In mammalian cells, immune responses to viral infection often invoke germ-line–encoded nucleic acid-binding pattern-recognition receptors, the engagement of which results in the induction of type I interferons (IFNs). Following the discovery that intracellular DNA could trigger Toll-like receptor-independent IFN production in a variety of mammalian cells, the relevant signaling pathway was shown to involve TBK1, IRF3, and STING (1–4). However, the major sensor of cytoplasmic DNA remained elusive until the ground-breaking discovery by James Chen and his colleagues of the nucleotidyltransferase cGAS (cGMP-AMP synthase) (5, 6). In this system, recognition of cytoplasmic DNA by cGAS generates a second messenger molecule, cGAMP (cGMP-AMP), which in turn activates the endoplasmic reticulum-resident adaptor protein STING. Activated STING subsequently translocates from the endoplasmic reticulum to the Golgi compartment, where it recruits TBK1 and the transcription factor IRF3, resulting in antiviral gene expression.
Because nucleic acids are present in all viruses and are not easily mutated for the “purpose” of recognition-avoidance by a prospective host, innate immune detection of viral nucleic acids appears highly strategic in terms of antiviral defense. However, the use of nucleic acids as pathogen-associated molecular patterns has potential complications, in that self-derived nucleic acids might also pose a risk of triggering an antiviral response. One way of avoiding such inappropriate signaling would be through the degradation of self-DNA in a controlled fashion.
TREX1, previously referred to as DNaseIII, is an exonuclease that degrades cytoplasmic DNA (7). The TREX1 protein is encoded by a single exon on chromosome 3p21, in which mutations have been associated with several distinct, albeit in some cases overlapping, human disease phenotypes, including the type I IFN-related disorders Aicardi-Goutières syndrome (AGS), familial chilblain lupus (FCL), and systemic lupus erythematosus (SLE) (8). The Trex1-deficient mouse demonstrates a life-limiting inflammatory phenotype, where tissue damage is completely abrogated by a cross with mice deficient in the IFN receptor IFNAR1 (9). DNaseII is another nuclease, this time acting as an endonuclease located in lysosomes (10). DNaseII knockout mice die in utero because of an inability of macrophages to digest nuclear DNA expelled from erythroid precursor cells, thus inducing the embryotoxic production of type I IFNs via the same pathway delineated in the Trex1-null mouse model (11). No human Mendelian disease has yet been described due to dysfunction of DNaseII. However, it is of note that the retained nucleic acids in the DNaseII knockout animal also signal to the production of inflammatory cytokines, such as TNF-α. Thus, mice with a double deficiency of DNaseII and a functional type I IFN receptor do not die prenatally. Rather, they develop polyarthritis as they age, which phenotype bears remarkable resemblance to the human disorder rheumatoid arthritis (12).
In PNAS, Gao et al. (13) report their findings on crossing the cGAS knockout mouse with both Trex1- and DNaseII-deficient mice. Their data show that cGAS is central to the induction of an IFN-mediated autoimmune state, including the production of autoantibodies, in both models (Fig. 1). It is of further note that abrogation of cGAS activity completely rescues the two phenotypes that have been described in association with DNaseII deficiency (i.e., in the presence/absence of a functional IFN receptor), indicating that DNA signaling via cGAS is central to both IFN and inflammatory cytokine induction in this setting.
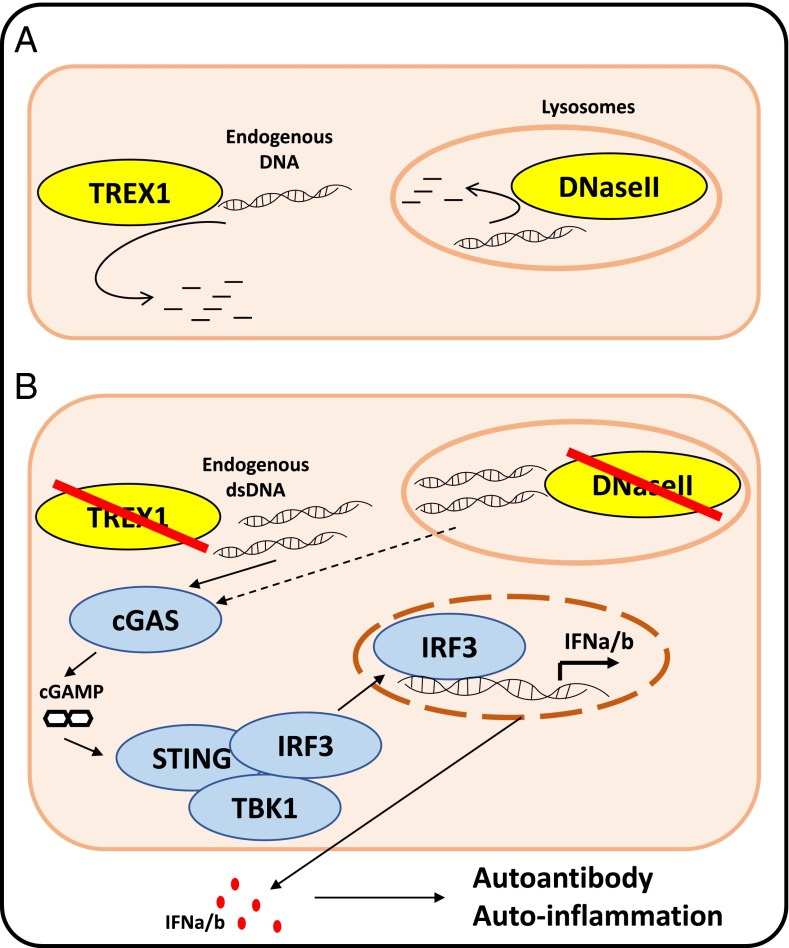
Fig. 1.
(A) Under physiological conditions, TREX1 degrades cytosolic DNAs, whereas DNaseII degrades lysosomal DNA. (B) TREX1 or DNaseII inhibition results in the accumulation of endogenous DNA, in response to which the DNA sensor cGAS synthesizes the second messenger cGAMP. Through binding to STING, cGAMP promotes a phosphorylation cascade resulting in IRF3 translocation to the nucleus. IRF3 is responsible for the transcription of IFN-α and -β, which trigger autoantibody production and systemic autoinflammation.
It has already been shown that STING is essential for the development of a phenotype in the context of Trex1 and DNaseII deficiency (14, 15). One unexpected finding of the current study (13) is the observation that, when crossed to a Sting null model, both Trex1 and DNaseII null mice demonstrate a marked elevation of cGAMP levels, over and above those observed in the single null animals. These data indicate that STING is not only triggered by cGAMP, but also plays a role in facilitating cGAMP clearance by a currently unknown mechanism. Considering that the double-knockout null mice do not display any phenotype, it appears that high levels of cGAMP per se do not cause any adverse effects.
AGS and FCL represent examples of the so-called type I interferonopathies, a recently suggested disease grouping in which an up-regulation of type I IFN is considered central to disease pathogenesis (16). AGS and FCL are rare. However, highly penetrant mutations in TREX1 were reported to be responsible for up to 2% of nonsyndromic SLE (17, 18), and DNA signaling is likely relevant in the broader category of lupus (19). The recognition of phenotypes as type I interferonopathies will be of importance as the possibility of “anti-IFN therapies” becomes a reality. Such strategies could include the use of anti-IFN antibodies, JAK inhibitors, and molecules blocking components of the innate immune response pathway, including cGAS.
The results now reported by Gao et al. (13) in relation to the role of cGAS in murine Trex1 deficiency corroborate data recently published by the team of Dan Stetson (20). Gao et al. (13) emphasize the salvage of the Trex1-associated phenotype even on the background of heterozygosity for a cGAS knockout allele. This latter point is of potential major significance if it translates to the human system; because it suggests that future therapies designed to block cGAS might be efficacious at doses that may not entail the risk of iatrogenic immunodeficiency consequent upon a loss of signaling to viral DNA. We look forward to the development of such therapeutic molecules to test this hypothesis.
Acknowledgments
This study was supported in part by a European Research Council Grant GA 309449 (to Y.J.C.) and a state subsidy managed by the National Research Agency (France) under the “Investments for the Future” program, reference ANR-10-IAHU-01 (to Y.J.C.).
Footnotes
The authors declare no conflict of interest.
See companion article on page E5699.
References
- 1.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25(3):373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202(10):1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7(1):40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 4.Goubau D, Deddouche S, Reis e Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38(5):855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindahl T, Gally JA, Edelman GM. Properties of deoxyribonuclease 3 from mammalian tissues. J Biol Chem. 1969;244(18):5014–5019. [PubMed] [Google Scholar]
- 8.Rice GI, Rodero MP, Crow YJ. Human disease phenotypes associated with mutations in TREX1. J Clin Immunol. 2015;35(3):235–243. doi: 10.1007/s10875-015-0147-3. [DOI] [PubMed] [Google Scholar]
- 9.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134(4):587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu MF, et al. The functions of Deoxyribonuclease II in immunity and development. DNA Cell Biol. 2008;27(5):223–228. doi: 10.1089/dna.2007.0691. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6(1):49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 12.Kawane K, et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443(7114):998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 13.Gao D, et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci USA. 2015;112:E5699–E5705. doi: 10.1073/pnas.1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gall A, et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36(1):120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci USA. 2012;109(47):19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crow YJ. Type I interferonopathies: Mendelian type I interferon up-regulation. Curr Opin Immunol. 2015;32:7–12. doi: 10.1016/j.coi.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Lee-Kirsch MA, et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet. 2007;39(9):1065–1067. doi: 10.1038/ng2091. [DOI] [PubMed] [Google Scholar]
- 18.Namjou B, et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 2011;12(4):270–279. doi: 10.1038/gene.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn J, Barber GN. Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease. Curr Opin Immunol. 2014;31:121–126. doi: 10.1016/j.coi.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Gray EE, Treuting PM, Woodward JJ, Stetson DB. Cutting edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi-Goutières Syndrome. J Immunol. 2015;195(5):1939–1943. doi: 10.4049/jimmunol.1500969. [DOI] [PMC free article] [PubMed] [Google Scholar]