Significance
Studies of antibiotic resistance are usually initiated in earnest only after resistance has become established in clinical pathogens. Here, we forewarn of a resistance mechanism to the novel antibiotics ketolides, which are only coming into broad medical practice. We show that the balanced activities and coordinated expression of two genes, pikR1 and pikR2, provide efficient protection to Streptomyces venezuelae, a bacterial producer of natural ketolides. Expression of the more potent gene, pikR2, is supported by pikR1 and specifically induced by ketolides. The resistance mechanism remains fully functional when pikR1 and pikR2 are transferred to other bacterial species and affords protection against clinical ketolides. These findings emphasize the need for the preemptive development of antibiotics that can overcome this resistance mechanism.
Keywords: ribosome, antibiotics, ketolides, resistance, macrolides
Abstract
Ketolides are promising new antimicrobials effective against a broad range of Gram-positive pathogens, in part because of the low propensity of these drugs to trigger the expression of resistance genes. A natural ketolide pikromycin and a related compound methymycin are produced by Streptomyces venezuelae strain ATCC 15439. The producer avoids the inhibitory effects of its own antibiotics by expressing two paralogous rRNA methylase genes pikR1 and pikR2 with seemingly redundant functions. We show here that the PikR1 and PikR2 enzymes mono- and dimethylate, respectively, the N6 amino group in 23S rRNA nucleotide A2058. PikR1 monomethylase is constitutively expressed; it confers low resistance at low fitness cost and is required for ketolide-induced activation of pikR2 to attain high-level resistance. The regulatory mechanism controlling pikR2 expression has been evolutionary optimized for preferential activation by ketolide antibiotics. The resistance genes and the induction mechanism remain fully functional when transferred to heterologous bacterial hosts. The anticipated wide use of ketolide antibiotics could promote horizontal transfer of these highly efficient resistance genes to pathogens. Taken together, these findings emphasized the need for surveillance of pikR1/pikR2-based bacterial resistance and the preemptive development of drugs that can remain effective against the ketolide-specific resistance mechanism.
The prototypes of most of the clinically useful antibiotics, including the diverse group of protein synthesis inhibitors, have been discovered among the secondary metabolites of bacterial species. Antibiotic-producing bacteria have developed an array of resistance genes to avoid committing suicide (1). The wide medical use of antibiotics has created a strong selective pressure for such resistance genes to transfer and integrate into the genomes of bacterial pathogens, curbing the beneficial effects of the drugs and shortening their clinical lifespan. Consequently, antibiotic producers are not only our allies in providing useful drugs but also, play an adversary role by facilitating the spread of resistance.
Macrolides are among the most medically successful antibiotics originating from the secondary metabolites of actinomycetes. They inhibit translation by binding in the nascent peptide exit tunnel (NPET) close to the peptidyl transferase center (PTC) of the large ribosomal subunit (2–4). The most common mechanism of macrolide resistance involves dimethylation of an rRNA nucleotide in the drug binding site (A2058 in the Escherichia coli 23S rRNA) by Erm methyltransferases (5). In the absence of antibiotic, A2058 dimethylation is deleterious for the cell, because modification of a residue in a functional site of the NPET distorts production of a subset of proteins (6). Therefore, to reduce the fitness cost associated with resistance, expression of the erm genes is often inducible and activated only when antibiotic is present. Such induction operates through antibiotic-controlled ribosome stalling at an upstream leader ORF (5, 7).
Macrolide antibiotics are built on a 14- to 16-atom macrolactone ring decorated with various side chains. The prototype 14-atom ring macrolide erythromycin (ERY) and its second generation derivatives carry cladinose at the C3-hydroxyl position of the ring and a desosamine sugar linked to the C5-hydroxyl group (Fig. 1B). In the newest generation of drugs, the ketolides, the C3-cladinose is replaced with a keto function. Ketolides are viewed as one of the most promising classes of antibiotics presently under development and offer broad medical application (8). The first medically useful ketolide telithromycin (TEL) and newer ketolides currently in clinical trials show dramatically improved antibacterial activity compared with earlier generations of macrolides, and to a large extent, because of their reduced propensity to activate inducible resistance genes (9, 10).
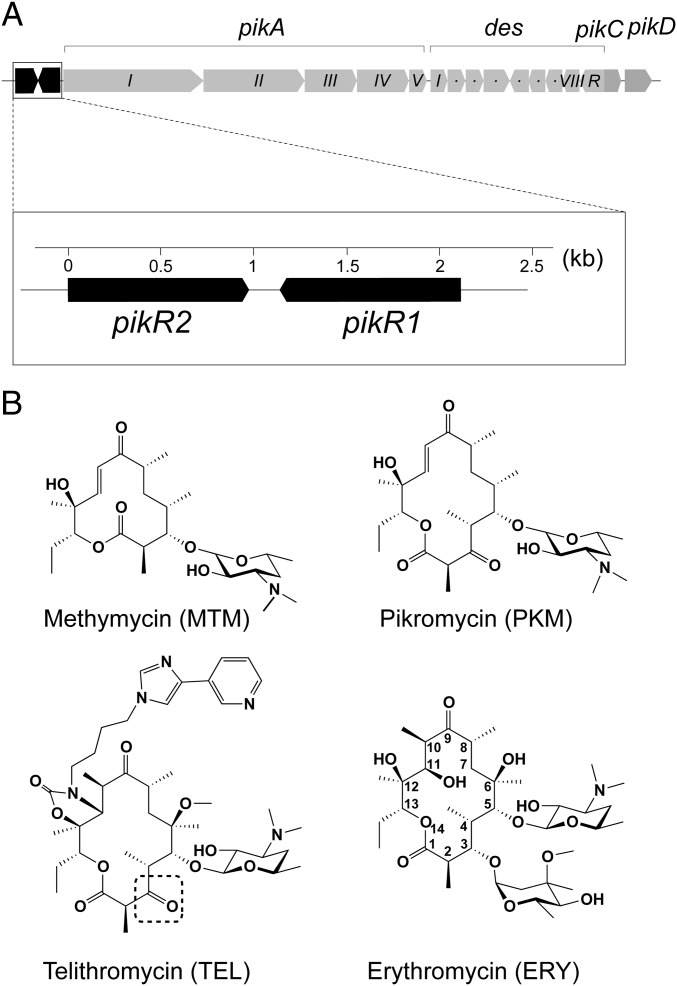
Fig. 1.
S. venezuelae pikR resistance genes and ketolide antibiotics. (A) The structure of the MTM/PKM biosynthetic gene cluster in S. venezuelae ATCC 15439. The polyketide synthase (pikA) and desosamine biosynthesis (des) gene operons along with the pikC and pikD genes are required for production of the active MTM and PKM antibiotics. The putative resistance genes pikR1 and pikR2 precede the MTM/PKM biosynthesis operon. (B) Structures of the natural antibiotics MTM and PKM produced by S. venezuelae ATCC 15439, the semisynthetic clinical ketolide TEL, and the C3-cladinose containing macrolide ERY. The dotted box in the TEL structure highlights the keto group, which replaces the cladinose sugar.
All of the clinically relevant ketolides are synthetic or semisynthetic derivatives of natural macrolides. The only naturally occurring 14-atom macrolactone ring ketolide that is presently known is pikromycin (PKM) (11) produced by Streptomyces venezuelae (strain ATCC 15439) (Fig. 1). The biosynthesis pathway of PKM is unique because of a modular polyketide synthesis skipping mechanism that can divert the pathway toward production of a second macrolide molecule, methymycin (MTM), which has a smaller macrolactone ring of 12 atoms (Fig. 1B) (12, 13). Although both PKM and MTM possess antibacterial activity, their modes of binding to the ribosome and their mechanisms of action remain unclear.
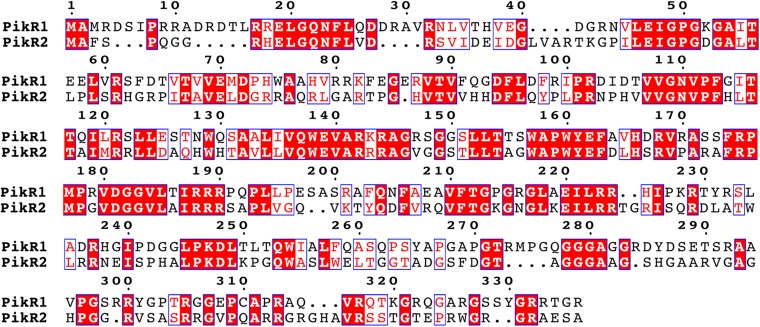
The modular polyketide synthesis genes pikAI-pikAV in the biosynthetic cluster of S. venezuelae ATCC 15439 are preceded by two putative resistance genes, pikR1 and pikR2, arranged tail to tail (Fig. 1A). The protein products PikR1 and PikR2 are 42% identical (Fig. S1) and show similarity to Erm-type rRNA methyltransferases (e.g., 30% and 28% identities with ErmE for PikR1 and PikR2, respectively) (14, 15). Given the significant fitness cost associated with erm-based resistance (6), the duplication of PikR enzymatic function in S. venezuelae is puzzling. Other than being of general biological interest, the mechanism by which a natural producer of ketolides attains resistance is of significant medical importance. Not in the least, it can be envisioned that selective pressure imposed by broad medical use of antibiotics could promote the transfer of natural resistance genes to pathogens. Understanding the function and regulation of pikR1/pikR2 and similar genes would facilitate their surveillance and possibly, help curb their dissemination. Study of pikR1/pikR2-like genes might, in turn, stimulate preemptive development of drugs that retain antimicrobial activity against strains harboring these resistance mechanisms.
Fig. S1.
Similarity of proteins encoded in the pikR1 and pikR2 genes in S. venezuelae ATCC 15439.
In this paper, we show that the pikR1 and pikR2 genes render the ketolide-producing S. venezuelae cells resistant to PKM and MTM as well as clinical ketolide antibiotics. Having determined the target, the mode of action, and the regulation of these genes, we reveal how S. venezuelae achieves ketolide resistance at a low fitness cost by balancing the activities of PikR1 and PikR2 and timing their expression. We present evidence that the expression of pikR2 gene has been optimized through evolution to respond to ketolide antibiotics. Finally, we show that transfer of pikR2 to other bacteria renders them resistant to ketolides, and this effect is accentuated in combination with pikR1. These findings illuminate resistance mechanisms that could potentially be acquired by clinical pathogens on launching new ketolide antibiotics.
Results
pikR1 and pikR2 Confer Resistance to MTM and PKM.
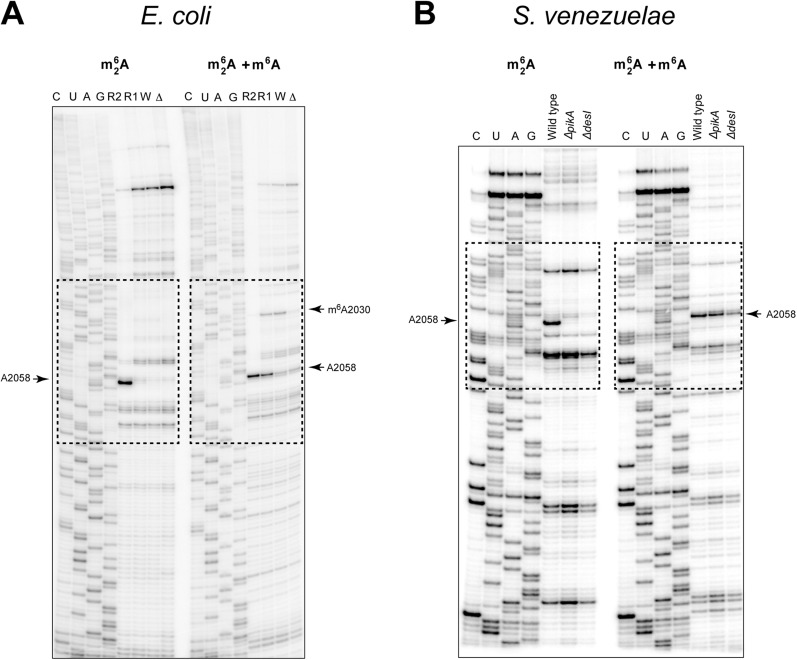
We first assessed whether pikR1 and pikR2, which show similarity to macrolide resistance erm genes (16), confer resistance to the natural ketolides MTM and PKM produced by S. venezuelae. The pikR ORFs were individually expressed from plasmids pPikR1 and pPikR2 (Table S1) in an E. coli strain hypersusceptible to macrolides. Although cells lacking these genes were completely inhibited by 4 µg/mL MTM or 8 µg/mL PKM, expression of pikR1 or pikR2 rendered E. coli resistant to >512 µg/mL either of the compounds. These data indicate that pikR1 and pikR2 confer resistance to natural ketolides and thus, have evolved or were acquired to protect S. venezuelae from its endogenous antibiotics.
Table S1.
Plasmids used in the study
| Plasmid name | Notes | Source |
| pERMZα | The reporter plasmid containing the regulatory region of the macrolide-inducible ermC gene, in which codons 3–244 of the ermC gene are replaced with 57 codons of lacZ γενε | 21 |
| pPikR1 | pERMZα derivative constitutively expressing the pikR1 gene under the control of the Ptac promoter and optimized Shine–Dalgarno sequence | This study |
| pPikR2 | pERMZα derivative constitutively expressing the pikR1 gene under the control of the Ptac promoter and optimized Shine–Dalgarno sequence | This study |
| pRL1 | pERMZα-derived reporter plasmid containing the regulatory region of the pikR1 gene, in which codons 6–331 of the pikR1 gene are replaced with 57 codons of lacZα; the transcription is driven by the Ptac promoter | This study |
| pRL2 | The reporter plasmid containing the regulatory region of the pikR1 gene, in which codons 6–317 of the pikR2 gene are replaced with 57 codons of lacZα; the transcription is driven by the Ptac promoter | This study |
| pRL2R2 | pERMZα-derived plasmid containing the pikR2 gene and 208 bp of the upstream regulatory region including the pikR2L regulatory ORF; the transcription is driven by the Ptac promoter | This study |
| pKC1139 | E. coli–Streptomyces shuttle vector pKC1139 containing a temperature-sensitive replicon | 36 |
| pSRP112 | pKC1139-derived plasmid containing flanking regions of the pikR2 gene; used for inactivation of pikR2 in the genome of S. venezuelae DHS2001 | This study |
| pSRP113 | pKC1139-derived plasmid containing flanking regions of the pikR1 gene; used for deletion of pikR1 from the genome of S. venezuelae DHS2001 | This study |
| pSRP114 | pKC1139 containing flanking regions of the pikR1-pikR2 gene cluster; used for deletion of the pikR1-pikR2 cluster from the genome of S. venezuelae DHS2001 | This study |
| pMIP12 | E. coli–M. smegmatis shuttle vector containing kanamycin resistance gene | 37 |
| pMR2 | pMIP12-derivative containing pikR2 with its regulatory region under the control of the PBlaF* promoter | This study |
| pMR1R2 | pMIP12-derivative containing pikR2 with its regulatory region under the control of the PBlaF* promoter | This study |
PikR1 and PikR2 Modify the Same 23S rRNA Nucleotide.
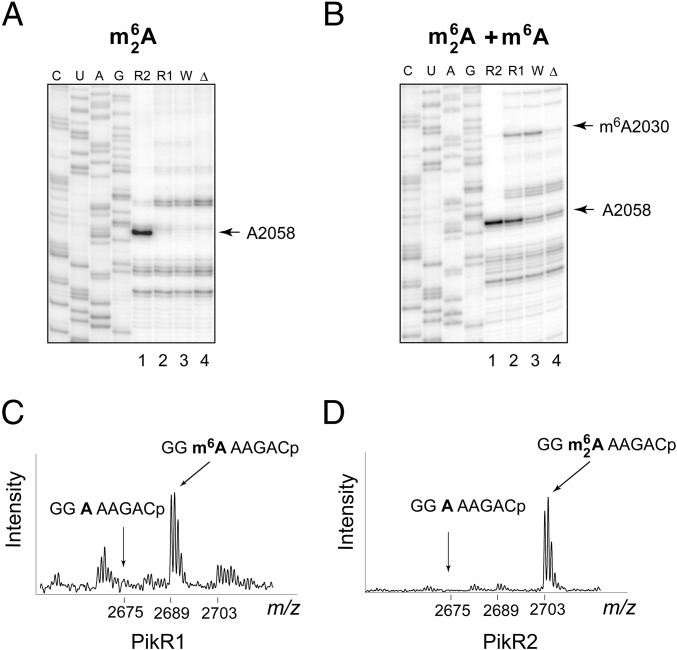
The presence of two resistance genes of potentially similar function may suggest that one of them is redundant, prompting us to dissect their individual functions. The binding sites of MTM and PKM in the bacterial ribosome have not been biochemically defined previously. However, structural evidence from the Deinococcus radiodurans large ribosomal subunit suggests that MTM binds in the PTC (17) rather than in the NPET, where all conventional macrolides and ketolides bind (2–4). Therefore, the site of action and the nature of the modifications introduced by PikR1 and PikR2 could not be predicted with any certainty. The majority of the investigated Erm-type enzymes confer resistance to macrolides by dimethylating the exocyclic amine of A2058 in the 23S rRNA (E. coli numbering throughout) (5). A2058 dimethylation (but not monomethylation) stalls the progress of reverse transcriptase (RT) on the RNA template and is readily detected by primer extension. A strong band corresponding to RT pausing at A2058 on rRNA extracted from E. coli cells expressing PikR2 indicated that it probably dimethylates this nucleotide (Fig. 2A and Fig. S2A). In contrast, under the standard primer extension conditions, no RT stop was detected at either A2058 or any other site within the PTC and the NPET on rRNA from cells with pPikR1 (Fig. 2A). However, when we optimized primer extension conditions for detecting N6 monomethylation of adenosine (Materials and Methods and Fig. 2B), RT pausing at A2058 was observed on rRNA from cells expressing PikR1 (Fig. 2B, lane 2), supporting the hypothesis that PikR1 monomethylates A2058.
Fig. 2.
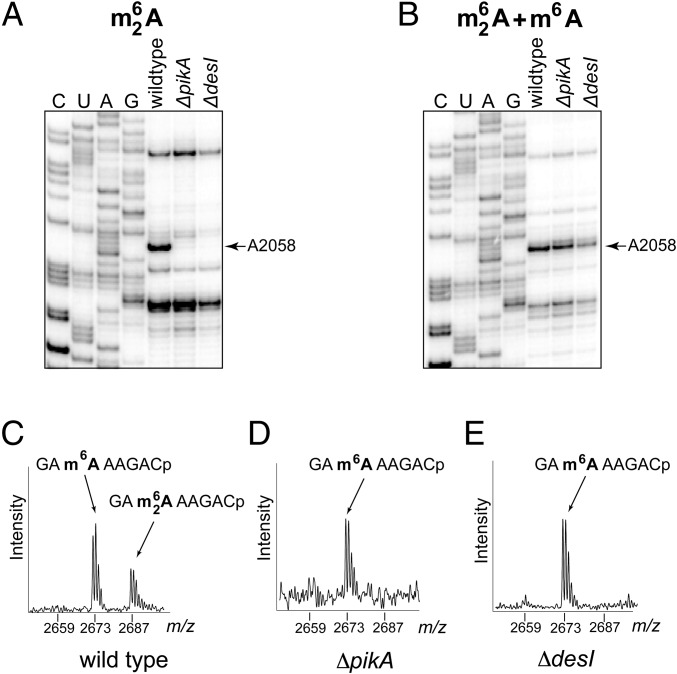
PikR1 and PikR2 RNA methyltransferases target A2058 in the 23S rRNA. (A) Primer extension analysis of m26A modification of rRNA extracted from WT E. coli cells (W; lane 3) or those constitutively expressing pikR1 (R1; lane 2) and pikR2 (R2; lane 1) genes. Sequencing lanes are marked C, U, A, G. Full gels are shown in Fig. S2A. (B) Primer extension analysis of the same samples as in A but carried out under conditions optimized for detection of m6A modification (Materials and Methods). The E. coli ΔrlmJ mutant, which lacks the native m6 modification of A2030 (33), was used as a control (Δ; lane 4). (C and D) MALDI-TOF analysis of the RNaseA-generated 23S rRNA fragment encompassing nucleotide A2058. rRNA samples were prepared from cells expressing (C) PikR1 or (D) PikR2.
Fig. S2.
Primer extension analysis of modification of rRNA extracted from (A) E. coli or (B) S. venezuelae. The segments of the full gels represented in Figs. 2 and 3 are boxed. Sequencing lanes are marked with the letters C, U, A, G according to the rRNA nucleotides. W, WT.
The nature of the reactions catalyzed by PikR1 and PikR2 was corroborated by MS. A 50-nt-long 23S rRNA fragment encompassing A2058 was isolated from rRNA extracted from E. coli cells expressing PikR1 or PikR2 and subjected to RNaseA digestion and MALDI-TOF analysis. In the PikR1 sample, the peak corresponding to the unmodified RNA fragment GGA2058AAGAC (m/z 2,675) was almost absent, and a new peak at m/z 2,689 appeared (Fig. 2C), indicating that a methyl group had been added. In the corresponding 23S rRNA fragment from the PikR2 sample, the unmethylated RNA peak was also absent and replaced by a peak at m/z 2,703 showing the addition of two methyl groups (Fig. 2D). Combined with the results of primer extension analysis, the MS data showed that PikR1 and PikR2 target the same rRNA nucleotide but generate two different products: PikR1 monomethylates A2058, whereas PikR2 dimethylates this nucleotide.
pikR1 Is Constitutively Expressed in the Native Host, Whereas pikR2 Is Activated only When Antibiotics Are Produced.
Why does a ketolide producer that is equipped with the A2058 dimethyltransferase gene pikR2 also need the pikR1 gene, which has a product that merely monomethylates the same nucleotide? To address this question, we examined the regulation of expression of pikR1 and pikR2 in S. venezuelae. When production of antibiotics was inactivated by deletion of either the pikAI-pikAIV genes (ΔpikA) or the desI gene (ΔdesI) in the biosynthetic operon (leaving the pikR1 and pikR2 resistance genes intact) (Table S2), A2058 was found to be fully monomethylated (Fig. 3 and Fig. S2B), but there was essentially no dimethylation of this residue (Fig. 3 A, D, and E). This result indicated that pikR1 is constitutively expressed, whereas pikR2 remains inactive when no antibiotic is produced. In contrast, in the antibiotic-producing WT S. venezuelae ATCC 15439, a significant fraction (∼30%) of A2058 was shown by primer extension to be converted to m26A (Fig. 3A and Fig. S3), an observation confirmed by MS (Fig. 3C). Thus, pikR2 is activated only when MTM and/or PKM are present in the cell. Because dimethylation of A2058 is known to reduce cell fitness (6), the inducible nature of pikR2 is consistent with an evolutionary adaptation by the antibiotic-producing host to achieve secure protection against endogenously produced ketolides while maintaining a low fitness cost for this service.
Table S2.
Strains used in the study
| Strain | Genotype | Source |
| E. coli JM109 | F′[traD36, proAB+ lacIq, ∆(lacZ)M15] endA1 recA1 hsdR17(rk−, mk+) mcrA supE44 λ-gyrA96 relA1 ∆(lac-proAB) thi-1 | 41 |
| E. coli JM109 ΔacrA | JM109, ΔacrA | This study |
| E. coli JM109 ΔacrB | JM109, ΔacrB | 42 |
| E. coli BW25113 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514 | 43 |
| E. coli JW3466 | BW25113, ΔrlmJ::kan | 44 |
| S. venezuelae ATCC15439 | WT | 12 |
| WT* (DHS2001) | S. venezuelae ATCC15439, ΔpikAI-pikAIV | 18 |
| DHS8708 | S. venezuelae ATCC15439, ΔdesI | 34 |
| R1 (DHS328) | DHS2001, ΔpikR2 | This study |
| R2 (DHS330) | DHS2001, ΔpikR1 | This study |
| Δ (DHS332) | DHS2001, ΔpikR1-ΔpikR2 | This study |
| ermKO-4 | M. smegmatis mc2155 Δerm38 | 38 |
Fig. 3.
Expression of pikR2 in S. venezuelae is activated during antibiotic production. (A and B) Primer extension analysis of rRNA extracted from WT S. venezuelae ATCC 15439 carried under conditions specific for detection of (A) m26A or (B) m26A and m6A modification. Full gels are shown in Fig. S2B. RNA was extracted from WT cells or mutants unable to produce active antibiotics because of deletion of the pikAI-pikAIV (ΔpikA) or desI (ΔdesI) genes. Sequencing lanes in A and B are marked C, U, A, G. (C–E) MALDI-TOF analysis of 23S rRNA fragments from the WT or the ΔpikA and ΔδεσI KO mutants of S. venezuelae; the fragments were generated with RNaseA and encompass nucleotide A2058.
Fig. S3.
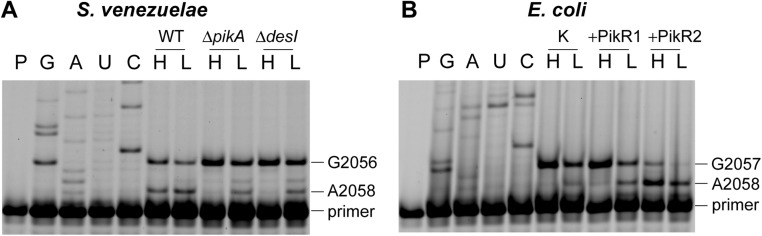
Primer extension on 23S rRNAs from (A) S. venezuelae and (B) E. coli. The extension reactions were performed with 1 mM dTTP and 1 mM ddCTP (H) or 0.01 mM dTTP and 1 mM ddCTP (L). The dideoxynucleotide completely stops reverse transcription at G2056 in S. venezuelae rRNA and at G2057 in E. coli rRNA. Gel band intensities were measured by scanning on a Typhoon FLA 9500 (GE Healthcare) and used to quantify the stops at A2058. The band at A2058 in extensions with 1 mM dTTP is caused by m62 dimethylation, whereas extensions with the lower dTTP concentration detect both monomethylated and dimethylated A2058. The proportion of A2058 nucleotides that were m62-dimethylated after ketolide induction was slightly above 30% (WT H lane). Note that, in contrast to adenine m62 dimethylation, m6A monomethylation does not completely arrest primer extension, even under low dTTP concentration conditions; therefore, the primer extension technique can be used to reliably quantify the extent of A2058 dimethylation but not monomethylation. G, A, U, and C are dideoxy sequencing lanes. K, E. coli cells with the empty vector; P, primer band. Sequencing lanes are marked G, A, U, C.
Expression of pikR2 Is Activated in the Natural Host by Clinically Relevant Ketolides.
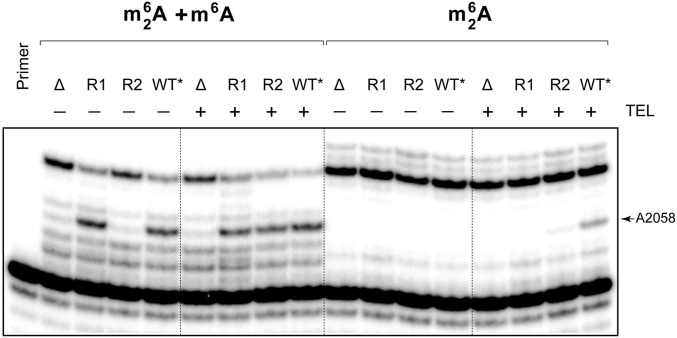
Induction of pikR2 by ketolides of its native host suggested that the gene could also be activated by medically relevant ketolides. We tested whether preincubation with the clinical ketolide TEL could induce pikR2 expression in S. venezuelae. Derivative strains containing only pikR1 (DHS328) or only pikR2 (DHS330) or lacking both genes (DHS332; designated here as R1, R2, or Δ, respectively) were prepared from the ΔpikA strain (DHS2001; designated here as WT*) (18). Without preincubation, the WT* and R1 cells showed an intermediate level of resistance to TEL [minimal inhibitory concentration (MIC) = 8 µg/mL], whereas R2 and Δ were highly sensitive (MIC = 0.25 µg/mL). Preincubation of the WT* and R2 strains with TEL concentrations of one-fourth MICs raised their respective resistance levels 8- and 64-fold, respectively (Table 1). The higher resistance of the WT* and R2 cells on exposure to TEL correlated with increased modification of nucleotide A2058 (Fig. S4). The microbiological and biochemical data thus show that, although constitutive expression of pikR1 provides some protection from the clinical ketolide TEL, resistance is dramatically increased by drug-mediated activation of pikR2.
Table 1.
MIC (micrograms per milliliter) of TEL or chloramphenicol for S. venezuelae ΔpikA strains containing different pikR resistance genes
| Strain† | Antibiotics | |||||
| TEL | Chloramphenicol | |||||
| Preincubation | Fold change | Preincubation | Fold change | |||
| No | Yes‡ | No | Yes‡ | |||
| WT* | 8 | 64 | 8 | 8 | 8 | 1 |
| Δ | 0.25 | 0.25 | 1 | 8 | 8 | 1 |
| R1 | 8 | 16 | 2 | 8 | 8 | 1 |
| R2 | 0.25 | 16 | 64 | 8 | 8 | 1 |
The ΔpikA strain, which contained both pikR1 and pikR2 resistance genes but was unable to produce antibiotics (DHS2001), was designated WT* control. Formal names of the other strains are DHS332 (Δ), DHGS328 (R1), and DHS330 (R2).
Cells were grown for 8 h in LB supplemented with one-fourth MIC of TEL (0.0625 µg/mL for Δ and R2 strains or 2 µg/mL for WT* and R1 strains) before MIC testing.
Fig. S4.
Primer extension analysis of mono- and dimethylation of A2058 in 23S rRNA extracted from different S. venezuelae mutants without (−) and with (+) preincubation for 10 h with one-fourth MIC of TEL. All of the strains used in the experiment were derivatives of the ΔpikA strain (WT*) containing only pikR1 (R1), only pikR2 (R2), or lacking both of the pikR genes (Δ).
Molecular Mechanism of pikR2 Induction.
Macrolide resistance genes are activated by programmed ribosome stalling at the upstream leader ORFs (5, 19). Many of the resistance genes are induced exclusively or preferentially by cladinose-containing macrolides, whereas ketolides are poor inducers (10, 20). Indeed, ketolides owe their high activity against many clinical isolates to their low propensity for activation of inducible resistance genes.
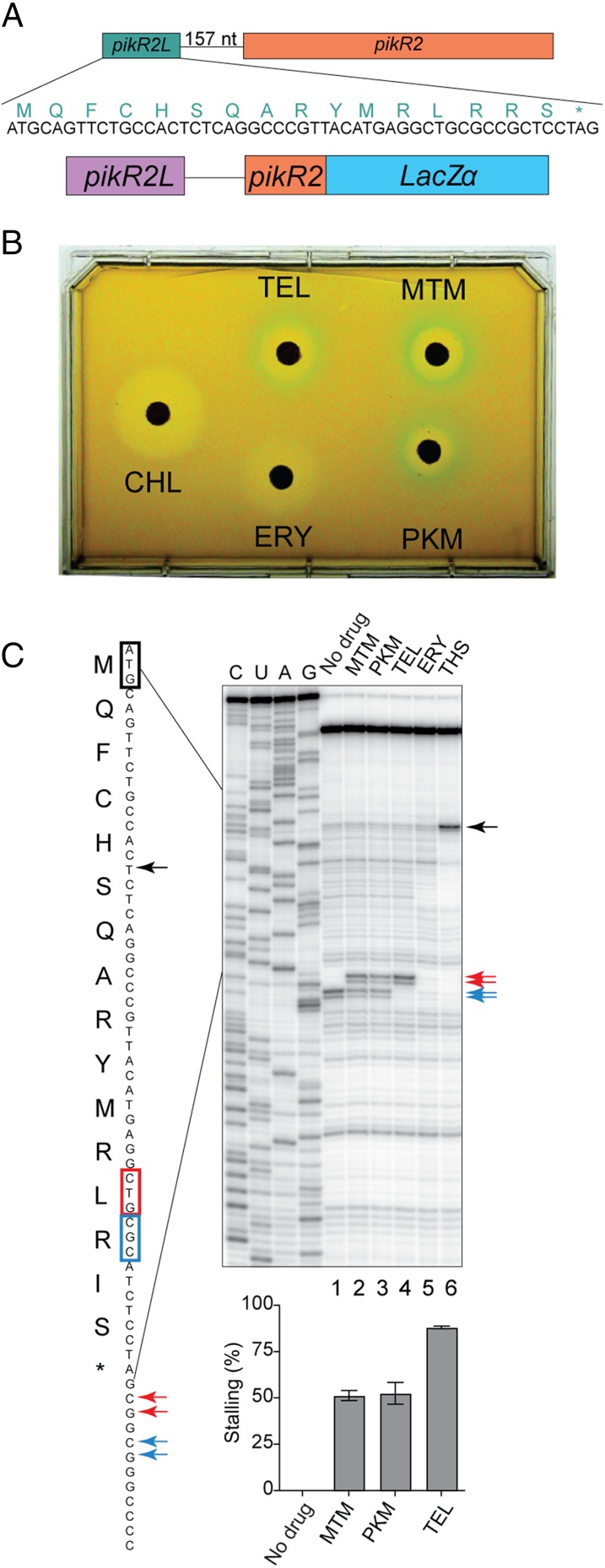
We were interested in elucidating the molecular mechanism of ketolide-dependent induction of the pikR2 gene. Examination of the pikR2 upstream regions revealed the presence of a 16-codon ORF (pikR2L) 157 bp upstream of pikR2 (Fig. 4A). The role of pikR2L in ketolide-mediated inducibility was investigated using a plasmid construct containing the pikR2L ORF, the intergenic region, and the first five codons of pikR2 fused to the lacZα reporter gene (Fig. 4B). An E. coli Ptac promoter drives transcription of the reporter system. Induction of the pikR2-lacZα chimera was tested in E. coli by a disk diffusion assay (21). Natural and semisynthetic clinical ketolide antibiotics but notably, not the cladinose-containing macrolide ERY activated expression of the pikR2L-based reporter system (Fig. 4B). These data showed that ketolide-specific induction of pikR2 occurs at the level of translation and is likely controlled by the upstream leader ORF.
Fig. 4.
The pikR2 regulatory region controls the inducible expression of the pikR2 resistance gene. (A) The putative leader ORF pikR2L precedes the pikR2 resistance gene. The nucleotide sequence of the ORF and the amino acid sequence of the encoded leader peptide are shown. (B) Antibiotic disk diffusion assay reveals inducibility of pikR2 in E. coli. In the reporter construct in E. coli cells, the pikR2 regulatory region controls expression of the lacZα reporter. The antibiotic disks contained TEL, MTM, PKM, ERY, or chloramphenicol (CHL). The clear areas around the disks contain antibiotic concentrations that inhibited cell growth. Blue halos around the ketolide-containing disks indicate drug-dependent induction of the reporter. (C, Upper) Toe-printing analysis shows ketolide-induced ribosome stalling at the Leu13 codon of the pikR2L ORF. The band of the ribosomes stalled by the control antibiotic thiostrepton (THS) at the initiation codon is indicated by black arrows. The ribosomes arrested with the Leu13 codon in their P site are shown by the red arrows. The ribosomes that reached the pikR2L 13th codon but failed to arrest translation were captured at the next Arg14 codon (blue arrows) because of the depletion of Ile-tRNA from the translation reaction by the presence of the Ile-tRNA synthetase inhibitor, mupirocin. The stop codon or the ORF is indicated with an asterisk. (C, Lower) Stalling efficiency calculated from the ratios of the intensity of the bands representing ketolide-dependent arrest (codon 13) vs. read through (codon 14). Error bars indicate data spreads in two independent experiments.
The mechanism of translational induction was investigated in a cell-free transcription–translation system using a primer extension inhibition assay (toe printing) (22, 23) to test for ketolide- and macrolide-induced ribosome stalling on the pikR2L mRNA. Both MTM and PKM induced strong translation arrest at the Leu13 codon of pikR2L (Fig. 4C, lanes 2 and 3). Unexpectedly, the clinical ketolide TEL was even more potent in arresting the ribosome at the Leu13 codon (Fig. 4C, lane 4). In contrast, consistent with the lack of in vivo induction of the pikR2L reporter system by ERY (Fig. 4B), this macrolide failed to induce ribosome stalling (Fig. 4C, lane 5). Taken together, these results suggest that pikR2 has been evolutionarily optimized for specific activation by natural ketolide antibiotics and that semisynthetic clinical ketolides can serve as even more potent inducers.
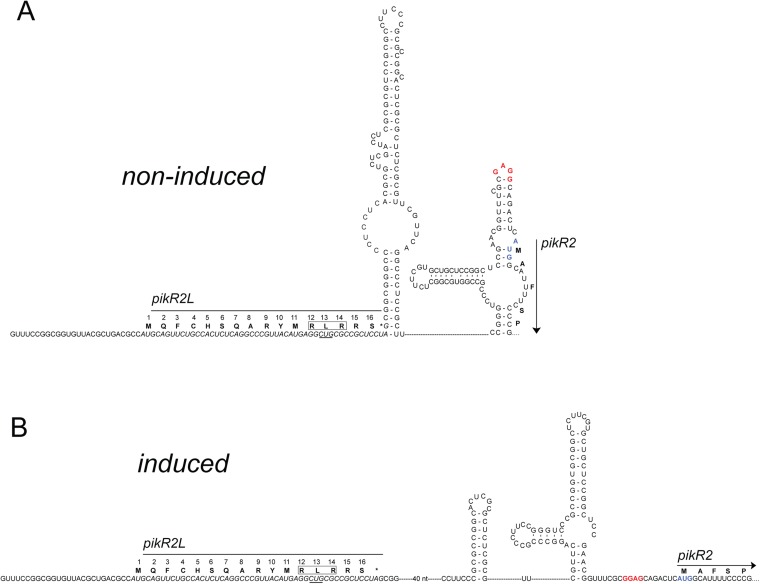
Computational analysis shows that a stable stem-loop configuration of mRNA in the pikR2L-pikR2 intergenic region may sequester the pikR2 ribosome binding site (Fig. S5A). Ketolide-induced ribosome stalling at the Leu13 codon of pikR2L would destabilize the proximal mRNA stem and promote an alternative conformation, in which the initiation region of pikR2 is accessible for translation (Fig. S5B).
Fig. S5.
The models of the secondary structure of pikR2 mRNA in the (A) noninduced and (B) induced states. The nucleotide sequence of the leader ORF pikR2L is italicized. The RLR sequence in the PikR2L leader peptide encompassing the Leu13 stalling codon is boxed, and the codon is underlined. The Shine–Dalgarno region of the pikR2 gene is shown in red, and the initiator codon is blue. The models are based on mfold predictions (40).
Inducible Expression of pikR2 Alone or Combined with pikR1 Confers a High Level of Ketolide Resistance in a Clinically Relevant Host.
After establishing that the natural resistance mechanisms conferred by pikR1 and pikR2 in the Streptomyces ketolide producer remain operational on transfer to E. coli, we extended the investigation to a heterologous Gram-positive model, because Gram-positive pathogens are the primary clinical targets of macrolides and ketolides. Mycobacterium tuberculosis is the etiological agent of tuberculosis and considered a possible target for ketolide therapy (24). We constructed strains of Mycobacterium smegmatis, a laboratory model of the pathogenic mycobacteria, which carried either the pikR2 gene controlled by its regulatory region or the entire pikR1-pikR2 cluster on a plasmid (Fig. 1A). The presence of pikR2 alone elevated the resistance of M. smegmatis to clinical ketolides by 16- to 32-fold (Table 2). The combination of pikR1 and pikR2 conferred a much higher level of resistance (exceeding 1 mg/mL for TEL). Constitutive monomethylation of A2058 by PikR1 most likely facilitates continued ribosome activity and expression of pikR2 on abrupt exposure of the cells to high concentrations of ketolides. When M. smegmatis cells with pikR2 or pikR1-pikR2 were preincubated with subinhibitory concentrations of TEL, resistance reached even higher levels, exceeding those of the control cells by several hundredfold (Table 2). Thus, the resistance genes originating in the producer of natural ketolides can render heterologous bacteria resistant to high concentrations of clinical ketolide antibiotics.
Table 2.
MIC (micrograms per milliliter) of clinically relevant ketolides for M. smegmatis harboring the pikR2 gene alone or the combination of pikR1 and pikR2 genes
| Plasmid | TEL | SOL | CET | |||
| Preincubation | Preincubation | Preincubation | ||||
| No | Yes* | No | Yes* | No | Yes* | |
| pMIP12 (vector) | 2 | 2 | 1 | 1 | 1 | 2 |
| pMR2 | 64 | 2,048 | 16 | 1,024 | 32 | 512 |
| pMR1R2 | 1,024 | 2,048 | 256 | 1,024 | 256 | 512 |
Antibiotics used were TEL, solithromycin (SOL), and cethromycin (CET). The M. smegmatis strain used in MIC testing was a derivative of the strain mc2155, in which the endogenous macrolide resistance gene erm38 was inactivated by allelic exchange (34).
Cells were grown for 72 h in the medium supplemented with one-eighth MIC of TEL (0.25 µg/mL for cells transformed with the empty vector, 8 µg/mL for cells transformed with pMR2, or 128 µg/mL for cells transformed with pMR1R2) before MIC testing.
Discussion
In this paper, we present evidence that the genes pikR1 and pikR2 have been evolutionary optimized to confer resistance to ketolide antibiotics. These genes not only render S. venezuelae ATCC 15439 resistant to its endogenously produced ketolides but also, confer appreciable resistance to clinical ketolides. The resistance conferred by pikR1 and pikR2 can be transferred to heterologous Gram-negative and -positive hosts. We further show that the fitness cost of resistance is economized by the ketolide-inducible expression of pikR2. Taking these factors into account, acquisition of the pikR1 and pikR2 genes is expected to confer a marked selective advantage on bacteria undergoing repeated exposure to ketolides, which could promote the transfer of these genes to pathogens when ketolides become more widely used in a clinical setting.
Our studies provide insights into the important question of why S. venezuelae, the producer of the natural ketolides MTM and PKM, maintains two resistance genes with seemingly overlapping functions. The constitutively expressed PikR1 monomethylates 23S rRNA nucleotide A2058 located at the site of ketolide action, and this modification confers an intermediate level of resistance. Higher levels of resistance are attained with the inducible PikR2, which adds a second methyl group to the same nucleotide. The combined action of two differentially expressed genes ensures active protein synthesis in the ketolide-producing cells across a broad range of the inhibitors concentrations.
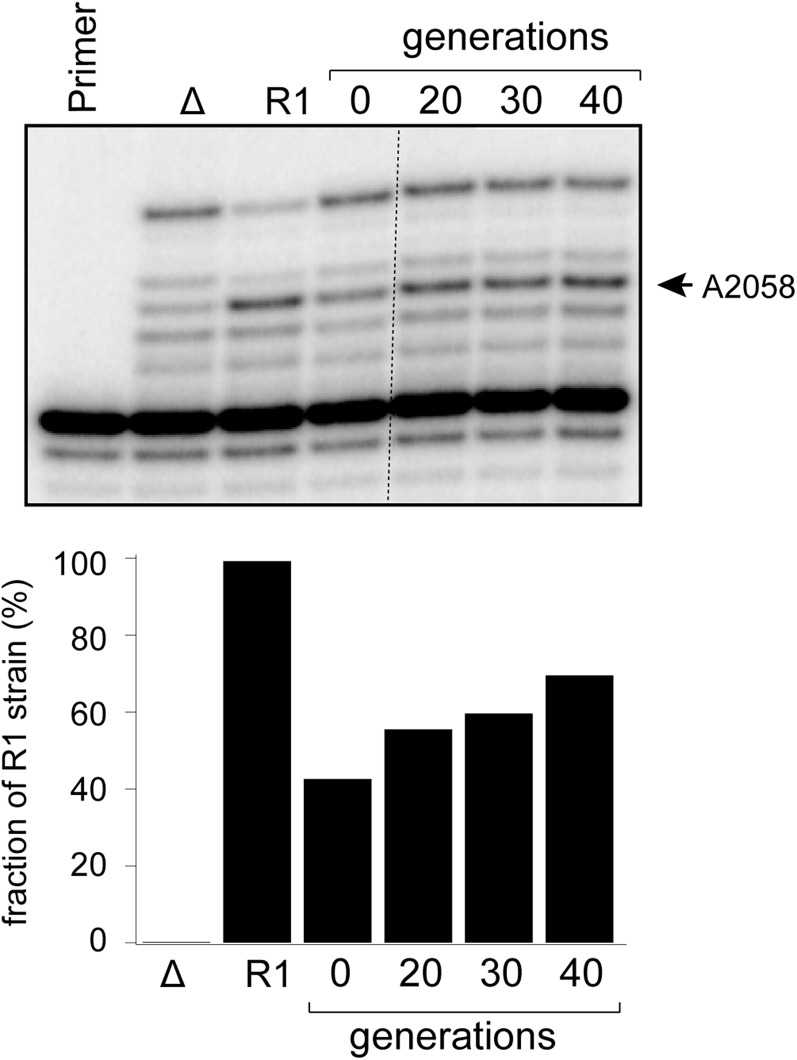
The inducibility of pikR2 is significant for the operation of the resistance mechanism. Although dimethylation of A2058, at least in Staphylococcus aureus, is known to significantly reduce cell fitness (6), monomethylation of the same nucleotide seems to have little, if any, effect on S. venezuelae fitness (Fig. S6). Thus, with a minimal toll in fitness cost for the constitutive PikR1 monomethyltransferase, S. venezuelae easily tolerates low concentrations of MTM and/or PKM. However, when S. venezuelae augments its production of these drugs, activation of the more costly PikR2 enzyme is required, resulting in A2058 dimethylation and higher resistance levels. Producers of other natural antibiotics, which carry more than one resistance gene, may use a similar strategy for achieving the cost-effective protection from inhibitors (25).
Fig. S6.
Growth competition reveals low fitness cost associated with A2058 monomethylation in S. venezuelae. S. venezuelae strains Δ and R1 deficient in antibiotic production and lacking both resistance genes (Δ) or carrying only pikR1 gene (R1) were mixed at approximately equal amounts and grown for the indicated number of generations with four consecutive passages with 1:1,000 dilutions. After isolation of the total RNA, the fraction of the R1 strain was assessed by the extent of A2058 monomethylation using primer extension. If RT did not pause because of A2058 modification, it would completely stop at C2055 because of incorporation of ddGTP. Primer extensions carried out on RNA prepared from the pure cultures of the Δ and R1 strains were used for calibration. The estimated fraction of the R1 strain in the co-growth culture is plotted.
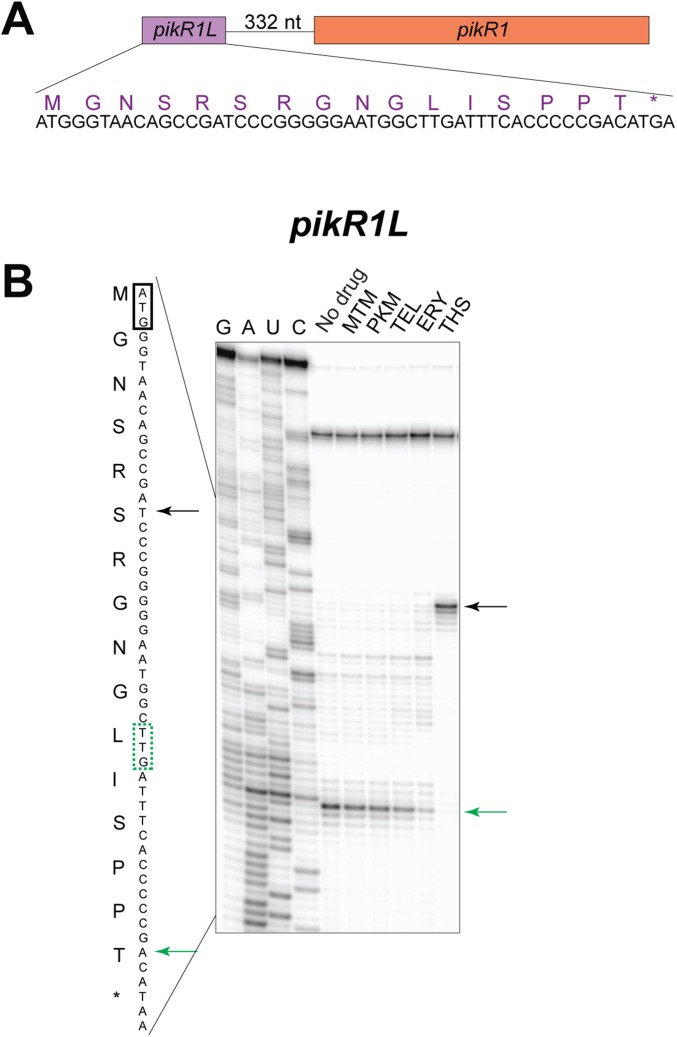
In contrast to other inducible erm genes, which respond primarily to macrolides bearing a C3-cladinose, the regulation of pikR2 has been evolutionary optimized for specific activation by ketolides (Fig. 4). Ketolide-specific pikR2 induction is most likely controlled by programmed translation arrest within the pikR2L leader ORF. Cladinose-containing macrolides are prone to promoting early peptidyl-tRNA drop off occurring within the first 6–10 codons (26). Consistently, the sites of programmed macrolide-dependent translation arrest within the leader ORFs of the resistant genes are located within the ORF’s first 10 codons, so that the drug-bound ribosome is able to reach the arrest site without prematurely dissociating from the mRNA (5, 19, 23, 27). Ribosomes translating pikR2L have to travel slightly farther and polymerize 13 amino acids before reaching the stalling codon. Because ketolides are less prone to promoting peptidyl-tRNA drop off (28), it is easier for ketolide-bound ribosomes than for ERY-bound ribosomes to polymerize the 13-aa-long nascent PikR2L chain. Indeed, the ERY-bound ribosome does not reach the stalling site (Fig. 4 and Fig. S5) and thus, fails to trigger pikR2 expression. Ketolide-induced stalling in the pikR2L ORF occurs within the motif RLR, which represents one of the most problematic sequences for translation by ketolide-bound ribosomes (28, 29). We note that a short ORF is also found in front of the constitutively expressed pikR1 gene (Fig. S7A), although this ORF does not promote macrolide- or ketolide-dependent stalling (Fig. S7B) and thus, seems to play no role in the expression of pikR1.
Fig. S7.
In contrast to the mechanism seen for pikR2, regulation of the pikR1 gene does not occur by stalling at its putative upstream ORF. (A) The sequence of the putative upstream ORF (pikR1L) and the encoded protein sequence. (B) Toe-printing analysis does not show any ketolide- or macrolide-specific stops during in vitro translation of the pikR1L ORF. The black arrows indicate the toe-printing band corresponding to the ribosome stalled by the control antibiotic thiostrepton (THS) at the pikR1L initiator codon (boxed). The green arrows show the band representing the ribosomes that were captured at the pikR1L 11th codon (boxed) because of the depletion of Ile-tRNA from the translation reaction by the presence of the Ile-RS inhibitor, mupirocin. Sequencing lanes are marked G, A, U, C.
The properties and regulation of the pikR1 and pikR2 genes are principally important for tolerating the broad range of inhibitor concentrations experienced by S. venezuelae. Constitutive monomethylation of A2058 by PikR1 renders ribosomes moderately resistant to ketolides, affording protection to S. venezuelae at the early stages of antibiotic production. Monomethylation of A2058 is a critical step toward acquisition of a high level of resistance, facilitating efficient translation of pikR2, despite the presence of a ketolide inhibitor. Indeed, on abrupt exposure of the S. venezuelae pikR1-null mutant (ΔpikR1) to TEL, the lone pikR2 gene was unable to provide any significant protection (Table 1), but when both pikR genes were present and cells were preincubated with low ketolide concentrations (mimicking the onset of antibiotic production), a high level of resistance was achieved. In the absence of pikR1, the resistance of S. venezuelae to TEL afforded by pikR2 alone, even on preincubation with the antibiotic, was no higher than that provided by the constitutively expressed monomethylase (Table 1). This intermediate level of resistance may be caused by either dimethylation of only a small fraction of ribosomes or mixed mono- and dimethylations, because Erm dimethyltransferases are known to add two methyl groups to A2058 in two consecutive reactions (30).
The synergy between the pikR1 and pikR2 genes was observed to an even greater extent in M. smegmatis, which was used to model the expression of the pikR genes in clinical pathogens. Here, the pikR2 gene alone conferred considerable resistance to clinical ketolides. However, it was the simultaneous presence of both pikR1 and pikR2 genes that provided the highest resistance (Table 2). The resistance to TEL afforded by pikR1 and pikR2 in M. smegmatis was significantly higher than in S. venezuelae. Factors that could contribute to this effect include the increased dosage of the resistance genes introduced into mycobacteria on a plasmid, the slower subunit assembly resulting in a higher proportion of methylated rRNA, or the lower affinity of the antibiotic for modified mycobacterial ribosomes.
The inducibility of pikR2, which lowers the fitness cost of the two-gene resistance mechanism, may facilitate not only its acquisition by a new host through horizontal gene transfer but also, its maintenance on discontinuation of the antibiotic treatment. Horizontal transfer of the pikR1/pikR2 gene pair may be further facilitated by their close physical proximity in the S. venezuelae chromosome (Fig. 1A). Despite the high GC content of pikR1 and pikR2, our results strongly suggest that their transfer into at least some clinical strains would render the strains highly resistant to ketolide therapy. These findings, thus, provide additional justification for renewed drug discovery efforts to identify novel antibiotics capable of overcoming protection rendered by natural resistance genes.
Materials and Methods
For isolation of the total RNA from S. venezuelae, cells were grown overnight in SGGP media, then diluted 1:100 in SCM media (31), and grown for 5 d at 30 °C with constant shaking. Cells were pelleted from 5-mL cultures, resuspended in 1 mL Tris-EDTA (TE) buffer (10 mM Tris⋅HCl, pH 8.0, 1 mM EDTA) containing 5 mg/mL lysozyme, incubated for 10 min at room temperature, and then, shaken for 10 min in the Minibead Breaker (Biospec Products) with 50 µL glass beads (particle size ≤106 μm; Sigma-Aldrich). Total RNA was isolated using the RNeasy Maxi Kit (Qiagen).
The RNeasy Plus Mini Kit was used to prepare total RNA from exponentially growing E. coli following the standard manufacturer’s protocol. Primer extension analysis under the standard conditions suitable for detection of adenine N6 dimethylation was carried out as previously described (21). In experiments where detection of N6 monomethylation was required, the concentration of dTTP was reduced from 1 to 0.01 mM with extension for 15 min at 37 °C instead of the standard 30 min at 42 °C. Primers L2180, L2563, and L2667 (Table S3) were used to examine domain V of 23S rRNA of E. coli. Primer L2405-R was used to check the modification status of A2058 in 23S rRNA of S. venezuelae.
Table S3.
Primers used in the study
| Name | Sequence |
| NdeI-pikR1-D2 | TCGTTCCATATGGCAATGCGCGACTCCAT |
| AflII-pikR1-R2 | CTTAAGCTTAAGCCAGACCAGCGGGAGGCGGA |
| NdeI-pikR2-D2 | GACTCCATATGGCATTTTCCCCGCAGGGCGG |
| AflII-pikR2-R2 | CCCACCTTAAGGGTCGGATCCGGCTCAGCAC |
| ORF-PikR1-d-new | CAGCTGCATATGGGTAACAGCCGATCCC |
| ORF-PikR1-R | TCTAGCTTAAGGTCGCGCATTGCCATGAACGATCCC |
| ORF2-PikR2-D | GACTATCATATGCAGTTCTGCCACTCTCAG |
| ORF2-PikR2-R | TACTAGCTTAAGCGGGGAAAATGCCATGAG |
| T7-pikR1-ORF1-fwd | ATTAATACGACTCACTATAGGGatataaggaggaaaaCatATGGGTAACAGCCGATCC |
| pikR1-ORF1-rev-spacer-NVI | GGTTATAATGAATTTTGCTTATTAACGATAGAATTCTATCACATTATGTCGGGGGTGAAATCAA |
| T7-SD-ORF2-pikR2 | ATTAATACGACTCACTATAGGGatataaggaggaaaaCatATGcagttc |
| O2-R2-IL-R | TTAACGATAGAATTCTATCACGGACGCGCGAGGATCGAGACGCGTGAGGAGGGGCCCGCCGCTAGGAGATGCGCAGCCTCATGTAACGGG |
| SvL2309 | TGTAGTAAAGGTCCCGGGG |
| Ecprotect | GCAGUGUACCCGCGGCAAGACGGAAAGACCCCGUGAACCUUUACUAUAG |
| Svprotect | GAGUAAAGAUGCUCGUUUCGCGCAGCAGGACGGAAAGACCCCGGGACCUUUACUACAG |
| T7 | ATTAATACGACTCACTATAGGG |
| O2-R2-NV1-R | GGTTATAATGAATTTTGCTTATTAACGATAGAATTCTATCACG |
| NV1 | GGTTATAATGAATTTTGCTTATTAAC |
| L2405-R | AGAGTGGTATTTCAACGGCGA |
| L2563 | TCGCGTACCACTTTA |
| L2667 | GGTCCTCTCGTACTAGGAGCAG |
| L2180 | GGGTGGTATTTCAAGGTCGG |
| SR199 | GCTATGACATGATTACGATTCGTCCCGGAGCGCCACACG |
| SR200 | ACCGCATGCACCAGGCCGTCGATCTCG |
| SR201 | GCCTGGTGCATGCGGTACGGAGCTCC |
| SR202 | CGACGGCCAGTGCCAAGCTTGCGGCGGAAATTCGAAGG |
| SR216 | GCTATGACATGATTACGAATTCGGAGTACTGGCCATCCGGC |
| SR217 | CGAGGCGATCGTCGTACGGACGCCGC |
| SR218 | TACGACGATCGCCTCGGTATGGAGTCG |
| SR219 | CGACGGCCAGTGCCAAGCTTGTCTCCGGAAGCCGCGCT |
| SR220 | GCTATGACATGATTACGAATTCCCACGACCCGACGCAG |
| SR221 | CGAGGCGAGGAAAATGCCATGAGTCTGCTCC |
| SR222 | GCATTTTCCTCGCCTCGGTATGGAGTCG |
| pMIP12-R2-D2 | ATGGATTAGAAGGAGAAGTACCGATGGGATTCTGCCACTCTCAGGCCCGTTACA |
| pMIP12-R2-R | TCGCCCGATCCCGTGTTTCGCTATTTCACGCGCTCTCCGCCCGCC |
| pMIP12-R2-D2-short | TTCTGCCACTCTCAGGCCCGTTACA |
| pMIP12-R2-R-short | TCACGCGCTCTCCGCCCGCC |
| pMIP12-R1-R | TCGCCCGATCCCGTGTTTCGCTATTACGAATTCCTCGGACTCACTCTTGGAC |
| pMIP12-R1-R-short | ACGAATTCCTCGGACTCACTCTTGGAC |
| acrAup | CATATGTTCGTGAATTTACAG |
| acrAdown | GCAATCGTAGGATATTGCG |
Toe printing was carried out as described previously (23). The status of rRNA modification in E. coli and S. venezuelae was analyzed using the approach described in ref. 32.
Other experimental details are provided in SI Materials and Methods.
SI Materials and Methods
Antibiotics, Enzymes, and Chemicals.
MTM and PKM were synthesized chemically as previously described (35) and repurified by preparatory HPLC using a Phenomenex Luna 5u C18 250 × 21.2-mm column (serial 444304–4) monitored at 250 nm at a flow rate of 9 mL/min with an isocratic mobile phase of H2O:MeCN (45:55) and a 0.1% NEt3 modifier. TEL, cethromycin, and solithromycin were from Cempra, Inc.; and ERY, clindamyicn, chloramphenicol, and thiostrepton were from Sigma Aldrich. Enzymes used for DNA cloning were from Fermentas. [γ32P]-ATP (specific activity of 6,000 Ci/mmol) was from MP Biomedicals. Other reagents and chemicals were purchased from either Fisher Scientific or Sigma Aldrich. All oligonucleotides used in the study were synthesized by Integrated DNA Technologies and are shown in Table S3.
Strains and Plasmids.
To generate Escherichia coli strains constitutively expressing PikR1 and PikR2 methyltransferase enzymes, their corresponding genes were PCR-amplified from the Streptomyces venezuelae strain ATCC 15439 genomic DNA using the primers NdeI-pikR1-D2 and AflII-pikR1-R2 or NdeI-pikR2-D2 and AflII-pikR2-R2 (Table S3), respectively, which introduced strong Shine-Dalgarno sequence upstream from the initiator AUG codons. The PCR products were digested with NdeI and AflII and cloned in the corresponding sites of the plasmid pERMZα (21) (Table S1) behind the Ptac promoter. The resulting plasmids pPikR1 and pPikR2 were used to transform the JM109 ΔacrA strain, and the transformants were used for MIC testing and RNA preparation.
To study the inducibility of pikR1 and pikR2 genes, their regulatory regions (including the putative leader ORFs, the intergenic region, and the first five codons of the resistance genes) were PCR-amplified from the DNA of S. venezuelae ATCC15439 using the primers ORF-PikR1-d-new and ORF-PikR1-R or ORF2-PikR2-D and ORF2-PikR2-R, respectively. The PCR products were digested with NdeI and AflII and cloned in the corresponding sites of plasmid pERMZα. The JM109 ΔacrB strain was transformed with the resulting plasmids pRL1 and pRL2, and the transformants were used for antibiotic disk diffusion experiments.
For studying the expression of the individual resistance genes in S. venezuelae, strains that lack pikR1, pikR2, or both of them were engineered. To prepare the strain that had only pikR1, the pikR2 gene in the S. venezuelae DHS2001 strain (Table S2) was inactivated by in-frame deletion of 271 codons (codons 31–301) to avoid any polar effects. To construct this deletion, the plasmid pSRP112 (Table S1), based on the E. coli–Streptomyces shuttle vector pKC1139 (36), was constructed by amplifying and cloning left- and right-flanking regions of the pikR2 genes using the genomic DNA of S. venezuelae DHS2001 as a template, primer pairs SR199–SR200 and SR201–SR202, and KOD Xtreme DNA Polymerase (Novagen). The plasmid was assembled using Gibson assembly (New England Biolabs Kit) according to the manufacturer’s instructions. Briefly, the amplified PCR products of flanking regions of pikR2 and linearized pKC1139 vector with EcoRI and HindIII were incubated at 50 °C for 2 h. After incubation, the samples were transformed into E. coli DH5α. Restriction digestion and sequencing verified the isolated pSRP112 plasmid. The plasmid pSRP112 was then introduced into the S. venezuelae DHS2001 by protoplasts-based transformation. A strain in which a single cross-over between the pSRP112 plasmid and the S. venezuelae DHS2001 chromosome had occurred was selected by cultivation of antibiotic-resistant transconjugants at 37 °C (the nonpermissive temperature for the pSG5-based replicon) in the presence of apramycin. Cells from one colony were subjected to second rounds of propagation in the absence of apramycin to allow for the second cross-over. The desired double cross-over mutant ΔpikR2 was selected by its apramycin-sensitive phenotype and verified by PCR. The resulting pikR2 deletion mutant of S. venezuelae DHS2001 was designated R1 (DHS328). The ΔpikR1 and ΔpikR1-pikR2 mutants were generated in the same way as described above using the primer pairs SR216–SR217 and SR218–SR219 for ΔpikR1 and SR219–SR220 and SR221–SR222 for ΔpikR1-pikR2. The pikR1 in-frame deletion encompassed codons 11–327, and the pikR1-pikR2 deletion left intact the first 10 codons of pikR1 and pikR2, removing the entire DNA segment in between them. The resulting pikR1- and pikR1-pikR2 deletion mutants of S. venezuelae DHS2001 were designated R2 (DHS330) and Δ (DHS332), respectively.
To check the resistance conferred by the pikR2 gene in Mycobacterium smegmatis, pikR2 was PCR-amplified with its regulatory region (including the pikR2L and the intergenic region) from the DNA of S. venezuelae strain ATCC15439 using the primers pMIP12-R2-D2-short and pMIP12-R2-R-short. The PCR product was gel-purified and used as a template for the second PCR using the primers pMIP12-R2-D2 and pMIP12-R2-R. The PCR product was gel-purified and introduced by Gibson assembly into the pMIP12 plasmid (37) cut with BamHI and SpeI restriction enzymes. The reaction mixture was transformed into E. coli JM109. The recombinant plasmid designated pMR2 (Table S1) was isolated, sequenced, and introduced into the Δerm38 strain of M. smegmatis (strain mc2155 ermKO-4) (38) (Table S2) by electroporation. The transformed cells were plated on agar plates containing 15 µg/mL kanamycin.
To clone the pikR2-pikR1 tandem (that contains pikR2 with its leader ORF and pikR1 with it native promoter), a 3.25-kb DNA fragment from the genomic DNA of S. venezuelae was initially PCR-amplified using the primers pMIP12-R2-D2-short and pMIP12-R1-R-short. The purified PCR product was reamplified using the primers pMIP12-R2-D2 and pMIP12-R1-R to introduce flanking regions suitable for cloning in the pMIP12 vector. The resulting PCR product was introduced into the BamHI and SpeI cut plasmid pMIP12 by Gibson assembly as described above. The resulting plasmid, pMR1R2 (Table S1), was eventually introduced into M. smegmatis strain mc2155 ermKO-4.
Toe-Printing Assay.
Toe printing was carried out as described previously (23). The templates containing genes coding for the putative PikR leader peptides under the control of the T7 promoter were generated by PCR using primers T7-pikR1-ORF1-fwd, pikR1-ORF1-rev-spacer-NVI (pikR1L), T7-SD-ORF2-pikR2, O2-R2-IL-R, T7, and O2-R2-NV1-R (piKR2L) (Table S3). The reverse PCR primer replaced the penultimate pikR2 Arg codon (CGC) with an Ile codon (ATC) to enable macrolide-independent translation arrest at this codon. The templates were translated in 5 µL PURExpress cell-free translation reactions (New England Biolabs) for 30 min at 37 °C. All of the reactions contained 50 µМ mupirocin, an Ile-tRNA synthetase inhibitor. When needed, other antibiotics (MTM, PKM, ERY, TEL, or thiostrepton) were added to the final concentration of 50 µM. After the addition of 5′-[32P]–radiolabeled primer NV1, reverse transcription was carried out for 15 min at 37 °C. Samples were then processed and analyzed as described in ref. 23.
MS Analysis of rRNA Modification.
The status of rRNA modification in E. coli and S. venezuelae was analyzed using the approach described previously (32). Briefly, total RNA was extracted from cells (see above) and hybridized with the DNA oligonucleotides Ecprotect and SVprotect (Table S3) complementary to the 23S rRNA sequence C2035-C2084 (E. coli) or C2025-C2083 (S. venezuelae). rRNA regions that were not protected by hybridization were digested away with nucleases, and the protected rRNA fragments were isolated by gel electrophoresis. The purified material was digested with RNaseT1 or RNaseA and subjected to MALDI-TOF on a Bruker Daltronics Ultraflextreme Spectrometer recording in reflector and positive ion modes (39). Spectra were analyzed using Flexanalysis software (Bruker).
Microbiological Testing.
MICs of antibiotics were determined by the broth microdilution assay. The MIC values were read after an overnight incubation (E. coli and S. venezuelae) or a 3-d incubation (M. smegmatis) at 37 °C.
Disk diffusion assays for testing the inducibility of the pRL1 and pRL2 reporters (Table S1) were carried out essentially as described previously (21), with the exception that the JM109 ΔacrB strain was used as the host. Briefly, cells transformed with reporter plasmids were grown overnight in the presence of 100 µg/mL ampicillin and 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG), and then, 1.5 mL cell cultures were mixed with 8.5 mL soft agar [0.6% (wt/vol) LB agar at 50 °C] and overlaid on agar plates containing 100 μg/mL ampicillin, 0.5 mM IPTG, and 80 μg/mL X-Gal. After the soft agar solidified, 5 mm Whatmann 3MM Paper Disks containing 32 μg MTM, 32 μg PKM, 32 μg ERY, 32 μg TEL, or 8 μg chloramphenicol were placed on top of the agar; plates were incubated for 18–24 h at 37 °C and then, photographed.
Growth Competition.
S. venezuelae Δ and R1 strains were grown separately overnight at 30 °C with shaking in CRM medium (10 g/L glucose, 103 g/L sucrose, 10.12 g/L MgCl2⋅6H2O, 15 g/L tryptic soy broth, 5 g/L yeast extract). The overnight cultures were diluted to A600 of 0.04 in fresh CRM medium and grown at 30 °C with shaking until they reached A600 of 0.5. Equal culture volumes of Δ and R1 strains were mixed and grown overnight with shaking at 30 °C. At each cycle, the culture was diluted 1,000-fold into fresh CRM medium and grown for 18 h. The culture was grown for a total of four passages corresponding to ∼40 cell generations. The ratio of cells was determined at cycles 2–4 by isolating total RNA from the coculture and examining the extent of monomethylation of A2058 in 23S rRNA by primer extension (Fig. S6). Pure cultures of Δ and R1 strains were used to prepare control RNA that was used for calibration.
Analysis of the Extent of A2058 Modification.
The extent of 23S rRNA A2058 mono- or dimethylation was assessed by primer extension as described previously (21) with minor modifications. Specifically, 0.5 pmol 5′-[32P]–labeled primer SvL2309 (Table S3) was annealed to 1 μg total S. venezuelae RNA and extended with AMV Reverse Transcriptase (Roche) in the presence of 1 mM dCTP and dATP, 1 mM ddGTP, and 0.001 mM dTTP (for A2058 monomethylation) or 0.25 mM dTTP (for A2058 dimethylation). The cDNA products were purified by phenol extraction, precipitated, resolved in a denaturing 12% polyacrylamide gel, and visualized by phosphoimaging.
Acknowledgments
We thank Brian Murphy, Skylar Carlson, and Xiaomei Wei for help in analysis of pikromycin (PKM) and methymycin (MTM); Dorota Klepacki for the E. coli ΔacrA strain; and Prabha Fernandes (Cempra, Inc.) for providing clinical ketolides. M.M.A. was supported by the King Saud University Scholarship for Graduate Studies. D.A.H. was supported by a University of Michigan Rackham Merit Predoctoral Fellowship. This work was supported by National Institutes of Health Grants R01 GM106386 (to N.V.-L. and A.S.M.) and R01 GM076477 (to D.H.S.), Danish Research Agency Forskningsrådet for Natur og Univers (FNU)-Rammebevilling Grant 10-084554 (to S.D.), and the Hans W. Vahlteich Professorship (D.H.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512090112/-/DCSupplemental.
References
- 1.Cundliffe E. How antibiotic-producing organisms avoid suicide. Annu Rev Microbiol. 1989;43:207–233. doi: 10.1146/annurev.mi.43.100189.001231. [DOI] [PubMed] [Google Scholar]
- 2.Schlünzen F, et al. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001;413(6858):814–821. doi: 10.1038/35101544. [DOI] [PubMed] [Google Scholar]
- 3.Bulkley D, Innis CA, Blaha G, Steitz TA. Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc Natl Acad Sci USA. 2010;107(40):17158–17163. doi: 10.1073/pnas.1008685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunkle JA, Xiong L, Mankin AS, Cate JH. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc Natl Acad Sci USA. 2010;107(40):17152–17157. doi: 10.1073/pnas.1007988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39(3):577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta P, Sothiselvam S, Vázquez-Laslop N, Mankin AS. Deregulation of translation due to post-transcriptional modification of rRNA explains why erm genes are inducible. Nat Commun. 2013;4:1984. doi: 10.1038/ncomms2984. [DOI] [PubMed] [Google Scholar]
- 7.Vázquez-Laslop N, Ramu H, Mankin AS. Nascent peptide–mediated ribosome stalling promoted by antibiotics. In: Rodnina MV, Green R, Wintermeyer W, editors. Ribosomes: Structure, Function, and Dynamics. Springer; Vienna: 2011. [Google Scholar]
- 8.Sutcliffe JA. Antibiotics in development targeting protein synthesis. Ann N Y Acad Sci. 2011;1241:122–152. doi: 10.1111/j.1749-6632.2011.06323.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosato A, Vicarini H, Bonnefoy A, Chantot JF, Leclercq R. A new ketolide, HMR 3004, active against streptococci inducibly resistant to erythromycin. Antimicrob Agents Chemother. 1998;42(6):1392–1396. doi: 10.1128/aac.42.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnefoy A, Girard AM, Agouridas C, Chantot JF. Ketolides lack inducibility properties of MLS(B) resistance phenotype. J Antimicrob Chemother. 1997;40(1):85–90. doi: 10.1093/jac/40.1.85. [DOI] [PubMed] [Google Scholar]
- 11.Brockman H, Henkel W. Pikromycin, ein neues Antibiotikum aus Actinomyceten. Naturwissenschaften. 1950;37(6):138–139. [Google Scholar]
- 12.Lambalot RH, Cane DE. Isolation and characterization of 10-deoxymethynolide produced by Streptomyces venezuelae. J Antibiot (Tokyo) 1992;45(12):1981–1982. doi: 10.7164/antibiotics.45.1981. [DOI] [PubMed] [Google Scholar]
- 13.Xue Y, Wilson D, Sherman DH. Genetic architecture of the polyketide synthases for methymycin and pikromycin series macrolides. Gene. 2000;245(1):203–211. doi: 10.1016/s0378-1119(00)00003-2. [DOI] [PubMed] [Google Scholar]
- 14.Xue Y, Zhao L, Liu HW, Sherman DH. A gene cluster for macrolide antibiotic biosynthesis in Streptomyces venezuelae: Architecture of metabolic diversity. Proc Natl Acad Sci USA. 1998;95(21):12111–12116. doi: 10.1073/pnas.95.21.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kittendorf JD, Sherman DH. The methymycin/pikromycin pathway: A model for metabolic diversity in natural product biosynthesis. Bioorg Med Chem. 2009;17(6):2137–2146. doi: 10.1016/j.bmc.2008.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts MC. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol Lett. 2008;282(2):147–159. doi: 10.1111/j.1574-6968.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 17.Auerbach T, et al. Structural basis for the antibacterial activity of the 12-membered-ring mono-sugar macrolide methymycin. Biotechnologia. 2009;1(84):24–35. [Google Scholar]
- 18.Jung WS, et al. Heterologous expression of tylosin polyketide synthase and production of a hybrid bioactive macrolide in Streptomyces venezuelae. Appl Microbiol Biotechnol. 2006;72(4):763–769. doi: 10.1007/s00253-006-0318-5. [DOI] [PubMed] [Google Scholar]
- 19.Ramu H, Mankin A, Vazquez-Laslop N. Programmed drug-dependent ribosome stalling. Mol Microbiol. 2009;71(4):811–824. doi: 10.1111/j.1365-2958.2008.06576.x. [DOI] [PubMed] [Google Scholar]
- 20.Vázquez-Laslop N, et al. Role of antibiotic ligand in nascent peptide-dependent ribosome stalling. Proc Natl Acad Sci USA. 2011;108(26):10496–10501. doi: 10.1073/pnas.1103474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey M, Chettiath T, Mankin AS. Induction of erm(C) expression by noninducing antibiotics. Antimicrob Agents Chemother. 2008;52(3):866–874. doi: 10.1128/AAC.01266-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartz D, McPheeters DS, Traut R, Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez-Laslop N, Thum C, Mankin AS. Molecular mechanism of drug-dependent ribosome stalling. Mol Cell. 2008;30(2):190–202. doi: 10.1016/j.molcel.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Falzari K, et al. In vitro and in vivo activities of macrolide derivatives against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005;49(4):1447–1454. doi: 10.1128/AAC.49.4.1447-1454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Memili E, Weisblum B. Essential role of endogenously synthesized tylosin for induction of ermSF in Streptomyces fradiae. Antimicrob Agents Chemother. 1997;41(5):1203–1205. doi: 10.1128/aac.41.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenson T, Lovmar M, Ehrenberg M. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J Mol Biol. 2003;330(5):1005–1014. doi: 10.1016/s0022-2836(03)00662-4. [DOI] [PubMed] [Google Scholar]
- 27.Sothiselvam S, et al. Macrolide antibiotics allosterically predispose the ribosome for translation arrest. Proc Natl Acad Sci USA. 2014;111(27):9804–9809. doi: 10.1073/pnas.1403586111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannan K, et al. The general mode of translation inhibition by macrolide antibiotics. Proc Natl Acad Sci USA. 2014;111(45):15958–15963. doi: 10.1073/pnas.1417334111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis AR, Gohara DW, Yap MN. Sequence selectivity of macrolide-induced translational attenuation. Proc Natl Acad Sci USA. 2014;111(43):15379–15384. doi: 10.1073/pnas.1410356111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen CT, Jakobsen L, Douthwaite S. Mycobacterium smegmatis Erm(38) is a reluctant dimethyltransferase. Antimicrob Agents Chemother. 2005;49(9):3803–3809. doi: 10.1128/AAC.49.9.3803-3809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Sherman DH. Isolation and structure determination of novamethymycin, a new bioactive metabolite of the methymycin biosynthetic pathway in Streptomyces venezuelae. J Nat Prod. 2001;64(11):1447–1450. doi: 10.1021/np010146r. [DOI] [PubMed] [Google Scholar]
- 32.Douthwaite S, Kirpekar F. Identifying modifications in RNA by MALDI mass spectrometry. Methods Enzymol. 2007;425:3–20. doi: 10.1016/S0076-6879(07)25001-3. [DOI] [PubMed] [Google Scholar]
- 33.Golovina AY, et al. The last rRNA methyltransferase of E. coli revealed: The yhiR gene encodes adenine-N6 methyltransferase specific for modification of A2030 of 23S ribosomal RNA. RNA. 2012;18(9):1725–1734. doi: 10.1261/rna.034207.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen DA, Koch AA, Sherman DH. Substrate controlled divergence in polyketide synthase catalysis. J Am Chem Soc. 2015;137(11):3735–3738. doi: 10.1021/ja511743n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen DA, et al. Biocatalytic synthesis of pikromycin, methymycin, neomethymycin, novamethymycin, and ketomethymycin. J Am Chem Soc. 2013;135(30):11232–11238. doi: 10.1021/ja404134f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bierman M, et al. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116(1):43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 37.Le Dantec C, Winter N, Gicquel B, Vincent V, Picardeau M. Genomic sequence and transcriptional analysis of a 23-kilobase mycobacterial linear plasmid: Evidence for horizontal transfer and identification of plasmid maintenance systems. J Bacteriol. 2001;183(7):2157–2164. doi: 10.1128/JB.183.7.2157-2164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nash KA. Intrinsic macrolide resistance in Mycobacterium smegmatis is conferred by a novel erm gene, erm(38) Antimicrob Agents Chemother. 2003;47(10):3053–3060. doi: 10.1128/AAC.47.10.3053-3060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirpekar F, Douthwaite S, Roepstorff P. Mapping posttranscriptional modifications in 5S ribosomal RNA by MALDI mass spectrometry. RNA. 2000;6(2):296–306. doi: 10.1017/s1355838200992148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31(13):3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: Nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 42.Vázquez-Laslop N, Ramu H, Klepacki D, Kannan K, Mankin AS. The key function of a conserved and modified rRNA residue in the ribosomal response to the nascent peptide. EMBO J. 2010;29(18):3108–3117. doi: 10.1038/emboj.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2(2006):2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]