Significance
For tens of millions of years, cold conditions have excluded shell-crushing fish and crustaceans from the continental shelf surrounding Antarctica. Rapid warming is now allowing predatory crustaceans to return. Our study of the continental slope off the western Antarctic Peninsula showed that abundant, predatory king crabs comprise a reproductively viable population at 841- to 2,266-m depth. Depth profiles of temperature, salinity, habitat structure, food availability, and predators indicate that there are no barriers to prevent king crabs from moving upward onto the outer shelf at 400–550 m. A cold-water barrier above 200 m could be breached within the next few decades. Emergence of king crabs on the shelf could have catastrophic consequences for the unique seafloor communities of Antarctica.
Keywords: biological invasion, polar emergence, climate change, predation, Southern Ocean
Abstract
Cold-water conditions have excluded durophagous (skeleton-breaking) predators from the Antarctic seafloor for millions of years. Rapidly warming seas off the western Antarctic Peninsula could now facilitate their return to the continental shelf, with profound consequences for the endemic fauna. Among the likely first arrivals are king crabs (Lithodidae), which were discovered recently on the adjacent continental slope. During the austral summer of 2010‒2011, we used underwater imagery to survey a slope-dwelling population of the lithodid Paralomis birsteini off Marguerite Bay, western Antarctic Peninsula for environmental or trophic impediments to shoreward expansion. The population density averaged ∼4.5 individuals × 1,000 m−2 within a depth range of 1,100‒1,500 m (overall observed depth range 841–2,266 m). Images of juveniles, discarded molts, and precopulatory behavior, as well as gravid females in a trapping study, suggested a reproductively viable population on the slope. At the time of the survey, there was no thermal barrier to prevent the lithodids from expanding upward and emerging on the outer shelf (400- to 550-m depth); however, near-surface temperatures remained too cold for them to survive in inner-shelf and coastal environments (<200 m). Ambient salinity, composition of the substrate, and the depth distribution of potential predators likewise indicated no barriers to expansion of lithodids onto the outer shelf. Primary food resources for lithodids—echinoderms and mollusks—were abundant on the upper slope (550–800 m) and outer shelf. As sea temperatures continue to rise, lithodids will likely play an increasingly important role in the trophic structure of subtidal communities closer to shore.
Climate change is substantially altering the composition and function of marine and terrestrial ecosystems (1–5). Polar-marine communities are particularly vulnerable to climate change, which at this point is the principal threat to their persistence (6). The near-absence of seasonal variation in sea temperature has led to the dominance of cold-stenothermal faunas, which have limited capacities to acclimate to rapid warming (7–9). At polar latitudes, cold-stenothermy and increasing physical disturbance will shift and reduce the geographic ranges of high-latitude marine taxa, increasing their risk of extinction (10–12).
Globally accelerating temperature increases are already having profound effects on polar ecosystems. Those impacts extend beyond autecological responses to include top-down and bottom-up effects on marine food webs (13–17). The endemic faunas of the Antarctic continental shelf are now at risk of invasion by durophagous (skeleton-breaking) predators (18), a functional group that (based on the limited paleontological and genetic data available) may not have been ecologically significant there for as long as tens of millions of years (19–22).
“Reptant” (bottom-walking) decapod crustaceans, teleostean fishes, and neoselachian sharks and rays are key predators in shallow-subtidal communities worldwide but are at present physiologically excluded from nearshore environments in Antarctica (19). Reptant decapods, which include brachyuran crabs, anomuran crabs, and lobsters, typically cannot survive in waters colder than 1 °C, although adult stages of some of the more cold-tolerant taxa can survive down to 0.4 °C (23, 24). Their limited capacity to down-regulate naturally occurring magnesium ions in their hemolymph leads to paralysis and death at lower temperatures (25).
In the absence of durophagous predators, benthic faunas of the Antarctic shelf are lightly skeletonized and dominated by epifaunal suspension-feeders. The top predators are slow-moving invertebrates, such as seastars and nemertean worms. In some respects, therefore, Antarctic-shelf communities are reminiscent of low-predation communities living in contemporary deep-sea habitats, as well as communities from shallow, Paleozoic environments (26–30). Now, as sea temperatures rise, the reintroduction of durophagous predators could radically alter the composition and trophic structure of the shelf-benthos in Antarctica (19, 20, 31).
Lithodid crabs (Crustacea: Decapoda: Anomura) are common in the deep sea globally, as well as in shallow waters at subpolar latitudes (32). Also known as king crabs or stone crabs, lithodids feed on a broad range of skeletonized invertebrates, but especially on echinoderms and mollusks (33–36). They are well known from deep-sea habitats of the Southern Ocean and are common in shallow, subantarctic waters (23, 37). Two species, Paralomis birsteini and Neolithodes capensis, were first observed on the continental slope adjacent to the western Antarctic Peninsula (WAP) in 2003, and these sightings were confirmed a few years later (38, 39). P. birsteini appears to be widespread in the Bellingshausen Sea and is the most commonly recorded lithodid species on the slope south of 60° S (18, 23, 37).
Phylogenetic evidence suggests multiple radiations of deep-sea lineages of lithodids into the Southern Ocean (32). How long they have been present on the Antarctic continental slope remains controversial (37), and there is virtually no information available on the status and viability of their populations. At some point, adult or larval Neolithodes yaldwyni must have moved over the shelf-break at ∼500 m to establish a reproductively viable population at slope-depths >850 m in Palmer Deep, a trough carved into the shelf of the WAP by glacial movement (40). Lithodids are, however, currently excluded from nearshore, shallow-shelf environments along the WAP, because there the shallowest waters over the shelf are colder than the slope-waters (41–44). Although they are the most cold-tolerant of the reptant decapods, lithodids are still physiologically incapable of surviving below ∼0.4 °C (18, 24, 32).
The bathymetric distribution of lithodids could change in the next few decades, however. Summertime sea-surface temperatures and temperatures of the Antarctic Shelf Bottom Waters off the WAP have risen by nearly 1.5 °C over the past 50 y, approximately double the globally averaged rate (45). Rising temperatures in shallow waters off the WAP will likely remove the thermal barrier to lithodids (and other reptant decapods) within the next several decades, facilitating their expansion into shallow, nearshore habitats (18). Judging from the strong, predatory role of invasive lithodids in Arctic food webs (33, 46), as well as the predatory impacts of the brachyuran snow crab Chionoecetes opilio in the Arctic (47), the effects of predation by lithodids could be severe in shallow-benthic communities in Antarctica (19, 40).
A recent critique (37) argued correctly that records of lithodids from the Antarctic slope and Palmer Deep do not by themselves constitute evidence that they could or will expand onto the shelf. Such a prediction could, however, be tested with time-series data on the population status and ecology of bathyal lithodids in Antarctica (37). Here, we take a significant first step in addressing that challenge. We describe a dense, reproductively viable population of P. birsteini living on the continental slope off the WAP. We show that there are no apparent physical or ecological barriers to impede the population from immediately expanding upward onto the deeper parts of the adjacent continental shelf, with potentially catastrophic impacts on the existing benthic fauna.
Results and Discussion
Population Structure of P. birsteini.
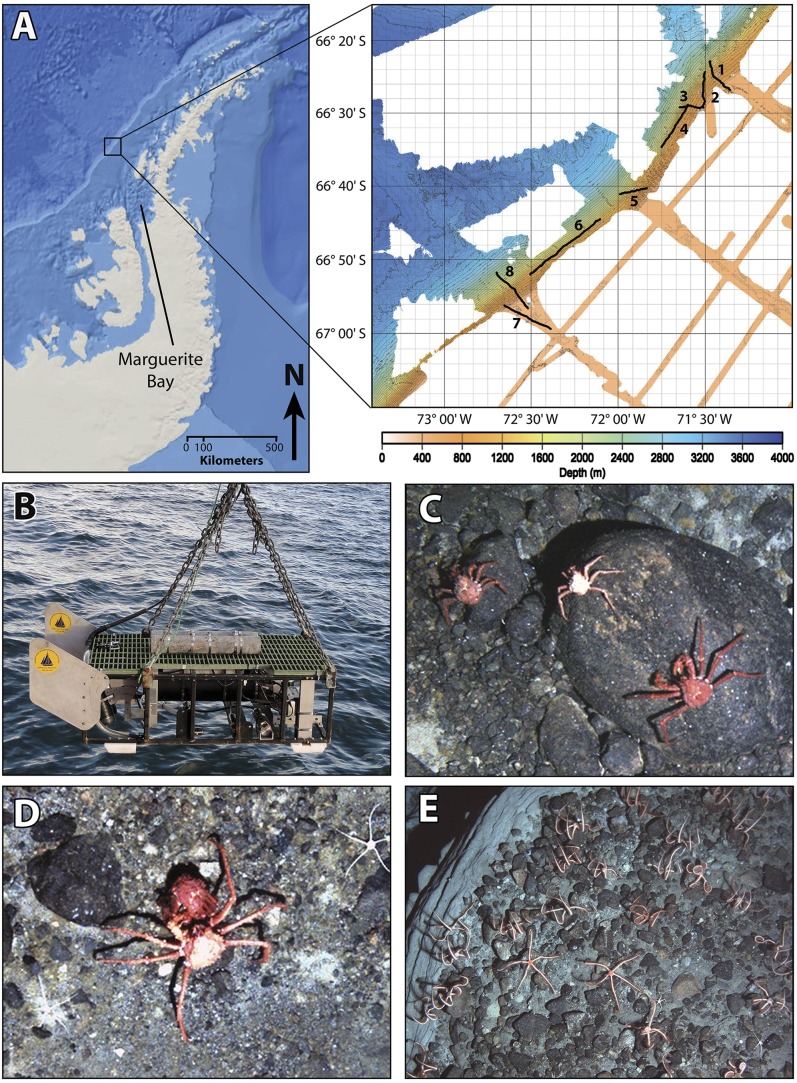
In 2010, we conducted the first comprehensive survey of a population of lithodids on the Antarctic slope by towing the camera-vehicle SeaSled from the RV Nathaniel B. Palmer off Marguerite Bay, WAP (66°42′ S, 72°12′ W) (Methods and Fig. 1 A and B). SeaSled was towed at an average altitude of 3 m above the sediment–water interface. We completed eight photographic transects of the seafloor across the outer shelf, slope, and continental rise, covering a depth range of 385–2,285 m, a total distance of 72 km, and a total area of 183,653 m2 (Fig. 1A, and Tables S1 and S2). The 38,018 pairs of overlapping, digital images we collected at altitudes of 0.5‒6.0 m above the substrate were used to estimate population densities of P. birsteini across depths and assess reproductive viability. Tandem ecological assessments enabled us to evaluate the potential for lithodids to emerge on the shelf (Fig. 2).
Fig. 1.
Study site, SeaSled, and images from the seafloor. (A) Location of study site along the western Antarctic Peninsula. Expanded view shows known bathymetry of the study site, based primarily on multibeam data collected during the cruise. Numbered lines denote the eight transects (see Tables S1 and S2 for details). The depth gradient runs southeast to northwest; white areas represent unmapped portions of the seafloor. Map of Antarctic Peninsula on left produced in ArcGIS 10.2. (B) Towed camera-vehicle SeaSled being deployed. (C) Three P. birsteini at 1,356-m depth. (D) Pair of P. birsteini in precopulatory embrace at 841 m. Larger animal is the male. The female has recently molted, as inferred from her dark-red coloration. (E) Ophiuroids on the seafloor at 1,134 m.
Table S1.
Details of the eight transects imaged within the 100 × 100-km study area off Marguerite Bay, western Antarctic Peninsula
| Transect | Minimum depth (m) | Maximum depth (m) | Length of transect (m) | Number of image pairs | Area covered (m2) | Start latitude/longitude | End latitude/longitude |
| 1 | 497 | 1,390 | 6,950 | 4,619 | 21,817 | 66°26′37′′ S | 66°22′53′′ S |
| 71°21′52′′ W | 71°28′28′ W | ||||||
| 2 | 562 | 1,535 | 7,326 | 4,189 | 19,436 | 66°24′35′′ S | 66°29′42′′ S |
| 71°30′09′′ W | 71°30′38′′ W | ||||||
| 3 | 586 | 1,310 | 3,987 | 2,370 | 13,468 | 66°29′39′′ S | 66°29′57′′ S |
| 71°31′09′′ W | 71°38′40′′ W | ||||||
| 4 | 1,037 | 1,316 | 7,884 | 3,610 | 18,516 | 66°29′24′′ S | 66°34′35′′ S |
| 71°36′39′′ W | 71°44′51′′ W | ||||||
| 5 | 626 | 1,527 | 6,090 | 3,262 | 17,154 | 66°40′32′′ S | 66°41′36′′ S |
| 71°48′23′′ W | 71°59′57′′ W | ||||||
| 6 | 1,109 | 1,398 | 18,664 | 10,438 | 45,535 | 66°44′29′′ S | 66°52′00′′ S |
| 72°06′20′′ W | 72°30′46′′ W | ||||||
| 7 | 385 | 642 | 11,114 | 5,403 | 25,656 | 66°56′12′′ S | 66°59′25′′ S |
| 72°39′26′′ W | 72°23′14′′ W | ||||||
| 8 | 409 | 2,285 | 9,566 | 4,127 | 22,071 | 66°52′17′′ S | 66°56′30′′ S |
| 72°40′49′′ W | 72°31′11′′ W | ||||||
| TOTAL | 71,581 | 38,018 | 183,653 |
Table S2.
Area per depth range for the eight transects imaged within the 100 × 100-km study area off Marguerite Bay, western Antarctic Peninsula
| Area (m2) per depth range | |||||||||
| Depth (m) | Dive 1 | Dive 2 | Dive 3 | Dive 4 | Dive 5 | Dive 6 | Dive 7 | Dive 8 | Total area (m2) |
| 300–399 | — | — | — | — | — | — | 3,884 | — | 3,884 |
| 400–499 | 286 | — | — | — | — | — | 20,564 | 6,218 | 27,068 |
| 500–599 | 5,387 | 4,086 | 301 | — | — | — | 790 | 869 | 11,433 |
| 600–699 | 2,376 | 2,286 | 2,865 | — | 2,036 | — | 418 | 674 | 10,655 |
| 700–799 | 2,218 | 2,946 | 2,291 | — | 2,407 | — | — | 1,299 | 11,161 |
| 800–899 | 2,771 | 3,432 | 1,567 | — | 2,738 | — | — | 1,159 | 11,667 |
| 900–999 | 2,222 | 1,064 | 1,915 | — | 1,523 | — | — | 859 | 7,583 |
| 1,000–1,099 | 2,129 | 884 | 1,678 | 7,732 | 2,181 | — | — | 687 | 15,291 |
| 1,100–1,199 | 1,737 | — | 1,922 | 4,850 | 1,681 | 8,730 | — | 552 | 19,472 |
| 1,200–1,299 | 1,871 | 843 | 842 | 5,578 | 1,423 | 9,769 | — | 608 | 20,934 |
| 1,300–1,399 | 820 | 1,907 | 87 | 356 | 1,314 | 27,036 | — | 987 | 32,507 |
| 1,400–1,499 | — | 1,246 | — | — | 1,438 | — | — | 807 | 3,491 |
| 1,500–1,599 | — | 742 | — | — | 413 | — | — | 768 | 1,923 |
| 1,600–1,699 | — | — | — | — | — | — | — | 681 | 681 |
| 1,700–1,799 | — | — | — | — | — | — | — | 843 | 843 |
| 1,800–1,899 | — | — | — | — | — | — | — | 765 | 765 |
| 1,900–1,999 | — | — | — | — | — | — | — | 971 | 971 |
| 2,000–2,099 | — | — | — | — | — | — | — | 1,314 | 1,314 |
| 2,100–2,199 | — | — | — | — | — | — | — | 1,009 | 1,009 |
| 2,200–2,299 | — | — | — | — | — | — | — | 1,001 | 1,001 |
| Total area (m2) | 21,817 | 19,436 | 13,468 | 18,516 | 17,154 | 45,535 | 25,656 | 22,071 | |
Fig. 2.
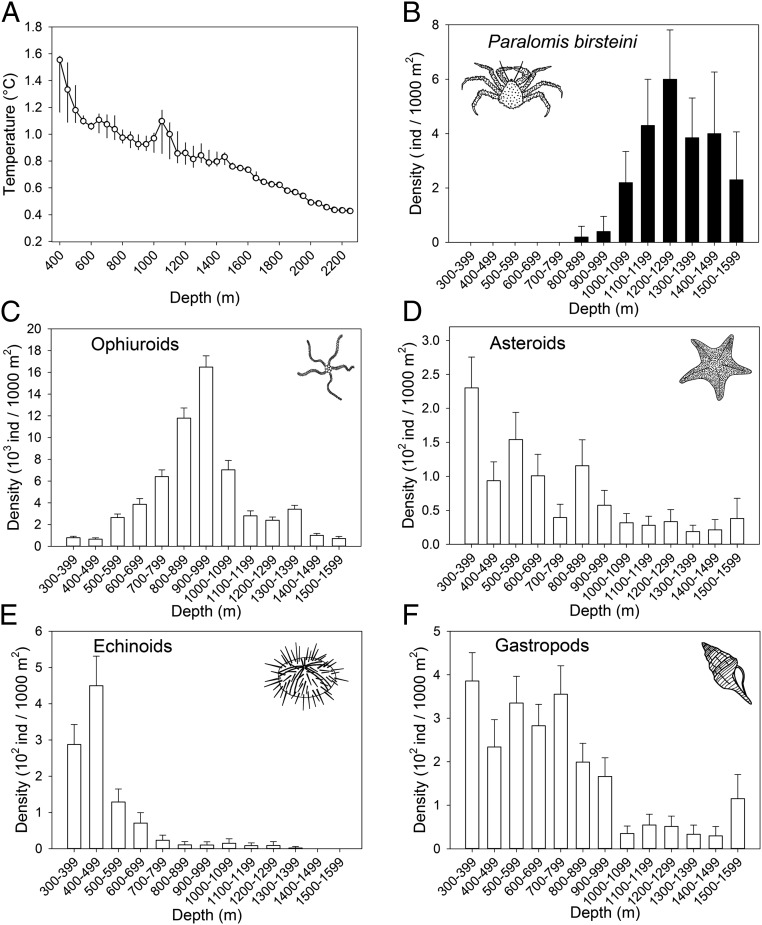
Physical attributes and densities of invertebrates in the study area off Marguerite Bay, Antarctica. (A) Mean temperature in 50-m depth increments over the depth range sampled. Vertical bars represent ranges. (B) Density of P. birsteini with depth, over the depth range for which accurate estimates were possible. Error bars represent 95% confidence intervals. Means and confidence intervals were calculated assuming the data are Poisson-distributed within depth bins. (C–F) Densities of potential prey as a function of depth. Error bars represent 95% confidence intervals. Means and confidence intervals were calculated assuming the data for each prey-taxon are normally distributed within depth bins. Note the different depth scale in A, and the different scales of density in B–F.
We identified two taxa of lithodids using morphological features plainly visible in the images and in specimens retrieved in a trapping study off Marguerite Bay in 2015. Almost all of the king crabs were referable to P. birsteini (based on ref. 39), of which 468 individuals were found at depths of 841–2,266 m on the continental slope and continental rise (Fig. 1C). A species of Neolithodes referable to N. yaldwyni was also present but relatively rare, with five individuals observed on the continental slope at 989–1,209 m. The following discussion, therefore, pertains to P. birsteini.
Lithodids were absent from the continental shelf, which breaks at 400–550 m depth off Marguerite Bay. (The continental shelf in Antarctica is deeper than continental shelves elsewhere as a result of isostatic depression by the polar ice cap and glacial erosion of the shelf sediments.) Densities of P. birsteini were 0.3 individuals × 1,000 m−2 at 841- to 1,000-m depth and increased to a mean of 4.5 individuals × 1,000 m−2 over a depth range on the slope of 1,100–1,500 m (Fig. 2B). The maximum density of ∼6 individuals × 1,000 m−2 at 1,200‒1,300 m was lower than the density of lithodids in Palmer Deep (64°57′ S, 64°17′ W), but similar to the densities of other lithodid populations (40). A few individuals were found deeper than 1,600 m, but the limited area surveyed at those depths (Table S2) precluded an accurate estimate of population density.
Three pairs of P. birsteini were photographed in precopulatory embrace, which is the prelude to spermatophore transfer, at 841-, 1,095-, and 1,297-m depth (Fig. 1D). As in other anomuran crabs, the male lithodid is about a third larger and grasps the chelipeds of the female until she molts her exoskeleton, rendering her receptive to spermatophore transfer. The dark-red color of the female in Fig. 1D indicates a recent molt. Three discarded exoskeletons observed between 1,163 and 1,307 m also indicate that P. birsteini in this population were successfully molting. Significantly, four of the nine female P. birsteini recovered in the trapping study in 2015 were gravid, including one that was carrying eggs in the later stages of development (with fully developed eyes).
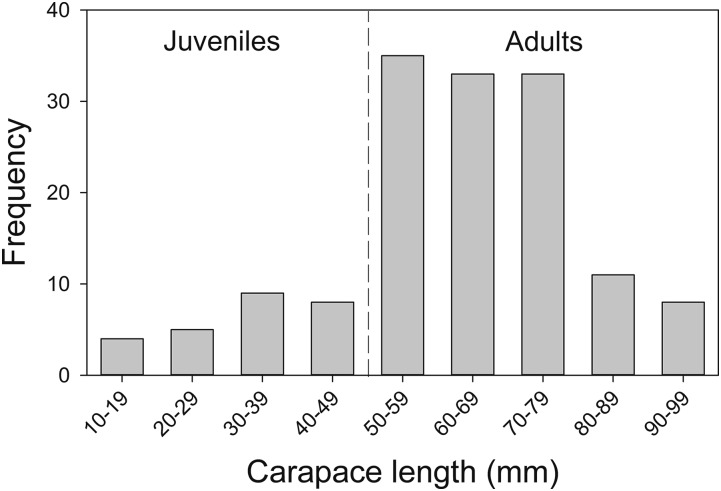
The mean carapace length (CL) of 146 P. birsteini that could be measured accurately from the images was 61.8 mm ± 0.15 SE (range 11.4–99.7 mm) (Fig. 3). Twenty-six individuals were <50-mm CL. Based on the age-structures of populations of similar-sized congeners in southern South America (48) and a single juvenile that was reported for this population in 2007 (39), these smaller individuals were classified as juveniles. The putative juveniles exhibited a broad bathymetric range of 1,126–2,266 m. Taken together with the large size of the population, our observations of precopulatory behavior, discarded molts, gravid females, and juveniles strongly suggest a reproductively viable population.
Fig. 3.
Carapace lengths of P. birsteini imaged off Marguerite Bay, western Antarctic Peninsula (n = 146 crabs that could be measured from the images; 1,075- to 2,266-m depth). Vertical dashed line denotes the maximum carapace length of putative juveniles.
Habitat Characteristics of the Slope and Shelf.
Water temperature.
Water temperatures were measured 1–6 m above the sediment–water interface using a CTD (conductivity–temperature–depth) instrument attached to SeaSled. Temperatures ranged from 0.43 to 1.6 °C at 385- to 2,285-m depth. The temperatures were highest on the continental shelf and negatively correlated with depth (Fig. 2A) (Pearson r = –0.962, P < 0.001). Along the same depth gradient, P. birsteini appeared on the slope at 841 m and increased rapidly in abundance, peaking at 1,200–1,300 m (Fig. 2B). P. birsteini were found at temperatures ranging from 0.43 °C at the deepest surveyed depth of 2,266 m on the continental rise to 1.16 °C at 1,039 m on the slope. The shallowest record was of two individuals at 841 m in an ambient temperature of 0.9 °C.
The thermal range of P. birsteini, ∼0.4–2.5 °C (24), as well as images collected off Anvers Island, 380 km to the northeast, strongly suggest that adults were actively feeding on the slope at temperatures at least as low as 0.82 °C (49). Water temperatures on the upper slope (550–800 m) and lower (outer) shelf (400–550 m) were, therefore, within the known thermal tolerance of this species at the time of the survey, which was conducted in the austral summer. Temperatures in the austral fall and winter are generally greater than 1.0 °C at shelf depths below 200 m off Marguerite Bay, because of localized intrusions of the Circumpolar Deep Water (CDW) and a general, multidecadal trend of shallowing of the CDW in the Bellingshausen Sea (41, 45, 50, 51). A cold-water barrier to lithodids remains at depths above 200 m, where Antarctic surface water and winter water typically keep ambient temperatures below 0 °C year-round (41, 43). In contrast, cold Antarctic surface water can persist as deep as 500 m in parts of the Weddell and Ross Seas, suggesting that king crabs may be physiologically excluded from outer-shelf habitats in those locations (52).
Salinity.
Salinity ranged narrowly from 34.9 to 36.0 psu over a depth range of 385‒2,285 m. Salinity generally does not drop below 34.7 psu at depths of 300 m or more (41). The range of observed salinities on the shelf and slope falls within the tolerances of lithodid crabs, including P. birsteini (www.iobis.org/mapper/).
Sedimentary composition.
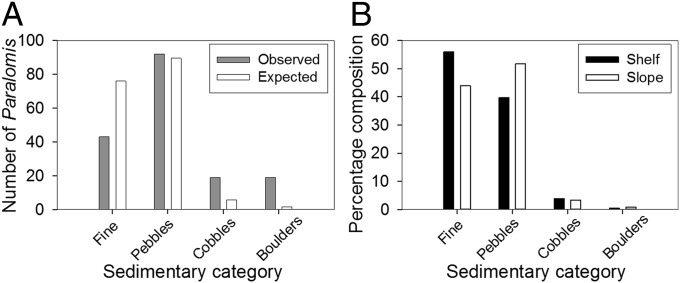
We assessed the substrate preferences of P. birsteini by comparing the observed frequencies of sedimentary size-classes on the seafloor with the size-classes on which the lithodids were observed (Fig. 4A). The availabilities of four size-classes of sediment—fine-grained sediment, pebbles, cobbles, and boulders—were quantified in the depth range of maximum density of P. birsteini, 1100‒1,500 m (Methods). The seafloor within that depth range was dominated by fine-grained sediment and pebbles (Fig. 4B). P. birsteini in 1,100‒1,500 m were found most frequently on pebbles, but a greater number were associated with cobble and boulder substrates than expected in the null hypothesis (G-test: G = 215.70, df = 3, P < 0.001) (Fig. 4A). There were no significant differences in the proportions of the four sedimentary categories between 1,100‒1,500 m and 400‒600 m (Fig. 4B) (Mantel test: r = ‒0.160, P = 0.22). Combining the distribution of P. birsteini on the sedimentary categories at 1,100- to 1,500-m depth with the relative abundances of those categories at 400‒600 m, we calculate that, absent other constraints, P. birsteini has the potential to reach densities of 4.5 individuals × 1,000 m−2 within that depth range.
Fig. 4.
Sedimentary composition and preferences of the lithodid crab P. birsteini along the western Antarctic Peninsula. (A) Observed and expected frequencies of P. birsteini on different sedimentary categories on the slope (1,100–1,500 m; n = 173). Expected values were calculated assuming the crabs showed no preferences for sedimentary categories. (B) Sedimentary composition on the continental shelf (400–600 m) and slope (1,100–1,500 m). Frequency data were converted to percentages for ease of comparison (n = 3,000 for the shelf; n = 6,000 for the slope).
Hydrostatic pressure.
We observed P. birsteini at a minimum depth of 841 m on the slope off Marguerite Bay. The shelf-break off Marguerite Bay extends at least to 550 m, which is well within the known depth range of the species. P. birsteini has been collected as shallow as 341 m on a seamount outside the Ross Sea (53), and other lithodids have been collected at 200 m or shallower off islands and seamounts surrounding Antarctica (23, 37, 54). Deep-water lithodids can be maintained for months in the laboratory at atmospheric pressure without any noticeable adverse effects (55, 56). Hydrostatic pressure should not, therefore, present a barrier to P. birsteini expanding onto the outer shelf off Marguerite Bay.
Many benthic invertebrates in Antarctica are eurybathic because the water column is uniformly cold. Ecological and evolutionary emergence of deep-water taxa in shallow water and submergence of shallow-water taxa in deep water are historical phenomena more common in Antarctica than at lower latitudes. These bathymetric shifts have been driven by glacial dynamics in the Neogene and have not been impeded by the effects of temperature or hydrostatic pressure (27, 57). Furthermore, movement of lithodids onto the outer Antarctic shelf—and shoreward as sea temperatures rise—would repeat their expansion onto the Patagonian shelf during deglaciation 10,000 y ago (20). Clearly the emergence of lithodids is physiologically and ecologically feasible. The acceleration of that emergence by anthropogenic climate change and the capacity of the lithodids for durophagous predation are features that would make their appearance on the Antarctic shelf ecologically novel and potentially transformative.
Distribution of prey resources.
The availability of food resources across depths was evaluated by estimating the population densities of the known prey of lithodids visible in images taken 0.5‒3.0 m above the substrate: ophiuroids, asteroids, echinoids, and gastropods. Potential prey that were not visible in the images, such as infaunal bivalves, could not be evaluated. The density of ophiuroids formed a unimodal distribution that peaked at 900–1,000 m (Figs. 1E and 2C). The overlapping tails of the depth distributions of ophiuroids and P. birsteini suggested an inverse relationship (Fig. 2 B and C). Asteroids, echinoids, and gastropods were more abundant at all shelf depths and generally more abundant on the upper slope than they were at 1,100‒1,500 m (Fig. 2 D‒F), suggesting that predation by P. birsteini may limit the distributions of these taxa. A similar disjunction in depth distribution of N. yaldwyni and echinoderms was observed in Palmer Deep (40), and C. opilio and ophiuroids were distributed in a complementary pattern in the Chukchi Sea (47). Considering the generality of diet in lithodids and their predilection for echinoderms and mollusks, there is no reason to expect that the population of P. birsteini would be food-limited were it to expand to the outer shelf.
Predation pressure.
Potential predators of benthic decapods include fishes, marine mammals, cephalopods, and other crustaceans in temperate, tropical, and Arctic marine ecosystems. The demersal fish fauna of Antarctica is comprised almost exclusively of three taxa of teleosts: the suborder Notothenioidei, distributed primarily in shelf environments; the family Zoarcidae on the slope; and the family Liparidae on the deep slope and continental rise. Fishes were rarely observed along the transects (58). Based on biomechanical analysis of the demersal teleosts, limited durophagous feeding is possible in notothenioids and zoarcids, but not in liparids (59). Some teleostean taxa might be capable of preying on the demersal larvae or juveniles of P. birsteini, but the few data available on their diets, distributions, and feeding biomechanics suggest that these fishes are not durophagous (59).
Nevertheless, published observations of predation on lithodids raise the possibility that their emergence onto the shelf may not be risk-free. The Antarctic toothfish Dissostichus mawsoni, a nektonic notothenioid, occasionally preys on lithodids at subantarctic latitudes, especially species such as P. birsteini that lack spiny exoskeletons; P. birsteini have been identified in the stomach contents of toothfish (54). Although D. mawsoni are present in the depth range in which we observed P. birsteini, they are extremely rare at all depths off Marguerite Bay (58) and are unlikely to pose a threat. Similarly, although skates (Rajidae) and larger octopods potentially could feed on P. birsteini in outer-shelf and slope habitats, they appeared only rarely in our images. Neither octopods nor skates play a significant role in Antarctic food webs (19).
Seals, which feed primarily on fish, cephalopods, and krill, could also prey on lithodids. Based on the diets of Antarctic seal species, the most likely candidates are Weddell seals, Leptonychotes weddellii, and southern elephant seals, Mirounga leonina. Weddell seals are known to feed on small crustaceans and can dive to at least 726 m, although their depth range is generally on the order of 100–200 m (60, 61). Male elephant seals, which dive to an average depth of 500 m, occasionally feed on benthic crustaceans (62). Neither species of seal is likely to pose a significant threat to P. birsteini on the outer shelf.
We estimated predation pressure on the population of P. birsteini by assessing the extent of sublethal damage, in the form of missing or regenerating chelipeds and walking legs. Injuries to limbs were rare: of 347 P. birsteini that could be assessed from the images, 31 individuals, or 8.9%, displayed one or more damaged limbs. Of the 2,776 limbs of these animals that were visible in the images, 34, or 1.2%, were damaged. This latter incidence of damage was low compared with temperate decapods [e.g., 22.8% in the blue crab Callinectes sapidus (63)], despite lower regeneration rates in colder, Antarctic waters. The incidence of damage in P. birsteini was also low compared with the lithodid Paralithodes camtschaticus in the Arctic [16.1% incidence (63)]. Chelipeds are often damaged in mating and other intraspecific interactions, so the low percentages for P. birsteini probably overestimate the frequency of interspecific, sublethal predation. Regardless, the low level of sublethal damage and the paucity of potential predators strongly suggest that both sublethal and lethal predation events are rare.
Conclusion
We found a reproductively viable population of the lithodid crab P. birsteini resident on the continental slope off Marguerite Bay, western Antarctic Peninsula. Although the history of this population is unknown, its contemporary ecology supports the idea that bathyal lithodids could expand upward within a few decades. Depth profiles of water temperature, salinity, sedimentary composition, and the availability of prey, as well as the general absence of predators, indicate conditions favorable to upward expansion of P. birsteini in this location, at least as shallow as the lower depths of the continental shelf. P. birsteini would likely have a pervasive impact on the resident shelf-benthos, as lithodids do in other low-temperature environments around the world. Long-term ecological monitoring off Marguerite Bay and additional locations around Antarctica will be the only way to test rigorously the hypothesis of an imminent or ongoing expansion of lithodids into shelf habitats (37). Our study provides the initial data for such a long-term study.
Declining temperatures in the Southern Ocean after the Eocene drove benthic communities on the Antarctic shelf from a typically Cenozoic structure and function to the retrograde, quasi-Paleozoic character they exhibit today. Anthropogenic climate change is now rapidly rewarming the Southern Ocean, potentially reversing this trend. At present rates of warming, lithodids should be able to survive in inner-shelf and coastal environments (above 200 m) within several decades. Emergence of predatory lithodids on the continental shelf could be a critical step toward remodernizing benthic communities in Antarctica and functionally homogenizing them with benthic-shelf communities at lower latitudes. Such changes would fundamentally alter the Antarctic benthos and diminish the global diversity of marine ecosystems.
Methods
A photographic survey of the continental slope and shelf off Marguerite Bay was conducted from the RV Nathaniel B. Palmer in early December 2010 during National Science Foundation cruise NBP10-05. Eight transects, averaging 9 km in length, were imaged within a 100 × 100-km study site (Fig. 1 and Table S1). Transects covered the outer continental shelf, slope, and rise from 385- to 2,285-m depth. Images of the seafloor were obtained using the towed camera-vehicle SeaSled, which is owned and operated by the Woods Hole Oceanographic Institution, Woods Hole, MA (58, 64). SeaSled was equipped with two cameras (each 1.4-megapixel, or 1,360 × 1,024 pixels), two strobes (150 w-s), an acoustic-Doppler current profiler (1,200 kHz; Teledyne RD Instruments), a depth sensor (Paroscientific), and a CTD (Seabird SBE-49 Fast CAT 16-Hz). Two overlapping, strobe-lit images were collected every 3 s at an average altitude of 3 m above the substrate. The altitudinal range of usable images was 0.5‒6.0 m above the substrate.
Density and Size Estimates.
Densities were estimated for P. birsteini and four taxa of benthic prey: ophiuroids, asteroids, echinoids, and gastropods. Image clarity was reduced with increasing altitude from the seafloor, and all images collected at an altitude above 6 m were discarded. The remaining images, which comprised 38,018 image pairs, were pooled, divided into 100-m depth bins, and used to estimate the density of P. birsteini.
A density estimate was calculated for P. birsteini within each depth bin in each transect. For each depth bin, the number of crabs in each transect was divided by the total area covered by the images from that transect within that depth bin, corrected for the overlap of the image pairs and between adjacent image pairs along the transect. The planar area of each image was determined trigonometrically from its dimensions and the altitude of the camera. The mean density of crabs per 1,000 m2 and the 95% confidence intervals within each depth bin were calculated assuming a Poisson distribution, because the crabs were rare in the images.
The potential prey were smaller and more abundant than P. birsteini, and accurate density estimates could only be obtained from images taken at a maximum altitude of 3 m. Densities were estimated using 250 randomly selected images from each depth bin, with the exception of 1,400–1,500 m and 1,500–1,600 m. Only 132 and 71 images, respectively, were available at altitudes of 3 m or less in those depth bins. The 95% confidence intervals were calculated assuming the prey taxa were normally distributed within each depth bin.
Where possible, the carapace lengths of the lithodids were measured based on the dimensions of the area captured in the image, using the software package Coral Point Count with Excel extensions (65). Measurements were only recorded for individuals observed on a horizontal plane and for which the entire carapace could be seen, at a maximum altitude of 3 m (i.e., the carapace and appendages had to appear symmetrical in the image).
Assessment of Benthic Habitat.
Temperature and salinity data recorded 1–6 m above the seafloor were pooled from all transects and averaged at each 50-m depth mark, from 400 to 2,250 m. For each 50-m depth mark, z, the temperature and salinity data from z ± 10 m were used.
The composition of the substrate was determined for the shelf (400–600 m) and slope (1,100–1,500 m) using 100 randomly selected images from a maximum altitude of 3 m from each 100-m depth bin. Fifteen points were randomly placed on each image within a standardized 1-m2 plot using Coral Point Count with Excel extensions. The grain of sediment beneath each point was measured and classified into one of four size-categories: fine-grained sediment (grain-size < 4 mm), pebble (4 to <64 mm), cobble (64 to <256 mm), or boulder (≥256 mm). In a pilot study using different numbers of random points, we determined that 15 points per image were sufficient to make accurate assessments of coverage of the different size-classes of sediments. Each P. birsteini photographed from a maximum altitude of 3 m was also analyzed to determine the size-class of sediment beneath it. A G-test was used to compare the observed frequency of P. birsteini on each size-class of sediment to the frequency expected under the assumption that they had no grain-size preference. The proportions of sedimentary size-classes between the shelf and slope were compared using a Mantel test.
Acknowledgments
This study was part of the fifth expedition to Antarctica carried out jointly by the US National Science Foundation, the Swedish Polar Research Secretariat, and the Swedish Research Council. We thank the crews of the RV Nathaniel B. Palmer and RVIB Oden, as well as the staff of Raytheon Polar Services Corporation, for logistical support in the field; K. Tönnesson, F. Weyer, and Y. S. Zhang for assistance; and M. Bansode, M. Bush, J. Eastman, and L. Toth for helpful discussion. Our research was supported by National Science Foundation Grants ANT-0838846 and ANT-1141877 (to R.B.A.), and ANT-0838844 and ANT-1141896 (to J.B.M.); and by Swedish Research Council Grant 824-2008-6429 (to P.-O.M. and J.N.H.). This is contribution 138 from the Institute for Research on Global Climate Change at the Florida Institute of Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513962112/-/DCSupplemental.
References
- 1.Williams JW, Jackson ST. Novel climates, no-analog communities, and ecological surprises. Front Ecol Environ. 2007;5(9):475–482. [Google Scholar]
- 2.Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S. Climate change and the past, present, and future of biotic interactions. Science. 2013;341(6145):499–504. doi: 10.1126/science.1237184. [DOI] [PubMed] [Google Scholar]
- 3.Norris RD, Turner SK, Hull PM, Ridgwell A. Marine ecosystem responses to Cenozoic global change. Science. 2013;341(6145):492–498. doi: 10.1126/science.1240543. [DOI] [PubMed] [Google Scholar]
- 4.Kidwell SM. Biology in the Anthropocene: Challenges and insights from young fossil records. Proc Natl Acad Sci USA. 2015;112(16):4922–4929. doi: 10.1073/pnas.1403660112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capinha C, Essl F, Seebens H, Moser D, Pereira HM. BIOGEOGRAPHY. The dispersal of alien species redefines biogeography in the Anthropocene. Science. 2015;348(6240):1248–1251. doi: 10.1126/science.aaa8913. [DOI] [PubMed] [Google Scholar]
- 6.Bennett JR, et al. Polar lessons learned: Long-term management based on shared threats in Arctic and Antarctic environments. Front Ecol Environ. 2015;13(6):316–324. [Google Scholar]
- 7.Peck LS, Convey P, Barnes DKA. Environmental constraints on life histories in Antarctic ecosystems: Tempos, timings and predictability. Biol Rev Camb Philos Soc. 2006;81(1):75–109. doi: 10.1017/S1464793105006871. [DOI] [PubMed] [Google Scholar]
- 8.Clarke A, et al. Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos Trans R Soc Lond B Biol Sci. 2007;362(1477):149–166. doi: 10.1098/rstb.2006.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung WWL, et al. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2009;10(3):235–251. [Google Scholar]
- 10.Brown A, Thatje S. The effects of changing climate on faunal depth distributions determine winners and losers. Glob Change Biol. 2015;21(1):173–180. doi: 10.1111/gcb.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutt J, et al. The Southern Ocean ecosystem under multiple climate change stresses—An integrated circumpolar assessment. Glob Change Biol. 2015;21(4):1434–1453. doi: 10.1111/gcb.12794. [DOI] [PubMed] [Google Scholar]
- 12.Urban MC. Climate change. Accelerating extinction risk from climate change. Science. 2015;348(6234):571–573. doi: 10.1126/science.aaa4984. [DOI] [PubMed] [Google Scholar]
- 13.Piepenburg D. Recent research on Arctic benthos: Common notions need to be revised. Polar Biol. 2005;28(10):733–755. [Google Scholar]
- 14.Doney SC. Oceanography: Plankton in a warmer world. Nature. 2006;444(7120):695–696. doi: 10.1038/444695a. [DOI] [PubMed] [Google Scholar]
- 15.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37(1):637–669. [Google Scholar]
- 16.McClintock JB, Angus RA, Ho C, Amsler CD, Baker BJ. A laboratory study of behavioral interactions of the Antarctic keystone sea star Odontaster validus with three sympatric predatory sea stars. Mar Biol. 2008;154(6):1077–1084. [Google Scholar]
- 17.Wassmann P, Duarte CM, Agustí S, Sejr MK. Footprints of climate change in the Arctic marine ecosystem. Glob Change Biol. 2011;17(2):1235–1249. [Google Scholar]
- 18.Thatje S, et al. Challenging the cold: Crabs reconquer the Antarctic. Ecology. 2005;86(3):619–625. [Google Scholar]
- 19.Aronson RB, et al. Climate change and invasibility in the Antarctic benthos. Annu Rev Ecol Evol Syst. 2007;38:129–154. [Google Scholar]
- 20.Aronson RB, Frederich M, Price R, Thatje S. Prospects for the return of shell-crushing crabs to Antarctica. J Biogeogr. 2015;42(1):1–7. [Google Scholar]
- 21.Near TJ, et al. Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proc Natl Acad Sci USA. 2012;109(9):3434–3439. doi: 10.1073/pnas.1115169109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whittle RJ, Quaglio F, Griffiths HJ, Linse K, Crame JA. The Early Miocene Cape Melville Formation fossil assemblage and the evolution of modern Antarctic marine communities. Naturwissenschaften. 2014;101(1):47–59. doi: 10.1007/s00114-013-1128-0. [DOI] [PubMed] [Google Scholar]
- 23.Thatje S, Arntz WE. Antarctic reptant decapods: More than a myth? Polar Biol. 2004;27(4):195–201. [Google Scholar]
- 24.Hall S, Thatje S. Temperature-driven biogeography of the deep-sea family Lithodidae (Crustacea: Decapoda: Anomura) in the Southern Ocean. Polar Biol. 2011;34(3):363–370. [Google Scholar]
- 25.Frederich M, Sartoris FJ, Pörtner HO. Distribution patterns of decapod crustaceans in polar areas: A result of magnesium regulation? Polar Biol. 2001;24(10):719–723. [Google Scholar]
- 26.Aronson RB, Blake DB. Global climate change and the origin of modern benthic communities in Antarctica. Am Zool. 2001;41(1):27–39. [Google Scholar]
- 27.Clarke A, Aronson RB, Crame JA, Gili JM, Blake DB. Evolution and diversity of the benthic fauna of the Southern Ocean continental shelf. Antarct Sci. 2004;16(4):559–568. [Google Scholar]
- 28.Gili J-M, et al. A unique assemblage of epibenthic sessile suspension feeders with archaic features in the high-Antarctic. Deep Sea Res Part II Top Stud Oceanogr. 2006;53(8):1029–1052. [Google Scholar]
- 29.Krug AZ, Jablonski D, Roy K, Beu AG. Differential extinction and the contrasting structure of polar marine faunas. PLoS One. 2010;5(12):e15362. doi: 10.1371/journal.pone.0015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crame JA. Evolutionary setting. In: De Broyer C, Koubbi P, editors. Biogeographic Atlas of the Southern Ocean. Scientific Committee on Antarctic Research; Cambridge, UK: 2014. pp. 32–35. [Google Scholar]
- 31.Aronson RB, et al. Climate change and trophic response of the Antarctic bottom fauna. PLoS One. 2009;4(2):e4385. doi: 10.1371/journal.pone.0004385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall S, Thatje S. Global bottlenecks in the distribution of marine Crustacea: Temperature constraints in the family Lithodidae. J Biogeogr. 2009;36(11):2125–2135. [Google Scholar]
- 33.Falk-Peterson J, Renaud P, Anisimova N. Establishment and ecosystem effects of the alien invasive red king crab (Paralithodes camtschaticus) in the Barents Sea—A review. ICES J Mar Sci. 2011;68(3):479–488. [Google Scholar]
- 34.Comoglio LI, Amin OA. Feeding habits of the false southern king crab Paralomis granulosa (Lithodidae) in the Beagle Channel, Tierra del Fuego, Argentina. Sci Mar. 1999;63(S1):361–366. [Google Scholar]
- 35.Jørgensen LL, Nilssen EM. The invasive history, impact and management of the red king crab Paralithodes camtschaticus off the coast of Norway. In: Galil BS, Clark PF, Carlton JT, editors. In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts. Springer; New York: 2011. pp. 521–536. [Google Scholar]
- 36.Boudreau SA, Worm B. Ecological role of large benthic decapods in marine ecosystems: A review. Mar Ecol Prog Ser. 2012;469:195–213. [Google Scholar]
- 37.Griffiths HJ, Whittle RJ, Roberts SJ, Belchier M, Linse K. Antarctic crabs: Invasion or endurance? PLoS One. 2013;8(7):e66981. doi: 10.1371/journal.pone.0066981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García Raso JE, Manjón-Cabeza ME, Ramos A, Olasi I. New record of Lithodidae (Crustacea, Decapoda, Anomura) from the Antarctic (Bellingshausen Sea) Polar Biol. 2005;28(8):642–646. [Google Scholar]
- 39.Thatje S, Hall S, Hauton C, Held C, Tyler P. Encounter of lithodid crab Paralomis birsteini on the continental slope of Antarctica, sampled by ROV. Polar Biol. 2008;31(9):1143–1148. [Google Scholar]
- 40.Smith CR, et al. A large population of king crabs in Palmer Deep on the west Antarctic Peninsula shelf and potential invasive impacts. Proc Biol Sci. 2012;279(1730):1017–1026. doi: 10.1098/rspb.2011.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klinck JM, Hofmann EE, Beardsley RC, Salihoglu B, Howard S. Water-mass properties and circulation on the west Antarctic Peninsula continental shelf in Austral fall and winter 2001. Deep Sea Res Part II Top Stud Oceanogr. 2004;51(17):1925–1946. [Google Scholar]
- 42.Barnes DKA, Peck LS. Vulnerability of Antarctic shelf biodiversity to predicted regional warming. Clim Res. 2008;37(2-3):149–163. [Google Scholar]
- 43.Jenkins A, Jacobs S. Circulation and melting beneath George VI Ice Shelf, Antarctica. J Geophys Res. 2008;113(C4):1–18. [Google Scholar]
- 44.Clarke A, Griffiths HJ, Barnes DKA, Meredith MP, Grant SM. Spatial variation in seabed temperatures in the Southern Ocean: Implications for benthic ecology and biogeography. J Geophys Res. 2009;114:G03003. [Google Scholar]
- 45.Schmidtko S, Heywood KJ, Thompson AF, Aoki S. Multidecadal warming of Antarctic waters. Science. 2014;346(6214):1227–1231. doi: 10.1126/science.1256117. [DOI] [PubMed] [Google Scholar]
- 46.Christiansen JS, Sparboe M, Sæther B-S, Siikavuopio SI. Thermal behavior and the prospect spread of an invasive benthic top predator onto the Euro-Arctic shelves. Divers Distrib. 2015;21(9):1004–1013. [Google Scholar]
- 47.Ravelo AM, Konar B, Trefry JH, Grebmeier JM. Epibenthic community variability in the northeastern Chukchi Sea. Deep Sea Res Part II Top Stud Oceanogr. 2014;102:119–131. [Google Scholar]
- 48.Hall S, Thatje S. King crabs up-close: Ontogenetic changes in ornamentation in the family Lithodidae (Crustacea, Decapoda, Anomura), with a focus on the genus Paralomis. Zoosystema. 2010;32(3):495–524. [Google Scholar]
- 49.Smith KE, et al. Discovery of a recent, natural whale fall on the continental slope off Anvers Island, western Antarctic Peninsula. Deep Sea Res Part I Oceanogr Res Pap. 2014;90:76–80. [Google Scholar]
- 50.Orsi AH, Whitworth T, III, Nowlin WD., Jr On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Res Part I Oceanogr Res Pap. 1995;42(5):641–673. [Google Scholar]
- 51.Martinson DG, McKee DC. Transport of warm upper circumpolar deep water onto the western Antarctic Peninsula continental shelf. Ocean Science. 2012;8(4):433–442. [Google Scholar]
- 52.Jacobs SS. Bottom water production and its links with the thermohaline circulation. Antarct Sci. 2004;16(4):427–437. [Google Scholar]
- 53.Ocean Survey 20/20 . International Polar Year and Census of Antarctic Marine Life Ross Sea voyage (TAN0802) Biodiversity Data. Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research; Wellington, New Zealand: 2013. [Google Scholar]
- 54.Ahyong ST, Dawson EW. Lithodidae from the Ross Sea, Antarctica, with descriptions of two new species (Crustacea: Decapoda: Anomura) Zootaxa. 2006;1303:45–68. [Google Scholar]
- 55.Watts J, Thatje S, Clarke S, Belchier M. A description of larval and early juvenile development in Paralomis spinosissima (Decapoda: Anomura: Paguroidea: Lithodidae) from South Georgia waters (Southern Ocean) Polar Biol. 2006;29(12):1028–1038. [Google Scholar]
- 56.Thatje S, Mestre NC. Energetic changes throughout lecithotrophic larval development in the deep-sea lithodid crab Paralomis spinosissima from the Southern Ocean. J Exp Mar Biol Ecol. 2010;386(1):119–124. [Google Scholar]
- 57.Brown A, Thatje S. Explaining bathymetric diversity patterns in marine benthic invertebrates and demersal fishes: physiological contributions to adaptation of life at depth. Biol Rev Camb Philos Soc. 2014;89(2):406–426. doi: 10.1111/brv.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eastman JT, et al. Photographic survey of benthos provides insights into the Antarctic fish fauna from the Marguerite Bay slope and the Amundsen Sea. Antarct Sci. 2013;25(1):31–43. [Google Scholar]
- 59.Bansode MA, Eastman JT, Aronson RB. Feeding biomechanics of five demersal Antarctic fishes. Polar Biol. 2014;37(12):1835–1848. [Google Scholar]
- 60.Schreer JF, Testa JW. Classification of Weddell seal diving behavior. Mar Mamm Sci. 1996;12(2):227–250. [Google Scholar]
- 61.Bestley S, Jonsen ID, Hindell MA, Harcourt RG, Gales NJ. Taking animal tracking to new depths: Synthesizing horizontal–vertical movement relationships for four marine predators. Ecology. 2015;96(2):417–427. doi: 10.1890/14-0469.1. [DOI] [PubMed] [Google Scholar]
- 62.Hindell MA, Slip DJ, Burton HR. The diving behavior of adult male and female southern elephant seals Mirounga leonina (Pinnipeda, Phocidae) Aust J Zool. 1991;39(5):595–619. [Google Scholar]
- 63.Juanes F, Smith LD. The ecological consequences of limb damage and loss in decapod crustaceans: A review and prospectus. J Exp Mar Biol Ecol. 1995;193(1):197–223. [Google Scholar]
- 64.Singh H, Roman C, Pizarro O, Eustice R, Can A. Towards high-resolution imaging from underwater vehicles. Int J Robot Res. 2007;26(1):55–74. [Google Scholar]
- 65.Kohler KE, Gill SM. Coral point count with Excel extensions (CPCe): A visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci. 2006;32(9):1259–1269. [Google Scholar]