Significance
The immune system detects microbial DNA in the cytosol of infected cells and mounts effective antimicrobial responses, including the production of type-I interferons. However, when self-DNA enters or accumulates in the cytosol, it can cause autoimmune diseases. Mutations of the exonuclease Trex1 in humans have been linked to autoimmune diseases including Aicardi–Goutieres Syndrome (AGS) and systemic lupus erythematosus (SLE). In mice, genetic deletion of Trex1 or the lysosomal nuclease DNaseII leads to lethal autoimmune diseases. Here we show that cyclic GMP-AMP synthase (cGAS) activation by self-DNA is responsible for the lethal autoimmune diseases in these models. These results provide the proof-of-concept that inhibition of cGAS may be an effective therapy for some autoimmune diseases such as AGS and SLE.
Keywords: cGAS, cGAMP, autoimmune disease, Trex1, DNaseII
Abstract
TREX1 is an exonuclease that digests DNA in the cytoplasm. Loss-of-function mutations of TREX1 are linked to Aicardi–Goutieres Syndrome (AGS) and systemic lupus erythematosus (SLE) in humans. Trex1−/− mice exhibit autoimmune and inflammatory phenotypes that are associated with elevated expression of interferon (IFN)-induced genes (ISGs). Cyclic GMP-AMP (cGAMP) synthase (cGAS) is a cytosolic DNA sensor that activates the IFN pathway. Upon binding to DNA, cGAS is activated to catalyze the synthesis of cGAMP, which functions as a second messenger that binds and activates the adaptor protein STING to induce IFNs and other cytokines. Here we show that genetic ablation of cGas in Trex1−/− mice eliminated all detectable pathological and molecular phenotypes, including ISG induction, autoantibody production, aberrant T-cell activation, and lethality. Even deletion of just one allele of cGas largely rescued the phenotypes of Trex1−/− mice. Similarly, deletion of cGas in mice lacking DNaseII, a lysosomal enzyme that digests DNA, rescued the lethal autoimmune phenotypes of the DNaseII−/− mice. Through quantitative mass spectrometry, we found that cGAMP accumulated in mouse tissues deficient in Trex1 or DNaseII and that this accumulation was dependent on cGAS. These results demonstrate that cGAS activation causes the autoimmune diseases in Trex1−/− and DNaseII−/− mice and suggest that inhibition of cGAS may lead to prevention and treatment of some human autoimmune diseases caused by self-DNA.
Recognition and elimination of invading genetic materials is a fundamental mechanism of host defense. In vertebrate animals, the immune system deploys sensors of DNA and RNA to detect microbial infections (1–3). In addition to a subset of Toll-like receptors that detect microbial nucleic acids in the lumen of endosomes, cytosolic nucleic acid sensors also play crucial roles in detecting pathogens, especially those that have successfully breached the membrane barriers and replicated in the interior of a cell. The cytosolic nucleic acid sensors include cyclic GMP-AMP (cGAMP) synthase (cGAS) and retinoic acid inducible gene I (RIG-I)-like receptors, which detect DNA and RNA, respectively, to induce type-I interferons (IFNs) and other inflammatory cytokines (4–6). cGAS binds to double-stranded DNA (dsDNA) in a sequence-independent manner (2, 7, 8). This binding causes a conformational change in the active site of cGAS, which then uses ATP and GTP as the substrates to synthesize cGAMP that contains mixed 2′–5′ and 3′–5′ phosphodiester bonds (9–15). cGAMP then binds to and activates the endoplasmic reticulum membrane protein STING (14, 16–18). STING in turn activates the protein kinases IKK and TBK1, which activate the transcription factors NF-κB and IRF3, respectively. NF-κB and IRF3 enter the nucleus and function together to induce IFNs and cytokines. RIG-I and its homolog MDA5 detect viral RNA in the cytoplasm and induce IFNs through a similar pathway, except that the essential adaptor protein functioning downstream of RIG-I and its homolog MDA5 is the mitochondrial membrane protein MAVS, not STING (2, 19).
Although detection of microbial nucleic acids provides a versatile and highly effective mechanism for the immune system to detect infections, inadvertent reactions to self nucleic acids pose a risk of triggering autoimmune and autoinflammatory diseases (20). In the case of RIG-I, the problem of avoiding activation by cytoplasmic self-RNA is solved by the ability of RIG-I to detect specifically viral RNA that contains 5′-triphosphate and -diphosphate (21–23). Cellular RNA contains modifications such as the 5′ cap in mRNA, which usually has 2′-O-methylation at the N1 position that prevents its recognition by RIG-I (24). For cGAS, which can be activated by dsDNA irrespective of its sequence or origin, avoidance of triggering autoimmunity is largely achieved by confining DNA in the nucleus, mitochondria, lysosome, and other membranous compartments, and by degrading DNA in the cytoplasm.
TREX1 is an exonuclease that degrades DNA in the cytoplasm (25–28). Trex1 deficiency in humans has been linked to several autoimmune and inflammatory diseases, including Aicardi–Goutieres Syndrome (AGS), systemic lupus erythematosus (SLE), familial chilblain lupus, and retinal vasculopathy with cerebral leukodystrophy (29). A common feature of these diseases is the elevated expression of IFN-stimulated genes (ISGs), suggesting that a defect in clearing cytosolic DNA leads to the activation of the IFN pathway. Trex1-deficient mice exhibit inflammatory diseases and premature death accompanied by elevated ISG expression. Despite some common autoimmune phenotypes, Trex1−/− mice manifest myocarditis, whereas human AGS patients suffer severe encephalopathy. The autoimmune and myocarditis phenotypes in Trex1−/− mice are rescued by deleting Sting, supporting an important role of the cytosolic DNA-sensing pathway in causing the inflammatory diseases in the absence of Trex1 (26, 27, 30). Interestingly, patients with gain-of-function mutations in STING also develop lupus-like syndromes; however, these patients also exhibit unique features of vascular and pulmonary inflammation (31, 32).
Another major cellular nuclease is DNaseII, which is localized in the lysosome and is largely responsible for the clearance of DNA of dead cells and expelled nuclei that are engulfed by macrophages (33–35). So far, no known DNaseII deficiency in humans has been reported. DNaseII−/− mice die in utero, and the embryos become severely anemic by embryonic day 17.5 (E17.5), owing to the inability of macrophages to digest nuclear DNA expelled from erythroid precursor cells. The undigested DNA induces excessive production of IFNs, which kill the embryos. Deletion of the IFN receptor IFNAR1 rescued the embryonic lethality of DNaseII−/− mice, but the double-knockout mice developed polyarthritis because of increased production of inflammatory cytokines such as TNF-α and IL6 (36). The increased production of these cytokines as well as IFNs is dependent on STING, because deletion of Sting in DNaseII−/− mice not only rescued the embryonic lethality but also prevented the development of polyarthritis (37).
A key question that remains is the identity of the DNA sensor that triggers the autoimmune diseases caused by defective clearance of self-DNA. Although cGAS is the dominant DNA sensor responsible for activating the IFN pathways in response to infections by a variety of pathogens, including DNA viruses, retroviruses, and some bacteria such as Mycobacterium tuberculosis (13, 38–43), the role of cGAS in autoimmune diseases has not been extensively investigated. Here we show that deletion of cGas in Trex1−/− and DNaseII−/− mice rescued the lethal autoimmune phenotypes of these mice and prevented the accumulation of cGAMP and the expression of ISGs in the mutant animals. These results demonstrate that cGAS activation causes the autoimmune diseases in Trex1−/− and DNaseII−/− mice.
Results
cGAS Is Responsible for Inflammatory Diseases and Death of Trex1−/− Mice.
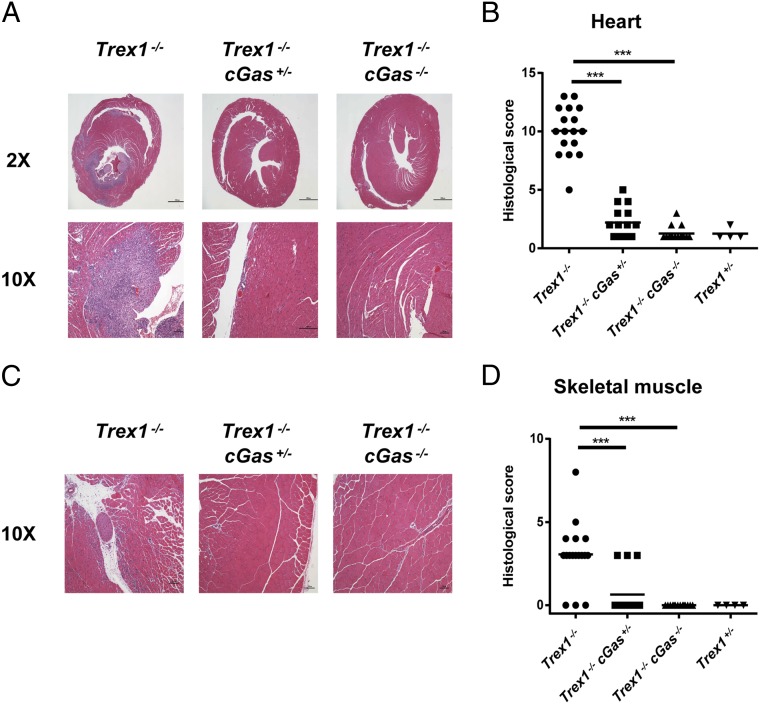
Trex1−/− deficient mice die within a few months after birth due to severe inflammation in multiple organs, especially in the heart (Fig. 1; refs. 26 and 27). To determine whether cGAS activation—presumably by self-DNA accumulated in the cytosol of Trex1−/− cells—is responsible for the inflammatory diseases and reduced survival of Trex1−/− mice, we generated Trex1−/− cGas−/− and Trex1−/−cGas+/− mice. Strikingly, all Trex1−/−cGas−/− mice that were monitored (89/89) survived >200 d after birth, whereas ∼39% (65/167) of Trex1−/− mice survived within the same period (Fig. 1A). Even removal of just one allele of cGas in Trex1−/− mice allowed ∼93% (91/98) of the mice to survive (Fig. 1). Hematoxylin and eosin (H&E) staining of the heart, skeletal muscle, skin, and kidney biopsies showed that the inflammatory pathology observed in these tissues of Trex1−/− mice was eliminated in Trex1−/−cGas−/− mice and markedly reduced in Trex1−/−cGas+/− mice (Fig. 2 and Fig. S1 A–E). Moreover, splenomegaly found in Trex1−/− mice was not observed in Trex1−/−cGas−/− mice (Fig. S1 F–H). These results demonstrate that cGAS is responsible for the inflammation and lethality of Trex1−/− mice.
Fig. 1.
cGAS deletion rescues the lethality of Trex1−/− mice. Survival curves of Trex1−/−, Trex1−/−cGas+/−, and Trex1−/−cGas−/− mice are shown. All mice were on a C57BL/6 background. Statistical analysis was performed with a Mantel–Cox test.
Fig. 2.
cGAS mediates multiorgan inflammation in Trex1−/− mice. (A and C) Representative H&E-stained heart (A) and skeletal muscle (C) sections from 12-wk-old Trex1−/−, Trex1−/−cGas+/−, and Trex1−/−cGas−/− mice. Blue-stained cells indicate leukocytes that infiltrate the heart. (B and D) Blinded analysis of the indicated tissues of 12-wk-old Trex1−/−, Trex1−/−cGas+/−, and Trex1−/−cGas−/− mice as well as Trex1+/− mice. Histological scores were calculated as described in Materials and Methods. Statistical analysis was performed with a two-tailed, unpaired Student's t test. ***P < 0.001.
Fig. S1.
cGAS is responsible for the pathology and splenomegaly of Trex1−/− mice. (A) Representative Picro-Sirius Red (PSR; to measure fibrosis)-stained heart sections from mice of indicated genotypes. (B) Representative H&E-stained skin sections. (C) Blinded analysis of the skin tissues represented in B. (D) Representative H&E-stained (Upper; ×10) and Periodic Acid Schiff (PAS; to detect glycogen) stained (Lower; ×10) kidney sections. (E) Blinded analysis of the kidney tissues represented in D. (F) Spleens from 12-wk-old Trex1−/− and Trex1−/−cGas−/− mice. (G and H) Spleen cell numbers (G) and spleen weights (H) were measured from 12-wk-old Trex1−/− and Trex1−/−cGas−/− mice. Statistical analysis was performed with a two-tailed, unpaired Student's t test. *P < 0.05; ***P < 0.001.
cGAS Deletion Abolishes the Expression of ISGs and Inflammatory Cytokines in Trex1−/− Mice.
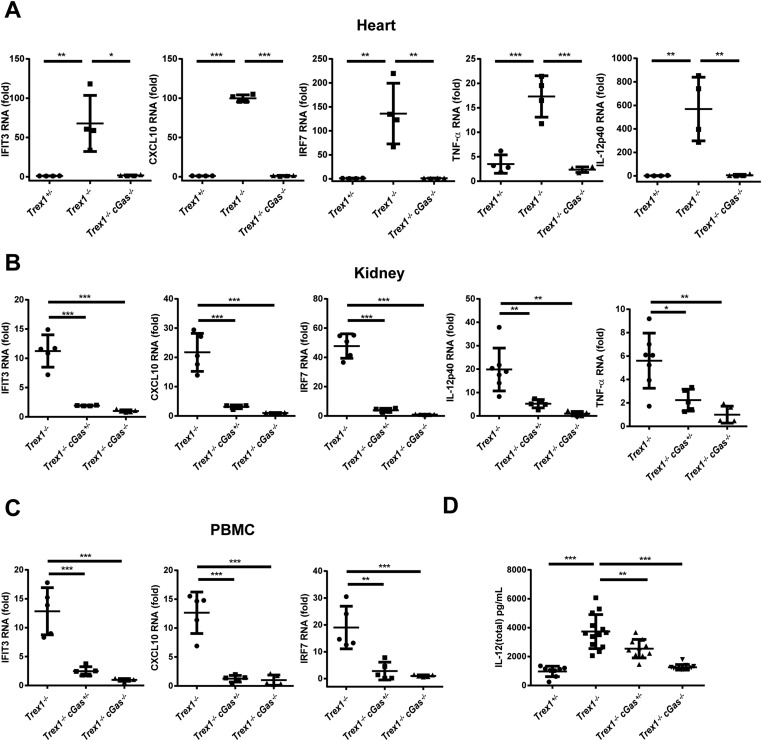
Gene-expression analysis by quantitative RT-PCR (qRT-PCR) of RNA from the hearts of 12-wk-old Trex1−/− and Trex1+/− mice showed that Trex1−/− mice had elevated levels of ISGs, including IFIT3, CXCL10, and IRF7, as well as the inflammatory cytokines TNF-α and IL-12 (Fig. S2A and Table S1). The expression of these ISGs and cytokines was largely abolished by removing one or both alleles of cGas in Trex1−/− mice (Fig. 3 A–E and Fig. S2A). Similar results were obtained by using kidneys and peripheral blood monocytes (PBMCs; Fig. S2 B and C) or by measurements of IL-12 protein in the mouse sera (Fig. S2D). Elevated levels of ISGs in bone marrow dendritic cells (BMDCs) (Fig. S3A), macrophages (Fig. S3B), and embryonic fibroblasts (Fig. S3C) from Trex1−/− mice were largely blunted by the deletion of cGas.
Fig. S2.
cGAS is essential for ISG expression in Trex1−/− mice. (A) qRT-PCR analysis of indicated ISGs, TNF-α, and IL12p40 in hearts from 12-wk-old mice of indicated genotypes. Fold changes are relative to Trex+/− mice. (B) qRT-PCR analysis of indicated ISGs, TNF-α, and IL12p40 in kidneys from 12-wk-old mice. Fold changes are relative to Trex1−/−cGas−/−mice. (C) qRT-PCR analysis of indicated ISGs in PBMCs from 5-wk-old mice. Fold changes are relative to Trex1−/−cGas−/−mice. (D) Measurement of IL-12 (total) in the plasma of 12-wk-old mice. Error bars represent SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Table S1.
qPCR primers
| Genes | Forward primers | Reverse primers |
| Rpl19 | AAATCGCCAATGCCAACTC | TCTTCCCTATGCCCATATGC |
| Cxcl10 | GCCGTCATTTTCTGCCTCA | CGTCCTTGCGAGAGGGATC |
| Ifng | CTTTGCAGCTCTTCCTCATGGCTGTTTCTG | TGACGCTTATGTTGTTGCTGATGGCCTG |
| Tnf | CACAGAAAGCATGATCCGCGACGT | CGGCAGAGAGGAGGTTGACTTTCT |
| Tbx21 | TCAGGACTAGGCGAAGGAGA | TAGTGGGCACCTTCCAATTC |
| Ifit3 | TGGCCTACATAAAGCACCTAGATGG | CGCAAACTTTTGGCAAACTTGTCT |
| Irf7 | ATGCACAGATCTTCAAGGCCTGGGC | GTGCTGTGGAGTGCACAGCGGAAGT |
| Il10 | CTATGCTGCCTGCTCTTACTG | AACCCAAGTAACCCTTAAAGTC |
| Il12p40 | GTTCAACATCAAGAGCAGTAGCA | CTGCAGACAGAGACGCCATT |
| Isg15 | GGAACGAAAGGGGCCACAGCA | CCTCCATGGGCCTTCCCTCGA |
Fig. 3.
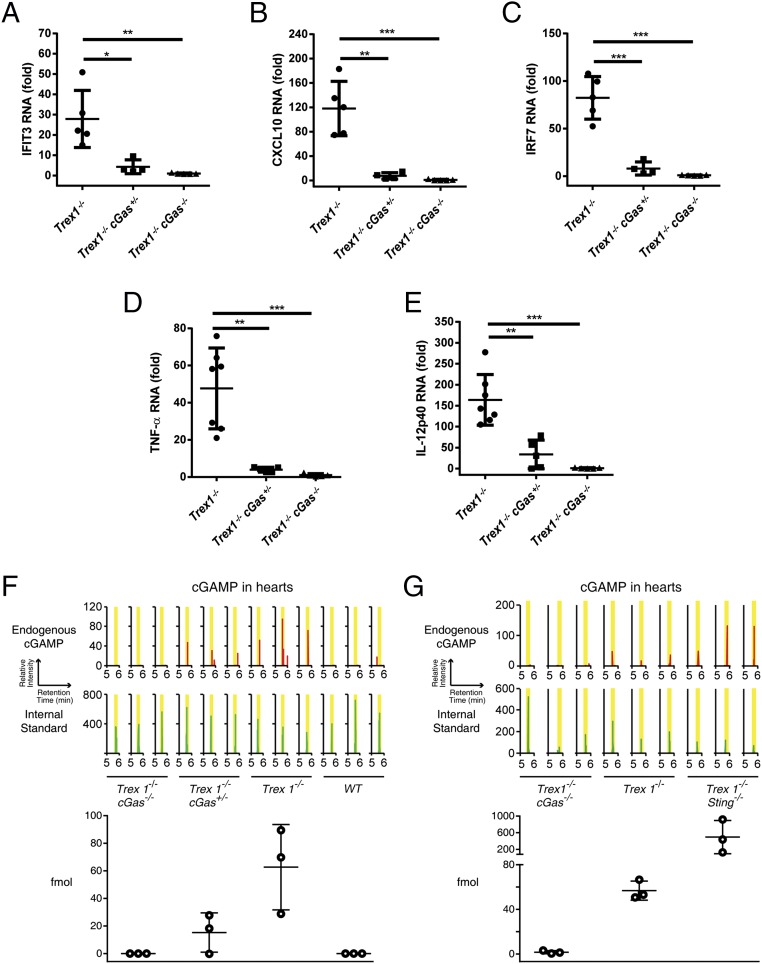
cGAS is essential for the expression of ISGs and inflammatory cytokines as well as cGAMP overproduction in Trex1−/− mice. (A–E) qRT-PCR analysis of indicated ISGs (A–C), TNF-α (D), and IL12p40 (E) in hearts from 12-wk-old mice of indicated genotypes. Fold changes are relative to Trex1−/−cGas−/−mice. Error bars represent SD. *P < 0.05; **P < 0.01; ***P < 0.001. (F and G) cGAMP levels in mouse hearts of indicated genotypes were quantified by LC-MS. Upper shows zoomed chromatograms displaying relative intensities of endogenous cGAMP (red) and internal standard (green). Lower shows the calculated amounts of cGAMP (fmol) in each sample. Error bars represent SEM.
Fig. S3.
cGAS is required for ISG induction in different cells types from Trex1−/− mice. GM-CSF–derived BMDCs (A), BMDMs (B), and mouse embryonic fibroblasts (MEF; C) from indicated genotypes were analyzed by qRT-PCR for expression of ISGs. Fold changes are relative to Trex1−/−cGas−/−mice. Error bars represent SD of triplicate assays. *P < 0.05; **P < 0.01; ***P < 0.001.
Trex1−/− Mice Overproduce cGAMP in a cGAS-Dependent Manner.
Quantitative mass spectrometry (MS) was used to measure the absolute amounts of cGAMP in mouse hearts. This measurement was made possible by spiking an internal standard of 13C1015N5-labeled cGAMP in the heart extracts (Materials and Methods). This measurement revealed elevated levels of cGAMP in Trex1−/− mice, which were markedly reduced in Trex1−/−cGas+/− mice and abolished in Trex1−/−cGas−/− mice (Fig. 3F). Interestingly, the levels of cGAMP in Trex1−/−Sting−/− mice were approximately eightfold higher than those in Trex1−/− mice (Fig. 3G), suggesting that STING facilitates the clearance of cGAMP through an unknown mechanism. Because Trex1−/−Sting−/− mice do not display any apparent inflammation or other abnormal phenotypes (30), these results imply that high levels of cGAMP do not appear to cause adverse effects in mice in the absence of STING.
cGAS Activation Causes Autoantibody Production and T-Cell Activation in Trex1−/− Mice.
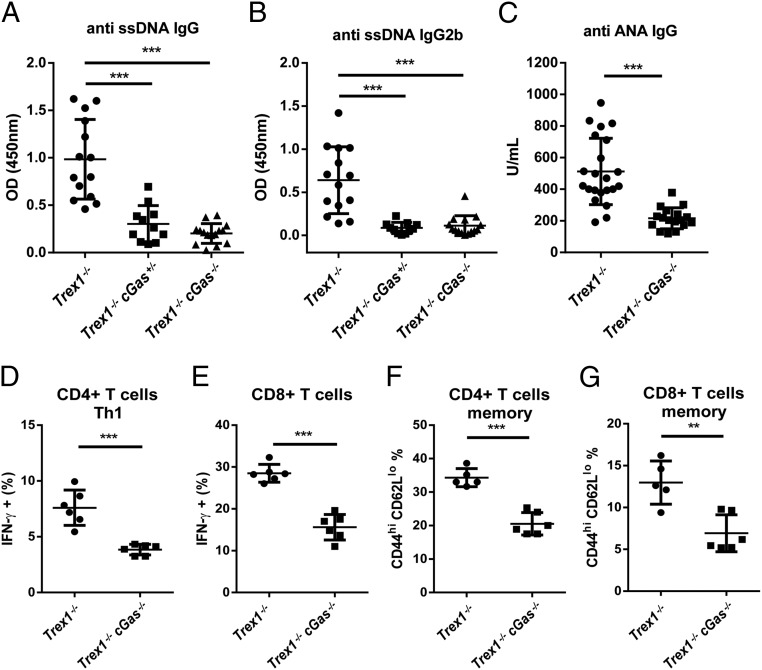
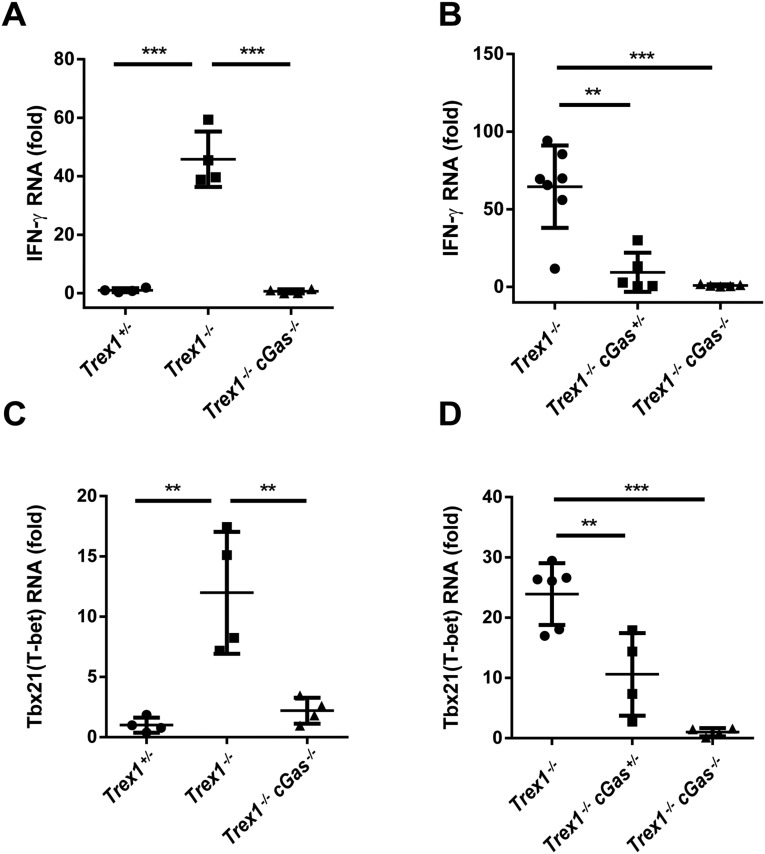
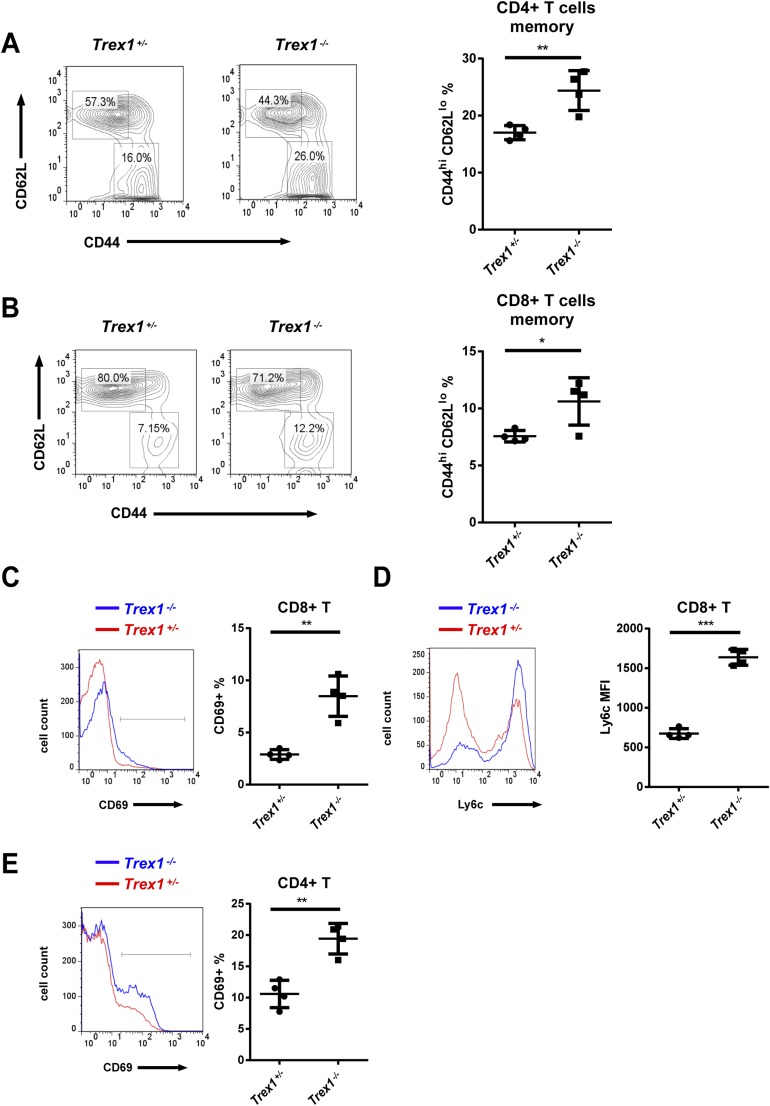
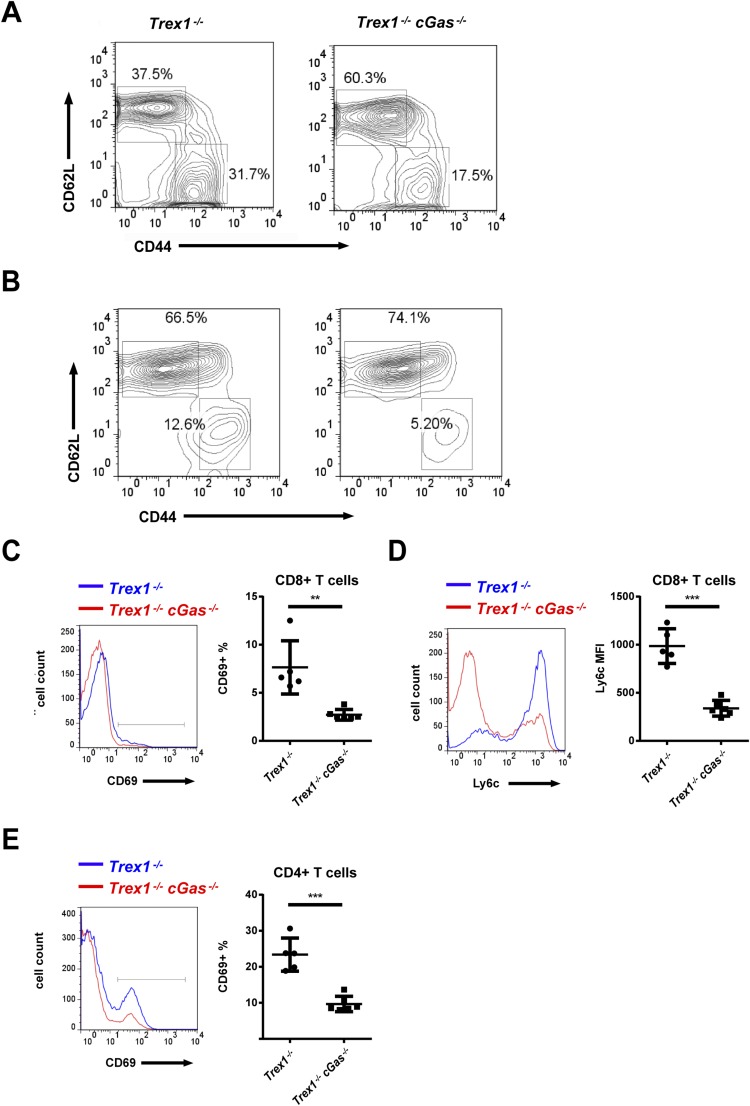
Trex1−/− mice, both 12-wk- and 5-mo-old, developed autoantibodies against DNA, nuclear antigens (ANAs), and other self-antigens, including histone H3, M2, and the U1 subunit of small nuclear ribonucleoprotein (U1-snRNP; Fig. 4 A–C and Fig. S4). All of these autoantibodies were greatly reduced by deleting one or both alleles of cGas. The CD4 and CD8 T cells from Trex1−/− mice had higher expression of T-bet than T cells from Trex1−/−cGas−/− mice and produced more IFN-γ in response to stimulation with phorbol myristate acetate (PMA) and ionomycin (Fig. 4 D and E; Fig. S5). Hearts from Trex1−/− mice, but not Trex1−/−cGas−/− mice, had elevated expression of IFN-γ and T-bet (also known as Tbx21) RNA, indicating spontaneous infiltration of activated immune cells to the heart, which was blocked by deleting cGas (Fig. S6). Autoreactive T cells in Trex1−/− mice were evident by enhanced surface expression of CD69 for CD4 and CD8 T cells and Ly6c for CD8 T cells (Fig. S7 C–E). Deletion of cGas in Trex1−/− mice markedly reduced the numbers of these activated T cells (Fig. S8 C–E). Elevated levels of memory CD4 and CD8 T cells (CD44hi/CD62Llo) in Trex1−/− mice were also reduced in Trex1−/−cGas−/− mice (Fig. 4 F and G; Fig. S7 A and B; Fig. S8 A and B). These results further demonstrate that cGAS activation causes the autoimmune phenotypes in Trex1−/− mice.
Fig. 4.
cGAS deletion mitigates autoimmunity in Trex1−/− mice. (A–C) Detection of autoantibodies, including ssDNA IgG (A), ssDNA IgG2b subtype (B), and ANA IgG (C) in the sera of 12-wk-old mice of indicated genotypes. (D and E) Flow-cytometric analysis of intracellular IFN-γ in response to PMA plus ionomycin in splenic CD4+ T cells (D) and CD8+ T cells (E) from 12-wk-old Trex1−/− and Trex1−/−cGas−/− mice. (F and G) Flow-cytometric analysis of CD44hiCD62lo cells in splenic CD4+ T cells (F) and CD8+ T cells (G) from 12-wk-old Trex1−/− and Trex1−/−cGas−/− mice. Error bars represent SD. **P < 0.01; ***P < 0.001.
Fig. S4.
cGAS is required for the generation of autoantibodies in Trex1−/− mice. Measurements of the indicated autoantibodies in the sera of 12-wk-old (A–C) and 5-mo-old (D–I) mice are shown. Error bars represent SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. S5.
cGAS is required for the generation of hyperreactive T cells in Trex1−/− mice. (A–C) Flow-cytometric analysis of intracellular IFN-γ in response to PMA plus ionomycin in splenic CD4+ T cells or CD8+ T cells from 12-wk-old mice of indicated genotypes. (D) Similar to C, except that CD8 T cells in PBMC were analyzed. (E and F) Flow-cytometric analysis of T-bet in splenic CD4 (E) and CD8 (F) T cells from 12-wk-old mice. Error bars represent SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. S6.
cGAS is essential for infiltration of autoreactive immune cells in the hearts of Trex1−/− mice. qRT-PCR analysis of IFN-γ (A and B) or T-bet (C and D) RNA in hearts from 12-wk-old mice of indicated genotypes is shown. Fold changes are relative to Trex1+/− (A and C) or Trex1−/−cGas−/− (B and D). Error bars represent SD. **P < 0.01; ***P < 0.001.
Fig. S7.
Trex1−/− mice have higher percentages of activated and memory T cells. (A and B) Flow-cytometric analysis of surface expression of CD62L and CD44 in splenic CD4+ T cells (A) and CD8+ T cells (B) from 12-wk-old Trex1+/− and Trex1−/− mice. (C–E) Flow-cytometric analysis of CD69 (C) and Ly6c (D) in splenic CD8+ T cells and CD69 (E) in splenic CD4+ T cells from 12-wk-old mice. Error bars represent SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. S8.
cGAS is responsible for higher percentages of activated and memory T cells in Trex1−/− mice. (A and B) Representative flow-cytometric analysis of CD62L and CD44 expression in splenic CD4+ (A) and CD8+ T cells (B) from 12-wk-old mice of indicated genotypes. (C–E) Flow-cytometric analysis of expression of CD69 (C) and Ly6c (D) in splenic CD8+ T cells and CD69 (E) in splenic CD4+ T cells. Error bars represent SD. **P < 0.01; ***P < 0.001.
cGAS Activation Causes Lethal Autoimmunity in DNaseII−/− Mice.
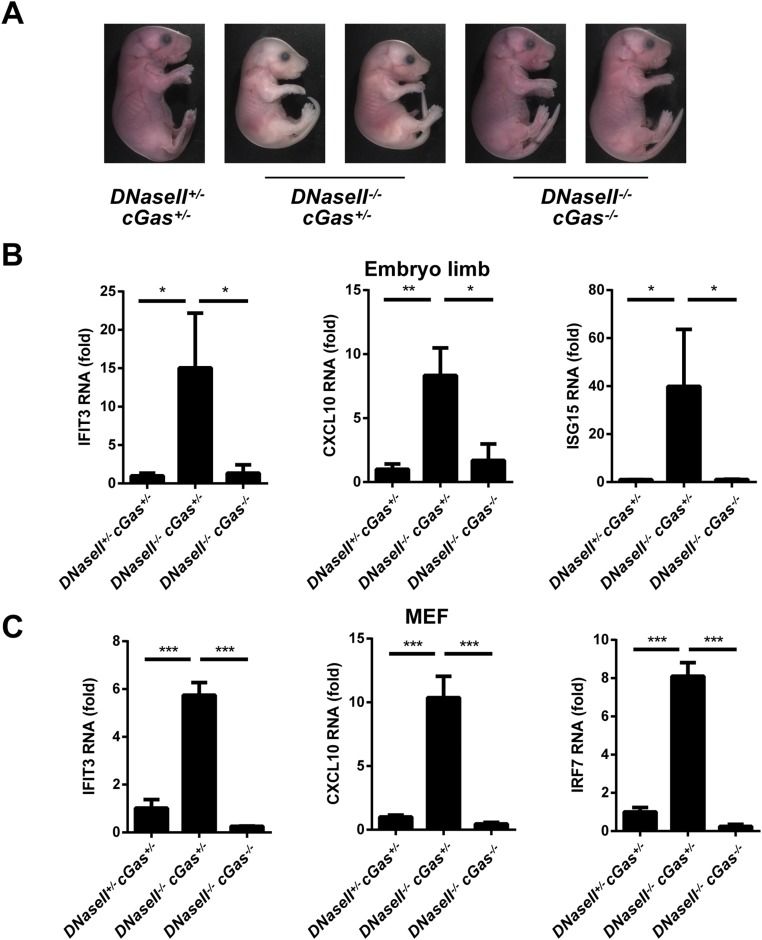
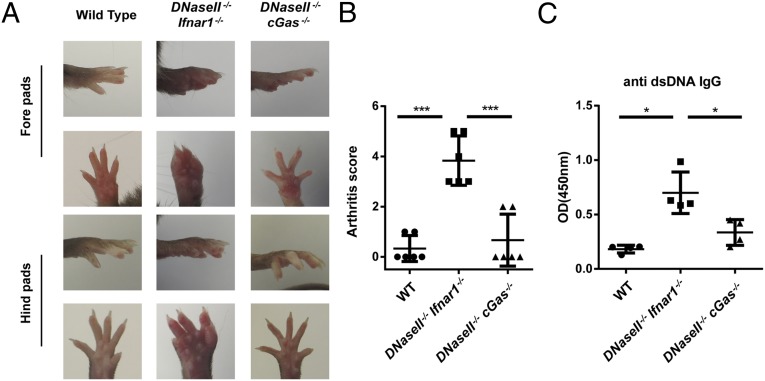
To investigate the role of cGAS in another autoimmune disease model, we deleted cGas in DNaseII−/− mice. DNaseII−/−cGas+/− mice were born dead, similar to DNaseII−/− mice (34). In contrast, DNaseII−/−cGas−/− mice appeared to be developmentally normal, similar to WT and DNaseII+/−cGas+/− mice. DNasII−/−cGas+/− embryos were also smaller and pale compared with DNaseII−/−cGas−/− and DNaseII+/−cGas+/− embryos (Fig. S9A). Thus, deletion of both cGas alleles is necessary to rescue the lethality of DNaseII−/− mice. As shown previously, DNaseII−/−Ifnar1−/− mice developed polyarthritis and produced antibodies against dsDNA (Fig. 5; ref. 36). In contrast, DNaseII−/−cGas−/− mice did not have apparent signs of arthritis, and the anti-DNA antibody was greatly reduced (Fig. 5 A–C). These results, together with the previous data showing that deletion of Sting rescued the phenotypes of DNaseII−/− mice, strongly suggest that cGAS is the dominant DNA sensor that activates STING to cause lethal autoimmunity in DNaseII−/− mice.
Fig. S9.
cGAS is responsible for embryonic lethality and ISG expression in DNaseII−/− mice. (A) Representative E17.5 embryos of indicated genotypes. (B and C) qRT-PCR analysis of indicated ISGs in E15.5 embryo limbs (B) and embryonic fibroblasts (C). Error bars represent SD *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 5.
cGAS activation causes polyarthritis in DNaseII−/−Ifnar1−/− mice. (A and B) Representative foot pad pictures (A) and arthritis scores (B) of 7-mo-old WT, DNaseII−/−Ifnar1−/−, and DNaseII−/−cGAS−/− mice. Arthritis scores were calculated as described in Materials and Methods. (C) Detection of serum autoantibodies against dsDNA. Statistical analysis was performed with a two-tailed, unpaired Student's t test. Error bars represent SD. *P < 0.05; ***P < 0.001.
qRT-PCR analyses of RNA isolated from the fetal liver, embryonic limb, and fibroblasts at E15.5 showed that the expression levels of IFIT3, CXCL10, and ISG15 were much higher in DNaseII−/−cGas+/− mice than in DNaseII+/−cGas+/− or DNaseII−/−cGas−/− mice (Fig. 6A; Fig. S9 B and C). Measurement of cGAMP in fetal livers showed that DNaseII−/−cGas+/− mice produced higher amounts of cGAMP than in DNaseII+/−cGas+/− mice. cGAMP was not detected in DNaseII−/−cGas−/− mice, but was strongly elevated in DNaseII−/−Sting−/− mice (Fig. 6B), consistent with higher levels of cGAMP in Trex1−/−Sting−/− mice (Fig. 3G). These results further suggest that cGAS is indispensable for the production of cGAMP in DNase-deficient cells, whereas STING appears to play a role in facilitating the clearance of cGAMP.
Fig. 6.
cGAS is essential for ISG up-regulation and cGAMP production in fetal livers of DNaseII−/− mice. (A) qRT-PCR analysis of indicated ISGs in fetal liver from E15.5 mouse embryos of indicated genotypes (n = 3). (B) cGAMP levels in mouse fetal livers of indicated genotypes were quantified by LC-MS. Upper shows zoomed chromatograms displaying relative intensities of endogenous cGAMP (red) and internal standard (green). Lower shows the calculated amounts of cGAMP in each sample. Error bars represent SD (A) or SEM (B). *P < 0.05; **P < 0.01.
Discussion
A number of DNA sensors have been proposed to induce type-I IFNs. Besides cGAS, several other proteins, including DAI, IFI16, DDX41, and DNA-PK, were reported to mediate IFN induction through STING (44). However, so far, only cGAS has been demonstrated by genetic experiments to function as a nonredundant and essential cytosolic DNA sensor that activates the type-I IFN pathway in vivo (13, 41). In particular, cGas−/− mice and cells derived from these mice failed to produce IFNs in response to infection by several DNA viruses. TLR9 is a membrane protein localized on the endosomal membrane, with its ligand-binding domain facing the lumen of endosome. TLR9 is activated by certain single-stranded DNA oligos that contain CpG-rich sequences. The binding of CpG DNA to TLR9 in plasmacytoid dendritic cells leads to production of copious amounts of IFN-α. However, induction of IFNs by mammalian DNA in myeloid dendritic cells was shown to be independent of TLR9 (45), and deletion of TLR9 in DNaseII−/− mice failed to rescue the lethal autoimmune diseases of these mice (46). Thus, another DNA sensor is likely responsible for triggering the inflammatory cascades when cellular DNA clearance is impeded.
Initial evidence that cGAS is responsible for activating the type-I IFN pathway in Trex1−/− cells was provided by findings that retroviruses such as HIV-1 robustly induced IFNs and ISGs in Trex1-deficient cells and that such induction was abolished by TALEN-mediated knockout of cGas (39). Subsequent studies further showed that deletion of cGas by the CRISPR/Cas9 technology in cell lines lacking Trex1 or DNaseII abrogated the induction of several ISGs (47, 48). In this study, we demonstrated that Trex1−/−cGas−/− mice were viable and free of any observable signs of diseases that would otherwise cause mortality in Trex1−/− mice. We further showed that cGas deletion rescued the embryonic lethality of DNaseII−/− mice and that the DNaseII−/−cGas−/− mice did not exhibit polyarthritis or other autoimmune phenotypes found in DNaseII−/−Ifnar1−/− mice. Taken together, these results provide the in vivo proof that cGAS activation is responsible for autoimmune diseases caused by defective clearance of self DNA.
Although the rescue of DNaseII−/− mice requires the deletion of both alleles of cGas, deletion of just one allele of cGas is sufficient to largely eliminate the inflammatory disease and rescue the lethality of Trex1−/− mice. It is not clear what causes the difference in these two models, but one possibility is that DNaseII−/− cells may contain more DNA in the cytoplasm than Trex1−/− cells, thereby causing strong inflammatory responses with just one allele of cGas. Alternatively, the cell types responsible for triggering the inflammatory responses may be different between DNaseII−/− and Trex1−/− mice; hence, the threshold of cGAS expression required to cause autoimmune diseases may vary in different cells. Future research should be directed at determining the cell types responsible for causing the autoimmune diseases in DNaseII−/− and Trex1−/− mice. Another important direction is to investigate the origin and nature of self-DNA that accumulates in these DNase-deficient mice.
Through quantitative MS, we have provided direct evidence that tissues from Trex1−/− and DNaseII−/− mice contain elevated levels of cGAMP, which explains why the IFN pathway is activated in these mice. Deletion of cGas ablated cGAMP production in these mice, formally demonstrating that cGAS is solely responsible for synthesizing cGAMP in response to accumulation of self-DNA in the cytosol. Interestingly, deletion of Sting further elevated the cGAMP levels in Trex1−/− and DNaseII−/− mice, suggesting that STING facilitates the clearance of cGAMP. The mechanism by which STING facilitates cGAMP clearance requires further investigation.
The demonstration that cGAS activation is responsible for the severe autoimmune diseases and lethality in Trex1−/− and DNaseII−/− mice strongly suggests that inhibition of cGAS could provide therapeutic benefits to patients with AGS and other autoimmune diseases such as SLE. cGAS is an enzyme that is likely amenable to inhibition by small-molecule drugs, and the recent determination of the high-resolution structures of cGAS in its apo- and DNA-bound forms should facilitate the development of these inhibitors (9–12, 14, 15, 49, 50). The patients who are most likely to benefit from cGAS inhibitors are those with elevated cGAMP production. Our finding that cGAMP levels are elevated in Trex1−/− and DNaseII−/− mice provides a proof of concept for extending such measurements to human patients with a variety of autoimmune diseases, especially those with interferonopathies (exhibiting IFN signatures).
Materials and Methods
Mice.
Trex1+/− mice were from Deborah Barnes (Cancer Research U.K., London) and Nan Yan (University of Texas Southwestern Medical Center), and DNaseII+/− mice were from Shigekazu Nagata (Kyoto University, Kyoto). cGas−/− mice were generated in our laboratory as described (13). All mice used in this study were on C57BL/6 background. The mice were bred and maintained under specific pathogen-free conditions in the animal facility of the University of Texas Southwestern Medical Center at Dallas according to experimental protocols approved by the Institutional Animal Care and Use Committee.
Pathology.
Tissues were fixed in 4% (wt/vol) paraformaldehyde, paraffin-embedded, cut into 5-μm sections, and stained with H&E. Heart tissues were stained with Picro-Sirius red, and kidney samples were stained with periodic acid–Schiff. Inflammation and fibrosis were evaluated based on degree of severity. The sum of individual scores was used to obtain a total tissue histological score. Specific information about the scoring criteria for each tissue was described (30). Clinical assessment of foot pads for arthritis was performed as described (36).
cGAMP Extraction and Quantification.
Fresh organs were snap-frozen in liquid nitrogen, minced immediately with dissecting scissors in cold 80% (vol/vol) methanol with 2% (vol/vol) acetic acid (HAc), and stored at −80 °C before further processing. On the day of analysis, the frozen samples were thawed on ice, and 13C1015N5-labeled cGAMP (+15 atomic mass units) internal standard was supplemented. Samples were then homogenized with a tissue tearor (Biospec Products) for 30 s and cleared by centrifugation (10,000 × g) for 10 min. The pellets were further extracted in 20% (vol/vol) methanol and 2% HAc for two more rounds, and all of the cleared extracts were combined. From these extracts, cGAMP was enriched by solid-phase extraction (SPE) using HyperSep Aminopropyl SPE Columns (Thermo Scientific). Briefly, the columns were first activated by methanol and washed twice with 2% HAc; after drawing through the extracts, columns were washed twice with 2% HAc and once with 80% methanol and, finally, eluted with 4% (vol/vol) ammonium hydroxide in 80% methanol. The eluents were spin-vacuumed to dryness, reconstituted in liquid chromatography (LC)/MS-grade water, cleared by centrifugation, and transferred to autosampler vials for MS analyses.
MS analyses were performed as described (38). Briefly, the SPE eluents were separated on an Xbridge Amide column (3.5 µm, 3.5 mm ID × 100 mm L; Waters) on a Dionex Ultimate 3000 Rapid Separation Liquid Chromatography system (Thermo Scientific). Mobile phase A was 20 mM ammonium bicarbonate with 20 mM ammonium hydroxide in water, and mobile phase B was acetonitrile. The separation ran at a flow rate of 400 µL/min for the first 14.5 min and 800 µL/min for the remaining 8.5 min, through the following gradient: 0 min, 85% B; 3 min, 85% B; 10 min, 2% B; 14 min, 2% B; 14.5 min, 85% B; and 23 min, 85% B.
The LC eluent was ionized by an Ion Max NG heated electrospray source, with a spray voltage of +3,750 V; an ion transfer tube temperature of 342 °C; a vaporizing temperature of 292 °C; and the sheath, auxiliary, and sweep gas at 45, 17, and 1 arbitrary units, respectively. The spray was analyzed online on a TSQ Quantiva triple quadruple mass spectrometer (Thermo Scientific), which performed continuous multiple reaction monitoring scans with a dwell time of 50 ms, Q1 and Q3 resolutions of 0.7 FWHM, and the collision-induced dissociation gas of 1.5 units. cGAMP and the internal standard were monitored in the positive mode with four transitions, respectively (cGAMP: 675–136, 675–152, 675–476, and 675–524; and the internal standard: 691–146, 691–152, 691–491, and 691–539). Raw MS Data were converted to the mzXML format with ReAdW and read into MATLAB for noise reduction and data processing. Absolute quantities of endogenous cGAMP were calculated with the light:heavy ratios and the molar quantity of supplemented internal standard.
Statistics.
Statistical analysis of mouse survival was performed by using the Mantel–Cox test. Other statistical analyses were performed with a two-tailed, unpaired Student's t test.
Note.
While our manuscript was in submission, Gray et al. independently showed that cGas ablation rescued the autoimmune phenotypes of Trex1−/− mice (51).
SI Materials and Methods
Cells.
Embryonic fibroblasts from mice of desired genotypes were prepared from day 15.5 embryos and cultured in DMEM supplemented with 10% (vol/vol) FBS. Bone marrow cells were collected from femurs and tibiae of mice. To obtain bone marrow-derived monocytes (BMDMs), ∼10 million bone marrow cells were cultured in DMEM containing 10% FBS, antibiotics, and conditioned medium from L929 cell culture. After 7 d, mature macrophages were harvested and cultured on 12-well plates for experiments. To obtain dendritic cells, bone marrow cells were cultured in RPMI 1640 containing 10% FBS, 10 mM Hepes (pH 7.4), 50 μM β-mercaptoethanol, and 10 ng/mL murine GM-CSF (peproTech). After 7 d, cells were collected and used as GM-CSF–induced BMDCs.
qRT-PCR.
Reverse transcription and real-time PCR (qPCR) reactions were carried out by using the iScript cDNA synthesis kit and iQ SYBR Green Supermix (Bio-Rad). qPCR was performed on an Applied Biosystems Vii7 using the primers shown in Table S1.
ELISA.
Serum anti-ssDNA IgG levels were quantified by sandwich ELISA. Briefly, 96-well ELISA plates (greiner bio-one) were coated with 1 μg/mL calf thymus ssDNA (Sigma-Aldrich) overnight at 4 °C. After blocking of the plates with 10% FBS, test sera were added at 1:100 dilution. HRP-conjugated goat anti-mouse IgG (Santa Cruz) was added at a dilution of 1:10,000. The reaction was developed with 3,3′,5,5′-tetramethylbenzidine substrate (Thermo Scientific), and the OD at 450 nm was measured. Serum IgG subclasses were quantified as above, except that the samples were incubated with goat Ab specific for IgG2b (Southern Biotech) at 1:10,000 dilution. For antihistone, anti-M2 antigen, anti-dsDNA, and anti–U1-snRNP assays, ELISA plates were coated overnight with 1 μg/mL histones H3 (Sigma), 0.5 μg/mL M2 antigen (Diarect), 1 μg /mL calf thymus DNA (Sigma-Aldrich), and 0.5 μg/mL U1-snRNP (Diarect), respectively. ANA IgG levels were determined by an anti-ANA bioassay ELISA kit (US Biological Life Sciences) according to the manufacturer’s instructions. Plasma IL-12 was measured by using the mouse Th1/Th2 9-Plex Ultra-sensitive Kit (Meso Scale Discovery) according to the manufacturer’s instructions.
Autoantibody Array Analysis.
Autoantibodies in mouse sera were detected by using autoantigen arrays as described (52). Selected autoantibodies were further validated by ELISA.
Flow Cytometry.
For surface staining, cells were washed in ice-cold FACS buffer [2% (wt/vol) BSA in PBS], then incubated with indicated antibody for 15 min and washed with FACS buffer. For intracellular IFN-γ staining, splenocytes or PBMCs were stimulated with 50 ng/mL PMA and 1 μM ionomycin in the presence of 1 μg/mL brefeldin A for 4.5 h, followed by surface staining with antibodies against CD3, CD4, and/or CD8. The cells were fixed with 2% (wt/vol) paraformaldehyde, permeabilized with 0.1% saponin, and stained for IFN-γ. For transcription factor staining, cells were stained by surface antibody, followed by intracellular staining for anti–T-bet (BioLegend). The stained cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed with FlowJo software. CD4-APC, CD4-FITC, CD3-Percp, and IFN-γ–PE antibodies were purchased from Biolegend; CD62L-PE, Ly6c-APC, CD8-FITC, and CD69-PE antibodies were purchased from eBioscience; and CD44-APC and CD8-APC antibodies were purchased from BD Bioscience.
Acknowledgments
We thank Drs. Deborah Barnes and Nan Yan for providing the Trex1+/− mice; Dr. Shigekazu Nagata for the DNaseII+/− mice; and Mr. John Shelton (University of Texas Southwestern) for performing the histology analyses of mouse tissues. This work was supported by National Institutes of Health Grant AI-93967, the Lupus Research Institute Distinguished Innovator Award, Welch Foundation Grant I-1389, and Cancer Research Prevention Institute of Texas Grant RP120718. T.L. was supported by a Cancer Research Institute Postdoctoral Fellowship. Z.J.C is a Howard Hughes Medical Institute Investigator.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 12903.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516465112/-/DCSupplemental.
References
- 1.Pandey S, Kawai T, Akira S. Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb Perspect Biol. 2015;7(1):a016246. doi: 10.1101/cshperspect.a016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 3.Goubau D, Deddouche S, Reis e Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38(5):855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014;54(2):289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Xiao TS, Fitzgerald KA. The cGAS-STING pathway for DNA sensing. Mol Cell. 2013;51(2):135–139. doi: 10.1016/j.molcel.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ablasser A, et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498(7454):380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Civril F, et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498(7454):332–337. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diner EJ, et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Reports. 2013;3(5):1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao P, et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153(5):1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XD, et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341(6152):1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51(2):226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, et al. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Reports. 2014;6(3):421–430. doi: 10.1016/j.celrep.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29(4):538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Jin L, et al. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28(16):5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoneyama M, Onomoto K, Jogi M, Akaboshi T, Fujita T. Viral RNA detection by RIG-I-like receptors. Curr Opin Immunol. 2015;32:48–53. doi: 10.1016/j.coi.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Crow YJ. Type I interferonopathies: Mendelian type I interferon up-regulation. Curr Opin Immunol. 2015;32:7–12. doi: 10.1016/j.coi.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Goubau D, et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature. 2014;514(7522):372–375. doi: 10.1038/nature13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornung V, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314(5801):994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 23.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314(5801):997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 24.Schuberth-Wagner C, et al. A conserved histidine in the RNA sensor RIG-I controls immune tolerance to N1-2'O-methylated self RNA. Immunity. 2015;43(1):41–51. doi: 10.1016/j.immuni.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazur DJ, Perrino FW. Identification and expression of the TREX1 and TREX2 cDNA sequences encoding mammalian 3′-->5′ exonucleases. J Biol Chem. 1999;274(28):19655–19660. doi: 10.1074/jbc.274.28.19655. [DOI] [PubMed] [Google Scholar]
- 26.Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131(5):873–886. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Morita M, et al. Gene-targeted mice lacking the Trex1 (DNase III) 3′-->5′ DNA exonuclease develop inflammatory myocarditis. Mol Cell Biol. 2004;24(15):6719–6727. doi: 10.1128/MCB.24.15.6719-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crow YJ, et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat Genet. 2006;38(8):917–920. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- 29.Rice GI, Rodero MP, Crow YJ. Human disease phenotypes associated with mutations in TREX1. J Clin Immunol. 2015;35(3):235–243. doi: 10.1007/s10875-015-0147-3. [DOI] [PubMed] [Google Scholar]
- 30.Gall A, et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36(1):120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeremiah N, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124(12):5516–5520. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371(6):507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang HC, Liao TH. Reassociation of deoxyribonuclease II with the lysosomal membrane isolated from porcine spleen. Arch Biochem Biophys. 1990;280(2):320–324. doi: 10.1016/0003-9861(90)90336-w. [DOI] [PubMed] [Google Scholar]
- 34.Kawane K, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292(5521):1546–1549. doi: 10.1126/science.292.5521.1546. [DOI] [PubMed] [Google Scholar]
- 35.Lan YY, Londono D, Hacohen N. Lysosomal DNaseII degrades nuclear DNA and prevents self DNA recognition. J Immunol. 2012;188(Meeting Abstract Suppl):159.17. [Google Scholar]
- 36.Kawane K, et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443(7114):998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 37.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci USA. 2012;109(47):19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins AC, et al. Cyclic GMP-AMP synthase is an innate immune DNA sensor for Mycobacterium tuberculosis. Cell Host Microbe. 2015;17(6):820–828. doi: 10.1016/j.chom.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao D, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341(6148):903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam E, Stein S, Falck-Pedersen E. Adenovirus detection by the cGAS/STING/TBK1 DNA sensing cascade. J Virol. 2014;88(2):974–981. doi: 10.1128/JVI.02702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoggins JW, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505(7485):691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wassermann R, et al. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 2015;17(6):799–810. doi: 10.1016/j.chom.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Watson RO, et al. The cytosolic sensor cGAS detects mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe. 2015;17(6):811–819. doi: 10.1016/j.chom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38(5):870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin DA, Elkon KB. Intracellular mammalian DNA stimulates myeloid dendritic cells to produce type I interferons predominantly through a toll-like receptor 9-independent pathway. Arthritis Rheum. 2006;54(3):951–962. doi: 10.1002/art.21677. [DOI] [PubMed] [Google Scholar]
- 46.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202(10):1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ablasser A, et al. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J Immunol. 2014;192(12):5993–5997. doi: 10.4049/jimmunol.1400737. [DOI] [PubMed] [Google Scholar]
- 48.Motani K, Ito S, Nagata S. DNA-mediated cyclic GMP-AMP synthase-dependent and -independent regulation of innate immune responses. J Immunol. 2015;194(10):4914–4923. doi: 10.4049/jimmunol.1402705. [DOI] [PubMed] [Google Scholar]
- 49.Li X, et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. 2013;39(6):1019–1031. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kranzusch PJ, Lee AS, Berger JM, Doudna JA. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Reports. 2013;3(5):1362–1368. doi: 10.1016/j.celrep.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray EE, Treuting PM, Woodward JJ, Stetson DB. Cutting edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi-Goutieres syndrome. J Immunol. 2015;195(5):1939–1943. doi: 10.4049/jimmunol.1500969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li QZ, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115(12):3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]