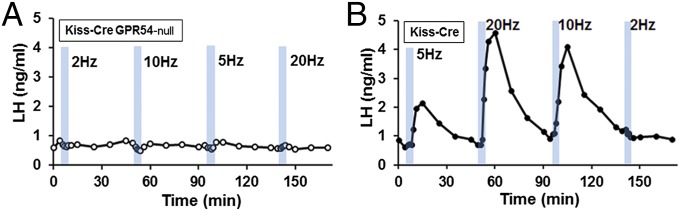
Effects of in vivo optogenetic activation of ARN kisspeptin neurons on LH secretion in GPR54-null mice. Representative examples of 5-min blue light activation of kisspeptin neurons (indicated by blue bars) at 2, 5, 10, and 20 Hz in (A) an AAV-injected Kiss1-IRES-Cre; Gpr54-null male mice and (B) in a control AAV-injected Kiss1-IRES-Cre;Gpr54+/− male mice.
Effects on LH secretion of activating ARN kisspeptin neurons for different durations.
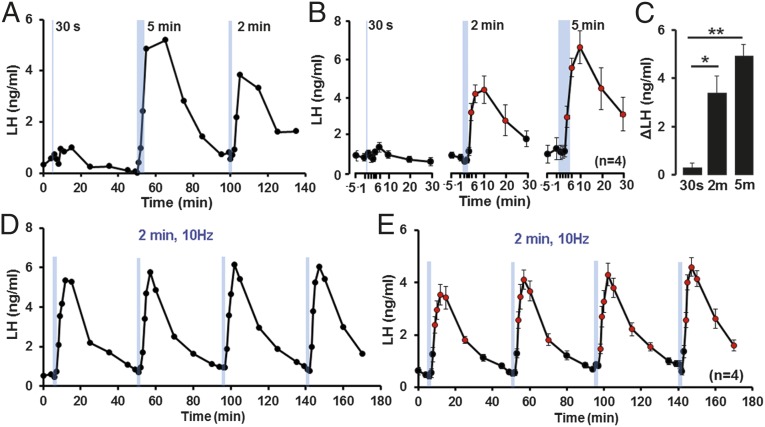
The above observations indicate that 10-Hz stimulation of the ARN kisspeptin neurons for 5 min is a potent activator of LH pulses. To examine the duration of activation required for a pulse of LH, anesthetized AAV-injected Kiss1-IRES-Cre male mice were stimulated at 10 Hz for 30 s, 2 min, and 5 min. Stimulation for 30 s showed no consistent or significant increase in LH (Fig. 5 A–C). In contrast, stimulation for 2 or 5 min evoked a rapid, pulse-like elevation in LH (P < 0.05, one-way repeated-measures ANOVA with Dunnett’s post hoc test) (Fig. 5B). The increase in LH evoked by 2-min (P < 0.05) and 5-min (P < 0.01) activation was significantly larger than that following 30 s with no difference in peak values between 2- and 5-min stimulation (Fig. 5C) (one-way ANOVA with Bonferroni’s post hoc test). The increment in LH evoked by 2-min stimulation exhibited the same pulse dynamics as those activated by 5-min stimulations (Fig. S2). This indicates that a 2-min 10-Hz activation of ARN kisspeptin neurons is sufficient to generate a pulse-like increment in LH secretion.
Fig. 5.
Effects of different durations of 10-Hz activation of ARN kisspeptin neurons on LH secretion in male mice. (A) Representative example of LH secretion evoked by blue light activation of kisspeptin neurons (indicated by blue bars) at 10 Hz for 30 s, 2 min, and 5 min in Kiss-IRES-Cre male mice. (B) Summary graphs showing mean ± SEM changes in LH secretion in response to 30-s, 2-min, and 5-min activation of ARN kisspeptin neurons at 10 Hz in male mice. Circles in red indicate LH levels significantly elevated compared with prestimulation values for each time interval (P < 0.05; one-way repeated-measures ANOVA with Dunnett’s post hoc test). (C) Histogram showing mean + SEM increase in LH secretion in the first 10 min after optogenetic stimulation for different durations in male mice (n = 4). *P < 0.05, **P < 0.01, one-way ANOVA with Bonferroni’s post hoc test. (D) Shows representative example of LH secretion evoked by 2-min blue light activation of kisspeptin neurons (indicated by blue bars) at 10-Hz repeated four times over 45-min intervals in a Kiss-IRES-Cre male mice. (E) Summary graph showing mean ± SEM evoked LH secretion in male mice (n = 4). Circles in red indicate LH levels significantly elevated compared with prestimulation values before each activation (P < 0.05; one-way repeated-measures ANOVA with Dunnett’s post hoc test).
Effects on LH secretion of repeated activation of ARN kisspeptin neurons.
Prior studies have shown that kisspeptin exerts profound long-lasting effects on GnRH neurons lasting over 1 h in vitro (28). However, repeated intravenous kisspeptin administration at short intervals can evoke repetitive LH pulses in monkeys (29) and other species (30). To examine the ability of repetitive endogenous kisspeptin to evoke LH pulses we repeatedly activated ARN kisspeptin neurons in male mice for 2 min at 10 Hz every 45 min. These studies showed that each of four 10-Hz activations over 3 h generated a remarkably consistent pulse-like increment in LH secretion (P < 0.05, one-way repeated-measures ANOVA with Dunnett’s post hoc test; n = 4) (Fig. 5 D and E) indicating the capacity of ARN kisspeptin neurons to generate repeated pulsatile LH secretion.
Discussion
We demonstrate here that the synchronous activation of ARN kisspeptin neurons in vivo evokes pulse-like increments in LH secretion. The AAV approach in Kiss1-IRES-Cre mice was effective at transducing 70% of kisspeptin neurons with ChR2 and electrophysiological brain-slice studies demonstrated kisspeptin neurons to exhibit high spike fidelity up to 30-Hz blue light activation. In vivo experiments in male mice showed that ARN kisspeptin neurons needed to be activated at ≥10 Hz to evoke reliable increments in LH secretion. Furthermore, whereas 30-s duration 10-Hz activation had no effect, a 2-min stimulation period was found to be as effective as 5 min in generating a pulse of LH. This finding is reminiscent of recent GnRH neuron optogenetic studies where a 2-min period of GnRH neuron activation was found to be the minimum activation interval required to evoke a pulse of LH secretion (25).
Unexpectedly, we found that the activation of ARN kisspeptin neurons in diestrous mice was less effective at modifying LH secretion than in males. In diestrous females, only 20-Hz activation was able to generate significant increases in LH and, even then, these changes were approximately fourfold lower in magnitude than those observed in males. To determine whether this sex difference in LH release resulted from sexually differentiated GnRH release or pituitary sensitivity to GnRH, we examined pituitary responses to exogenous GnRH. Interestingly, the magnitude of the pituitary response to GnRH in diestrous females was only ∼40% of that of males. To our knowledge, in vivo sex differences in pituitary sensitivity to GnRH have not been reported previously for mice. Nevertheless, our data suggest that the pituitary will underlie at least part of the sex differences observed here following activation of ARN kisspeptin neurons in intact male and female mice. Sex differences have occasionally been reported for ARN kisspeptin neurons themselves (31), including a 20-fold difference in spontaneous firing rate (32), and may also contribute to the sexually dimorphic optogenetic responses found here.
Previous investigations have shown that kisspeptin biosynthesis within the ARN is robustly suppressed by estradiol (33, 34). To examine whether different levels of kisspeptin peptide within ARN kisspeptin cells may impact upon their ability to regulate LH secretion, we compared OVX and intact diestrous female mice. Optogenetic activation of ARN kisspeptin neurons at 10 and 20 Hz in OVX mice generated >fourfold larger increments in LH secretion compared with intact females. As pituitary sensitivity to exogenous GnRH was not different between OVX and diestrous mice, it is likely that optogenetic activation evokes enhanced kisspeptin release in OVX mice, resulting in larger LH responses. Notably, 5-Hz stimulation was able to elevate LH release in OVX mice but not intact animals, suggesting that there is more efficient electrochemical coupling of kisspeptin release from kisspeptin nerve terminals in the OVX state. This would provide one mechanism through which estrogen negative feedback could occur in the absence of any changes in the actual firing rate of kisspeptin neurons (32).
The ARN kisspeptin, or KNDy cells, synthesize numerous neuropeptidergic and classic transmitters (20, 23, 35) and innervate only the distal processes of GnRH neurons in mice, either at the level of the dendron or their nerve terminals in the median eminence (36, 37). We found that 2-Hz stimulation of ARN KNDy neurons was completely ineffective in modulating LH secretion. Because low stimulation frequencies of 1–2 Hz typically evoke only classic neurotransmitter release (38, 39), this suggests that glutamate release from KNDy cells is insufficient on its own to modify LH secretion. To help decipher which KNDy neuropeptide is responsible for activating LH release, we examined the effects of optogenetic activation in the Gpr54-null mouse. Although the pituitary gland was still able to generate a small response to exogenous GnRH in Gpr54-null mice, optogenetic activation of ARN kisspeptin neurons had no effect at all on LH levels. This finding demonstrates that, even following 20-Hz activation of KNDy cells, kisspeptin is the key neuropeptide/transmitter released by these cells to generate an LH pulse. This finding is compatible with prior data showing that kisspeptin can act at the distal processes of GnRH neurons to modulate GnRH secretion (40, 41) and that the effects on LH secretion of coreleased neuropeptides, such as NKB, may be upstream of kisspeptin signaling (27, 42, 43).
There seems little doubt that kisspeptin signaling is essential for normal LH pulsatility in mammals (44–47), but an essential role for the ARN kisspeptin neurons has yet to be proven. Importantly, increases in extracellular kisspeptin levels within the region of the ARN can correlate with pulsatile GnRH secretion (48) and injection of Gpr54 antagonists into the ARN region decreases LH pulse frequency (49, 50). The location of Gpr54 antagonism within those studies is unknown and may possibly be at the level of the Gpr54-expressing GnRH neuron dendron/terminals within and adjacent to the ARN, where they would suppress the putative episodic kisspeptin drive to GnRH secretion. It is also noteworthy that the selective reduction of ARN kisspeptin levels by ∼30% results in a small but significant 8% slowing of LH pulse frequency in the rat (22). To our knowledge, we herein provide the first direct evidence that the synchronous activation of ARN kisspeptin neurons can generate pulsatile LH release. This represents critical support for the hypothesis that the ARN kisspeptin neurons are part of the GnRH pulse generator and demonstrates that kisspeptinergic innervation of GnRH neuron distal projections can be a remarkably potent neuronal construct for generating repeated LH pulses.
Materials and Methods
Animals.
Adult Kiss1-IRES-Cre+/−, Kiss1-IRES-Cre+/−;Rosa26-CAG-τGFP, Kiss1-IRES-Cre+/−;Gpr54−/−, Kiss1-IRES-Cre+/−;Gpr54+/−, and wild-type C57BL/6J mice (8- to 16-wk-old) were housed in a 12-h light/12-h dark cycle (lights on at 0600 hours and off at 1800 hours) with food and water available ad libitum. Where indicated, mice were bilaterally overiectomized under isoflurane anesthesia and used for experiments 3–10 wk later. For intact females, estrous cycle stage was determined by daily vaginal smear. All procedures were approved by the University of Otago Animal Ethics Committee.
Stereotaxic Injections of AAV.
Adult mice were anesthetized with isoflurane, placed in a stereotaxic apparatus, and given simultaneous bilateral 1-μL injections of AAV9-EF1-dflox-hChR2-(H134R)-mCherry-WPRE-hGH (4.35 × 1013 GC/mL; Penn Vector Core) into the ARN at a rate of 100 nL/min. The syringes were left in situ for 3 min before and 10 min after the injections. Coordinates according to the Paxinos mouse atlas (51) were 1.2-mm posterior to bregma, 0.3-mm lateral to midline, and 6.0-mm depth.
Brain Slice Electrophysiology.
Acute 200-μm-thick coronal brain slices containing the ARN were prepared between 0900 and 1100 hours, as reported previously (32). The 95%O2/5%CO2 equilibrated artificial cerebrospinal fluid contained: 120 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 1 mM NaH2PO4, 2.5 mM CaCl2, 1.2 mM MgCl2, and 10 mM glucose. Loose-seal cell-attached recordings (10–30 MΩ) were made from mCherry-fluorescent kisspeptin neurons visualized through an upright microscope fitted for epifluorescence (Olympus). Kisspeptin neurons were identified by the presence of mCherry, revealed by brief illumination with green light, and then patched under differential interference contrast optics, as reported previously (32). Action currents were recorded (2- to 5-MΩ pipettes) in voltage clamp mode with 0-mV command voltage. Recorded neurons were stimulated by delivering blue light (473 nm) from a 100-μm optic fiber coupled to DPSS laser (Ike-Cool), controlled by a Grass S88X stimulator. The light intensity at the tip of the optic fiber was 5 mW. Pulses of light (5-ms duration) were delivered at 1, 2, 5, 10, 20, 30, and 40 Hz for 1 s every 10 s, repeated six times over 1 min. Signals were acquired using a Multiclamp 700A amplifier (Molecular Devices) connected to a Digidata 1322A and filtered at 3 kHz before digitizing at 10 kHz.
Immunohistochemistry.
Intact male AAV-injected Kiss1-IRES-Cre+/−;Rosa26-CAG-τGFP mice were killed by overdose of sodium pentobarbital (3 mg/100 μL, i.p.) and transcardially perfused with 20 mL of 4% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer (pH 7.6). Three sets of 30-μm-thick coronal brain sections were cut and processed for free-floating GFP immunofluorescence using a polyclonal chicken anti-GFP antiserum (1:1,500; Chemicon International), biotinylated goat anti-chicken secondary immunoglobulins (1:400; Vector Laboratories), and streptavidin Alexa488 (Alexa Fluor; Molecular Probes). Three sections at the level of the rostral, middle, and caudal ARN were analyzed in each mouse by counting the total number of cells expressing GFP or mCherry.
In Vivo Optogenetic Activation.
Three to 10 wk after AAV injection, mice were anesthetized with isoflurane and an optical fiber (100-μm tip diameter) connected to a laser was implanted into the ARN (−1.2 mm AP, midline, 6.0 mm DV). Thirty minutes later the stimulation protocol commenced. This consisted of either (i) 5-ms pulses of blue light delivered at frequencies of 2, 5, 10, or 20 Hz for 5 min each in a randomized order; (ii) 5-ms pulses of blue light at 10 Hz delivered for 30 s, 2 min, or 5 min; or (iii) 5-ms pulses of blue light at 10 Hz delivered for 2 min repeated four times over 45-min intervals. Serial blood samples (5 μL each) were collected from the tail tip at −5, −1, 1, 2, 3, 4, 6, 10, 20, and 30 min, where time 0 is the start of the stimulation. To test the pituitary sensitivity to GnRH, baseline LH levels were determined from blood samples taken 5 and 0 min before subcutaneous injection of 200 ng/kg GnRH and compared with LH levels 15 min later. The blood samples were processed by ELISA as reported previously (45).
Statistical analysis of LH values at −5, −1, 1, 2, 3, 4, 6, 10, 20, and 30 min was undertaken using one-way repeated-measures ANOVA with Dunnett’s post hoc test, comparing values to the −5 min LH level. To compare between groups, the change in LH was determined by subtracting average baseline LH values at −5 and −1min from average evoked levels detected at 6 and 10 min in each mouse and these combined to provide mean ± SEM values for each group. Statistical comparisons were made by two-way repeated-measures ANOVA with Bonferroni’s post hoc tests. Pituitary responses to subcutaneous GnRH were determined by subtracting baseline LH values from that of 15 min after GnRH, and were analyzed with one-way ANOVA with Bonferroni’s post hoc test.
Acknowledgments
We thank Profs. Uli Boehm (Homburg, Germany) and Bill Colledge (Cambridge, United Kingdom) for provision of previously reported mouse lines; Pauline Campos, Rob Porteous, Jenny Clarkson, and Karl Iremonger for helpful advice and assistance. This work was supported by grants from the New Zealand Health Research Council and Royal Society Marsden Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512243112/-/DCSupplemental.
References
- 1.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 2.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 3.Levine JE, Pau KY, Ramirez VD, Jackson GL. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology. 1982;111(5):1449–1455. doi: 10.1210/endo-111-5-1449. [DOI] [PubMed] [Google Scholar]
- 4.Wetsel WC, et al. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc Natl Acad Sci USA. 1992;89(9):4149–4153. doi: 10.1073/pnas.89.9.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez de la Escalera G, Choi AL, Weiner RI. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: Intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc Natl Acad Sci USA. 1992;89(5):1852–1855. doi: 10.1073/pnas.89.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbison AE. Physiology of the adult GnRH neuronal network. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. 4th Ed. Vol 1. Academic; San Diego: 2015. pp. 399–467. [Google Scholar]
- 7.Goodman RL, Karsch FJ. The hypothalamic pulse generator: A key determinant of reproductive cycles in sheep. In: Follett BK, Follett DE, editors. Biological Clocks in Seasonal Reproductive Cycles. Vol Colson Papers No. 32. John Wright & Sons; Bristol, England: 1981. pp. 223–236. [Google Scholar]
- 8.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24(2):79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 9.Maeda K, et al. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res. 2010;1364:103–115. doi: 10.1016/j.brainres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Blake CA, Sawyer CH. Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology. 1974;94(3):730–736. doi: 10.1210/endo-94-3-730. [DOI] [PubMed] [Google Scholar]
- 11.Pau KF, Kuehl DE, Jackson GL. Effect of frontal hypothalamic deafferentation on luteinizing hormone secretion and seasonal breeding in the ewe. Biol Reprod. 1982;27(4):999–1009. doi: 10.1095/biolreprod27.4.999. [DOI] [PubMed] [Google Scholar]
- 12.Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96(5):1073–1087. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- 13.Soper BD, Weick RF. Hypothalamic and extrahypothalamic mediation of pulsatile discharges of luteinizing hormone in the ovariectomized rat. Endocrinology. 1980;106(1):348–355. doi: 10.1210/endo-106-1-348. [DOI] [PubMed] [Google Scholar]
- 14.Plant TM, et al. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta) Endocrinology. 1978;102(1):52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RC, et al. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39(3):256–260. doi: 10.1159/000123988. [DOI] [PubMed] [Google Scholar]
- 16.Thiéry JC, Pelletier J. Multiunit activity in the anterior median eminence and adjacent areas of the hypothalamus of the ewe in relation to LH secretion. Neuroendocrinology. 1981;32(4):217–224. doi: 10.1159/000123162. [DOI] [PubMed] [Google Scholar]
- 17.Mori Y, et al. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology. 1991;53(4):392–395. doi: 10.1159/000125746. [DOI] [PubMed] [Google Scholar]
- 18.Kimura F, Nishihara M, Hiruma H, Funabashi T. Naloxone increases the frequency of the electrical activity of luteinizing hormone-releasing hormone pulse generator in long-term ovariectomized rats. Neuroendocrinology. 1991;53(1):97–102. doi: 10.1159/000125704. [DOI] [PubMed] [Google Scholar]
- 19.Wakabayashi Y, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittelman-Smith MA, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu MH, et al. Relative importance of the arcuate and anteroventral periventricular kisspeptin neurons in control of puberty and reproductive function in female rats. Endocrinology. 2015;156(7):2619–2631. doi: 10.1210/en.2014-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro VM, et al. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. doi: 10.1210/en.2013-1231. [DOI] [PubMed] [Google Scholar]
- 25.Campos P, Herbison AE. Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA. 2014;111(51):18387–18392. doi: 10.1073/pnas.1415226112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman RL, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 27.Navarro VM, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152(11):4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han S-K, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147(2):1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 30.Tovar S, et al. Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology. 2006;147(6):2696–2704. doi: 10.1210/en.2005-1397. [DOI] [PubMed] [Google Scholar]
- 31.Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: Implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297(5):E1212–E1221. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Croft S, et al. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153(11):5384–5393. doi: 10.1210/en.2012-1616. [DOI] [PubMed] [Google Scholar]
- 33.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 34.Adachi S, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 35.Kalló I, et al. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol. 2012;24(3):464–476. doi: 10.1111/j.1365-2826.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- 36.Herde MK, Iremonger KJ, Constantin S, Herbison AE. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci. 2013;33(31):12689–12697. doi: 10.1523/JNEUROSCI.0579-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156(7):2582–2594. doi: 10.1210/en.2015-1131. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, et al. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci. 2011;31(7):2421–2430. doi: 10.1523/JNEUROSCI.5759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schöne C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Reports. 2014;7(3):697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149(8):3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glanowska KM, Moenter SM. Differential regulation of GnRH secretion in the preoptic area (POA) and the median eminence (ME) in male mice. Endocrinology. 2015;156(1):231–241. doi: 10.1210/en.2014-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94(3):237–245. doi: 10.1159/000329045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Galiano D, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–328. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 44.Roseweir AK, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steyn FJ, et al. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. doi: 10.1210/en.2013-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenenbaum-Rakover Y, et al. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92(3):1137–1144. doi: 10.1210/jc.2006-2147. [DOI] [PubMed] [Google Scholar]
- 47.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 48.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li XF, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4(12):e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodman RL, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. doi: 10.1210/en.2013-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic; San Diego: 2004. [Google Scholar]