Kidney diseases are a major problem worldwide and the number of patients with chronic kidney diseases and acute or chronic renal failure are rising each year (1). Hemodialysis and peritoneal dialysis are used to replace the detoxification function of kidneys in patients with end-stage renal disease (ESRD). Although these treatments are life-preserving, they are associated with a marked impairment of quality of life and fail to replace important kidney functions, such as endocrine activities (renin, erythropoietin, and activation of vitamin D) and fine regulation of electrolyte/water homeostasis. Kidney transplantation is so far the only option to restore all aspects of normal kidney function, providing much better quality of life and longer survival compared with dialysis. However, there is a severe shortage of human donor organs: in the United States ∼17,000 kidney transplantations were done in 2014, but at least fivefold more patients are on the waiting list, and each day 12 people die while waiting for a life-saving kidney transplant (2). Alternatives to human kidney transplants from living or deceased donors are thus urgently needed. Current strategies include xenotransplantation of kidneys from genetically engineered donor pigs (reviewed in ref. 3), use of extracorporal bioartificial kidneys with tubular cells grown in hollow fiber devices (4), development of bioengineered kidneys by repopulating the decellularized scaffold of cadaveric organs with endothelial and epithelial cells (5), and de novo kidney generation from stem cells and embryonic progenitor tissues (reviewed in ref. 6). In PNAS, Yokote et al. (7) use an elegant isogenic pig model system to demonstrate that functional de novo kidneys of sufficient size for human transplantation can be generated in principle, and that urine excretion can be facilitated by development of a stepwise peristaltic ureter (SWPU) system.
Because of its complex architecture and composition of numerous highly specialized and differentiated cell types, the kidney is one of the most difficult organs for de novo formation. Kidney development originates from the intermediate mesoderm, which differentiates into the nephric duct and the metanephric mesenchyme (MM). The nephric duct yields the ureteric bud (UB), which itself forms the renal collecting ducts and the lower urinary tract. In concerted reciprocal interactions with the UB, the MM finally forms the nephrons (the functional system of the kidney consisting of glomerulus, proximal tubule, Henle’s loop, and distal tubule) and the interstitial tissue of the kidney. Based on known mechanisms of mammalian nephrogenesis (reviewed in ref. 8), different experimental routes to achieve de novo formation of functional kidney tissue have been followed.
One approach is the directed stepwise differentiation of embryonic stem (ES) cells or induced pluripotent stem (iPS) cells by using specific growth factors and small chemical inhibitors of specific signaling pathways into renal progenitor cells, which—under appropriate conditions—can form complex tubular and glomerular structures (9). Formation of an almost entirely stem cell-derived organ can be achieved by injecting pluripotent stem cells into early embryos, which are genetically disabled to form a specific tissue or organ and thus provide an empty niche for whole-organ development. Kobayashi et al. (10) pioneered this so-called blastocyst complementation approach by demonstrating formation of an entire mouse or rat pancreas in chimeric mice produced by injection of mouse or rat pluripotent stem cells into blastocysts with defect copies of pancreatic and duodenal homeobox 1 (Pdx1), an essential gene for pancreas formation. As a first step toward clinical translation of this concept, Matsunari et al. (11) succeeded in producing allogeneic pig pancreas by injecting genetically labeled embryonic blastomeres into morula stage embryos from transgenic pancreatogenesis disabled pigs. To adapt this approach for de novo kidney formation, mouse ES or iPS cells were injected into mouse blastocysts lacking a functional spalt-like transcription factor 1 (Sall1) gene, resulting in impaired development of the MM-derived components of the kidney. Chimeric mice generated by this approach had kidneys that were almost entirely derived from the injected pluripotent cells, except for the renal vascular and nervous system (12). Injection of rat iPS cells into Sall1 mutant mouse blastocysts failed to generate rat kidneys in mouse, suggesting insufficient cross-talk between UB and MM from different species. In addition to such biological difficulties in a xenogeneic blastocyst complementation system, ethical problems arise if human pluripotent stem cells injected into organogenesis-impaired blastocysts form, for example, neurons or germ cells, eventually resulting in interspecific chimeras (13).
These problems can be avoided if an embryonic metanephros (the progenitor structure of the definitive kidney, including the UB and MM) is used to start growth of a de novo kidney. Rat embryonic metanephroi transplanted into the omentum or under the kidney capsule of adult rats were shown to obtain their blood supply from the recipient and to differentiate into functional tubular and glomerular structures with detoxification (14) and endocrine functions (secretion of erythropoietin and renin) (reviewed in ref. 6). Furthermore, metanephroi from porcine embryos developed fully functional nephrons after implantation into immunosuppressed or immunodeficient mice (reviewed in ref. 6). The need for immunosuppression might be avoided by using genetically engineered porcine donor embryos lacking major xeno-antigens (reviewed in ref. 3) and expressing immune modulatory proteins preventing rejection of the metanephros xenograft by humoral and cellular mechanisms (15).
Although the size of a metanephros-derived kidney tissue is primarily controlled by the metanephros donor species, growth of a porcine “neo-kidney” in a rodent host is obviously limited. Therefore, Yokote et al. (7) transplanted metanephroi from 30-d-old pig embryos (pregnancy duration in pigs is 114 d) into the omentum of recipient pigs. To avoid immunological complications, cloned donor embryos—isogenic to the recipients—were used. The grafted metanephroi showed substantial growth (5–7 mm after 3 wk, 3 cm after 8 wk), formation of kidney glomeruli and tubuli, and production of urine. However, because no functional urinary excretion system developed, the de novo kidney tissue underwent pressure atrophy (hydronephrosis). To overcome this problem, Yokote et al. tested a larger embryonic graft, including the metanephros and cloaca, which is a common opening to the urinary and digestive tracts during development and permits formation of a urinary bladder. This approach was first tested in a rat model and proved to be superior to metanephros-only transplantation with regard to neo-kidney development and urine production. To facilitate excretion of the urine, Yokote et al. next developed a procedure, the SWPU system, where the graft-derived neo-bladder was surgically connected to one ureter of the recipient rat and facilitated continuous discharge of graft-derived urine. Finally, Yokote et al. were able to translate the approach of combined metanephros plus cloaca transplantation and development of an SWPU system into the pig model, demonstrating that it has the potential to work in an animal with similar size to humans.
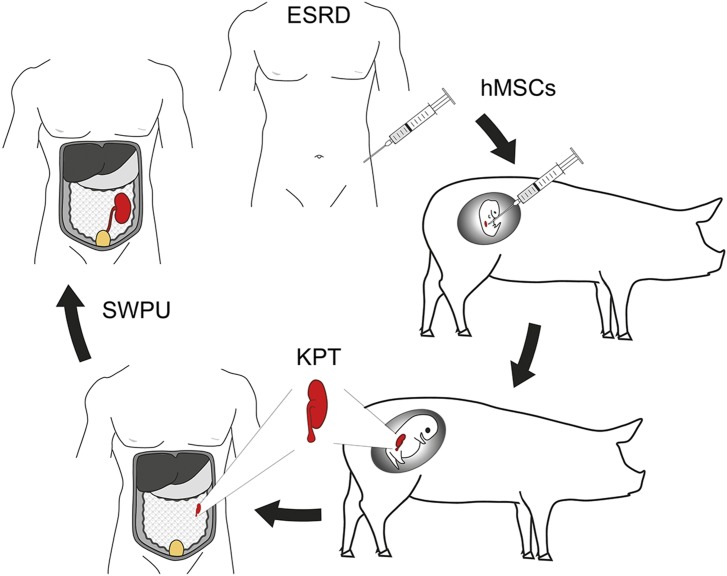
Yokote et al.’s (7) study has a number of important implications for future attempts of de novo human kidney formation to overcome the shortage of donor organs for kidney transplantation. Metanephroi cannot only form functional kidney tissue per se but also serve as a developmental niche inducing differentiation of injected human mesenchymal stem cells (hMSCs) into all kinds of specialized cell types of the kidney (16). Thus, a scenario can be envisaged where metanephroi in pig embryos are used as developmental niche for hMSCs to derive human kidney progenitor tissue, which can be transplanted into ESRD patients to form a de novo kidney (Fig. 1). Because hMSCs can be easily derived from bone marrow or adipose tissue, there is at least a theoretical possibility to generate patient-specific kidney progenitor tissue, although hMSCs from long-term dialysis patients were found to have abnormal gene-expression profiles, which may affect their differentiation capacity (17).
Fig. 1.
Concept of using pigs for de novo formation of human kidneys. hMSCs are injected into porcine embryonic metanephroi as a niche for generating hMSC-derived kidney progenitor tissue (KPT), which is grafted into the omentum of a patient with ESRD for further development and differentiation. The de novo originating kidney is finally connected to the patient’s ureter (e.g., by the SWPU system) to facilitate excretion of urine. Figure courtesy of Andreas Blutke.
Although the concept of using the nephrogenic niche in xeno-embryos for developing human kidney tissue from hMSCs is highly attractive and the work by Yokote et al. (7) is an important step toward its realization, a number of challenges are still remaining. In the original description of the method (16), hMSCs were injected at the site of sprouting of the UB in rat embryos recovered 11.5 d after conception. The embryos were then maintained for 48 h ex vivo in whole embryo culture, before chimeric metanephroi were isolated, cultured for another day, and then transplanted in the omentum of adult rats. Because of their larger size, whole-embryo culture of pig embryos of the corresponding stage is probably not possible, and sophisticated techniques need to be established for injecting the hMSCs into pig embryos in utero. Differentiation of hMSCs in isolated cultured pig metanephroi may be an alternative. Another issue may be that hMSC-derived de novo kidney tissue will likely be contaminated with porcine cells (cells from the embryonic organogenic niche, circulating MSCs, and vascular endothelial cells), which would induce immune rejection of the chimeric graft after transplantation to humans. This problem could be overcome by depleting the porcine cells by inducible expression of suicide genes (18). Alternatively, pig embryos from genetically modified pig lines generated for organ xenotransplantation (3, 15) could be used as organogenic niche to avoid rejection of chimeric de novo kidney tissue. In addition, Yokote et al. (7) discuss the possibility of generating fully human-derived neo-kidney using the blastocyst complementation technique: that is, injection of human stem cells into paired box 2/paired box 8 (PAX2/PAX8)-defective pig blastocysts. Mouse embryos lacking functional Pax2 and Pax8 genes are unable to form metanephroi and ureters (19), and loss-of-function mutations in the orthologous porcine genes can now be easily engineered, for example by using the CRISPR/Cas system (20).
The Yokote et al. (7) report opens new perspectives for de novo formation of functional human kidneys. This approach, along with progress in porcine kidney xenotransplantation and the development of bioengineered kidneys, may help to overcome the dramatic shortage of human donor organs for kidney transplantation.
Acknowledgments
We thank Dr. Andreas Blutke for creating Fig. 1. E.K. and E.W. are members of EU COST Action BM1308 “Sharing Advances on Large Animal Models – SALAAM”.
Footnotes
The authors declare no conflict of interest.
See companion article on page 12980.
References
- 1.Jha V, et al. Chronic kidney disease: Global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health & Human Services 2015 doi: 10.3109/15360288.2015.1037530. Organ Procurement and Transplantation Network. Available at optn.transplant.hrsa.gov/. Accessed April 22, 2015. [DOI] [PubMed]
- 3.Cowan PJ, Cooper DK, d’Apice AJ. Kidney xenotransplantation. Kidney Int. 2014;85(2):265–275. doi: 10.1038/ki.2013.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humes HD, Buffington DA, MacKay SM, Funke AJ, Weitzel WF. Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat Biotechnol. 1999;17(5):451–455. doi: 10.1038/8626. [DOI] [PubMed] [Google Scholar]
- 5.Song JJ, et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19(5):646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanaka S, Yokoo T. Current bioengineering methods for whole kidney regeneration. Stem Cells Int. 2015;2015:724047. doi: 10.1155/2015/724047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokote S, et al. Urine excretion strategy for stem cell-generated embryonic kidneys. Proc Natl Acad Sci USA. 2015;112:12980–12985. doi: 10.1073/pnas.1507803112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little MH, McMahon AP. Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012;4(5):a008300. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taguchi A, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14(1):53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142(5):787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Matsunari H, et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci USA. 2013;110(12):4557–4562. doi: 10.1073/pnas.1222902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usui J, et al. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012;180(6):2417–2426. doi: 10.1016/j.ajpath.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Hermerén G. Ethical considerations in chimera research. Development. 2015;142(1):3–5. doi: 10.1242/dev.119024. [DOI] [PubMed] [Google Scholar]
- 14.Rogers SA, Lowell JA, Hammerman NA, Hammerman MR. Transplantation of developing metanephroi into adult rats. Kidney Int. 1998;54(1):27–37. doi: 10.1046/j.1523-1755.1998.00971.x. [DOI] [PubMed] [Google Scholar]
- 15.Klymiuk N, et al. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes. 2012;61(6):1527–1532. doi: 10.2337/db11-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoo T, et al. Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci USA. 2005;102(9):3296–3300. doi: 10.1073/pnas.0406878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamanaka S, et al. Adipose tissue-derived mesenchymal stem cells in long-term dialysis patients display downregulation of PCAF expression and poor angiogenesis activation. PLoS One. 2014;9(7):e102311. doi: 10.1371/journal.pone.0102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto K, et al. Xenotransplanted embryonic kidney provides a niche for endogenous mesenchymal stem cell differentiation into erythropoietin-producing tissue. Stem Cells. 2012;30(6):1228–1235. doi: 10.1002/stem.1101. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard M, Souabni A, Mandler M, Neubüser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16(22):2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 2014;24(3):372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]