Significance
Estrogen regulation of tissue homeostasis, particularly the regulation of cell proliferation and differentiation in female reproductive organs, is interesting both in its basic biology and in several disease models and cancer biology. Using an epithelial estrogen receptor 1 (Esr1) conditional knockout mouse model, we showed that vaginal epithelial cells lacking ESR1 failed to activate growth factor signaling and remained undifferentiated and in a proliferative state throughout the epithelium. Thus, epithelial ESR1 mediates the switch between cell proliferation and squamous cell differentiation, demonstrating unique roles of ESR1 in the female reproductive tract.
Keywords: estrogen receptor, vagina, keratinization, amphiregulin, epithelium
Abstract
Estrogen-mediated actions in female reproductive organs are tightly regulated, mainly through estrogen receptor 1 (ESR1). The mouse vaginal epithelium cyclically exhibits cell proliferation and differentiation in response to estrogen and provides a unique model for analyzing the homeostasis of stratified squamous epithelia. To address the role of ESR1-mediated tissue events during homeostasis, we analyzed mice with a vaginal epithelium-specific knockout of Esr1 driven by keratin 5-Cre (K5-Esr1KO). We show here that loss of epithelial ESR1 in the vagina resulted in aberrant epithelial cell proliferation in the suprabasal cell layers and led to failure of keratinized differentiation. Gene expression analysis showed that several known estrogen target genes, including erbB growth factor ligands, were not induced by estrogen in the K5-Esr1KO mouse vagina. Organ culture experiments revealed that the addition of erbB growth factor ligands, such as amphiregulin, could activate keratinized differentiation in the absence of epithelial ESR1. Thus, epithelial ESR1 integrates estrogen and growth factor signaling to mediate regulation of cell proliferation in squamous differentiation, and our results provide new insights into estrogen-mediated homeostasis in female reproductive organs.
Estrogens play important roles in vertebrate reproductive biology, with effects principally mediated through the nuclear estrogen receptors (ESRs). ESRs are members of the nuclear receptor superfamily and typically up-regulate gene transcription upon ligand binding. Two different genes encoding the two ESR subtypes, ESR1 (formerly named ERα) and ESR2 (formerly named ERβ), have been identified from a variety of vertebrate species. ESR1 is the predominant subtype regulating estrogen-mediated proliferation and differentiation of female reproductive organs. Administration of estrogens increases organ weights and promotes cell proliferation and differentiation, whereas such events were not evoked in the Esr1-deficient mice (1–3).
Estrogen actions on the uterus and mammary gland have been extensively analyzed. During the first step of estrogen action, epithelial–stromal interactions play a pivotal role in epithelial cell proliferation. In vitro tissue recombination experiments with Esr1 knockout (KO)- and wild-type-derived epithelium and stroma revealed that the effects of estrogen on uterine and mammary epithelial cell proliferation are mediated primarily via stromally expressed ESR1 (4–6). Estrogen-induced growth factors secreted from the stroma subsequently promote epithelial cell proliferation (7–10). Experiments using epithelial cell-specific KO of Esr1 revealed that estrogen can induce proliferation of uterine epithelial cells despite the absence of epithelial ESR1 (11). In contrast, similar experiments using a mammary epithelium-specific Esr1 KO showed that ESR1 is required in the epithelial cells for mammary gland growth and ductal epithelial cell proliferation (12). It is uncertain whether these differences result from different requirements for epithelial ESR1 in the uterus versus the mammary gland or whether they result from methodological differences in tissue recombination experiments (e.g., age of donor, hormone treatment protocol) (13). Irrespective of these differences, there are definite requirements for epithelial ESR1 expression in uterine epithelial functionalization (e.g., lactoferrin gene expression and prevention of cell death in the uterus), as well as in ductal morphogenesis, alveologenesis, and lactation in the mammary gland (11, 12).
Another female reproductive organ, the mouse vagina, exhibits a unique estrogen-responsive feature. The vaginal epithelium exhibits cyclical, estrogen-dependent cell proliferation and squamous differentiation during the estrous cycle. The vaginae of ovariectomized (OVX) mice show an atrophied epithelium with two to three cell layers, whereas estrogen administration rapidly induces epithelial cell proliferation in the basal layer. The suprabasal cells are no longer mitogenic but differentiate while moving up through the epithelium, resulting in keratinization of apical cells. The fully stratified and keratinized vaginal epithelium resembles the typical stratified and keratinized epidermis found in the skin and other organs.
The formation and maintenance of such epithelia relies on a tightly balanced process of epithelial cell proliferation and squamous differentiation. Classical tissue recombination experiments suggest that epithelial ESR1 in the vagina is dispensable for cell proliferation but is indispensable for squamous differentiation (5, 14). Until now, no mouse model for tissue-specific KO of Esr1 in the vagina has been developed. In this study, we used genetic analysis of ESR1 function in the vagina by creating an epithelium-specific Esr1 KO to provide new insights into the potential tissue-specific function of ESR1. We found that estrogen can induce cell proliferation in the absence of epithelial ESR1, but that the epithelial cells showed severe defects in keratinocyte commitment. We also found that amphiregulin (Areg) is required downstream of epithelial ESR1 for terminal differentiation in the epithelium. Thus, epithelial ESR1 integrates estrogen and growth factor signaling to mediate the switch between cell proliferation and squamous cell differentiation in the mouse vagina.
Results
Phenotypes of Epithelial-Specific Esr1 KO Mice.
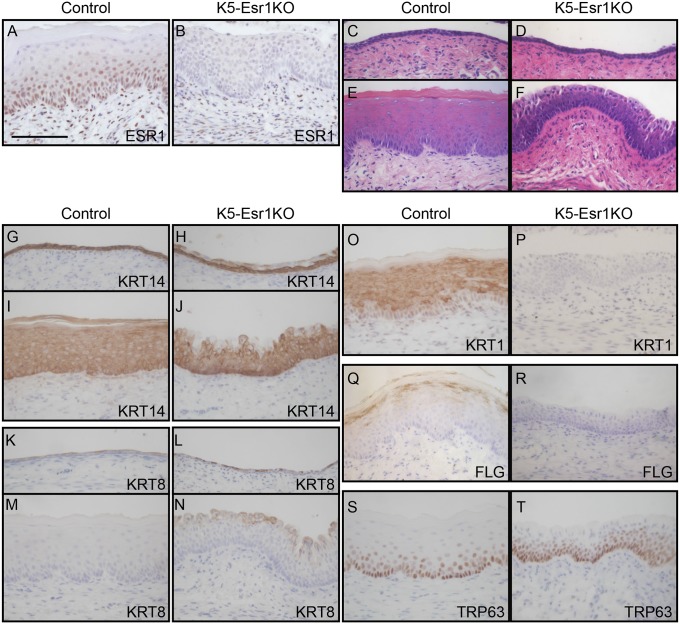
ESR1 protein is expressed in both the vaginal epithelium and the stroma in the wild-type mouse vagina (Fig. 1A). We generated a vaginal epithelial cell-specific Esr1 KO mouse model (K5-Esr1KO) by crossing Esr1-floxed mice with K5-Cre transgenic lines that express Cre recombinase in the vaginal epithelial cells (15). ESR1 protein was detected in the stroma, but not in the epithelium, demonstrating the epithelium-specificity of Esr1 loss of function in the K5-Esr1KO mouse vagina (Fig. 1B). We first tested whether estrogen induced cell proliferation and/or differentiation in the K5-Esr1KO mouse vagina, using OVX mice to avoid any confounding effects of the hypothalamus-pituitary-gonadal axis and to simplify analysis of estrogen effects. The vaginal epithelium of both 8-wk-old OVX control and K5-Esr1KO mice was composed of two to three layers of cuboidal cells (Fig. 1 C and D). 17β-Estradiol (E2) administration induced epithelial stratification and full keratinization in control mice (Fig. 1E), whereas the K5-Esr1KO mice exhibited stratified epithelia, but not keratinized differentiation (Fig. 1F). The epithelia of K5-Esr1KO mice treated with E2 were thinner than those of controls (58.4 ± 8.7 µm vs. 95.6 ± 8.4 µm; P < 0.05). These phenotypes were not a result of an insufficiency of E2 exposure (see the following long-term E2 exposure experiment). The K5-Esr1KO female mice are infertile, probably because of the expression of Cre recombinase in uterine epithelial cells (16).
Fig. 1.
Effects of epithelial cell-specific ablation of Esr1 on the mouse vagina. ESR1 protein expression was evaluated by immunohistochemistry in control (A) and K5-Esr1KO mice treated with E2 (B), indicating successful deletion of Esr1 specifically in the epithelial cells in the K5-Esr1KO mice. The vaginal epithelium of 8-wk-old OVX control (C) and K5-Esr1 (D) mice. E2 treatment induces vaginal epithelial stratification and keratinized differentiation in the control (E), but fails to such differentiation in the vaginal epithelium of K5-Esr1KO mice (F). Expression pattern of cell differentiation markers in vaginal epithelium of OVX (G, H, K, and L) and E2-treated (I, J, M–T) mice. Immunohistochemical staining for KRT14 (G–J), KRT8 (K–N), KRT1 (O and P), FLG (Q and R), and TRP63 (S and T). In K5-Esr1KO mice, differentiating cell-specific markers KRT1 and FLG are not expressed, even after E2 treatment (C–F). (Scale bar, 100 µm.)
Next we used immunohistochemical staining to further characterize the phenotypes of the K5-Esr1KO mouse vagina. The temporal and spatial expression profiles of specific keratins reflected the differentiation status of epithelial cells (17). The vaginal epithelial marker, keratin 14 (KRT14), was expressed throughout all layers of the vaginal epithelium (Fig. 1 G and I). KRT14 was expressed in basal and suprabasal cells but was absent in the apical cell layer in OVX and estrogen-treated K5-Esr1KO vaginae (Fig. 1 H and J). KRT8, a marker for undifferentiated cells, was expressed in a few suprabasal cells in the control OVX mouse vagina, but its expression was augmented in the OVX K5-Esr1KO mice (Fig. 1L). KRT8-positive cells were persistent in the apical layer of cells, even after estrogen treatment in K5-EsR1KO mice (Fig. 1 M and N). The stratified and squamous differentiation marker, KRT1, was expressed in the differentiating suprabasal cells upon estrogen-treatment in the control mouse vagina. Intriguingly, it was not detected in the K5-Esr1KO mice, although these animals did exhibit stratified epithelium (Fig. 1 O and P). Expression of the terminal differentiation marker, filaggrin (FLG), was absent in the K5-Esr1KO mice (Fig. 1 Q and R).
TRP63 is a crucial regulator of squamous differentiation and is no longer expressed once cells are committed to squamous epithelial lineages (18, 19). TRP63 was mainly expressed in the basal cells and in the two to three upper layers of cells adjacent to the basement membrane in the vagina of control mice (Fig. 1S) (20). Intriguingly, TRP63 expression was augmented and expanded into the superficial layer of the K5-Esr1KO mouse vagina (Fig. 1T). We infer from these results that epithelial cells in the K5-Esr1KO mouse vagina exhibit impaired commitment toward squamous differentiation and, therefore, are maintained in an undifferentiated state.
Loss of Epithelial ESR1 Impaired Cell Proliferation and Cell Death in Vagina.
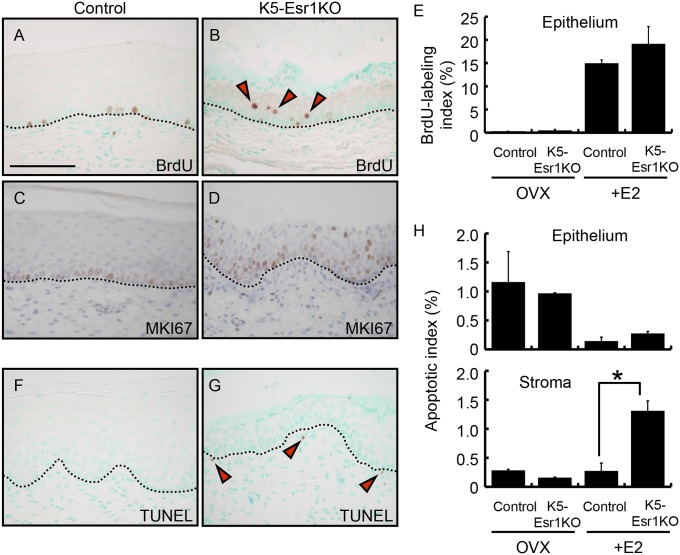
We investigated the proliferation status of vaginal epithelial cells using BrdU incorporation. The proliferation indices were not significantly changed between control and K5-Esr1KO vaginae with or without E2 treatment (Fig. 2 A, B, and E). Although cell proliferation was restricted in the basal cells of E2-treated control mouse vagina, several BrdU-positive cells were detected in the suprabasal cells in K5-Esr1KO mice (red arrowheads in Fig. 2B). Likewise, MKI67, another cell proliferation marker, was expressed in the basal cells in control vaginal epithelium, but it was robustly detected in the upper layer cells of vaginal epithelium in K5-Esr1KO mice (Fig. 2 C and D). Thus, suprabasal cells in K5-Esr1KO mouse vagina show proliferative potency.
Fig. 2.
Ectopic cell proliferation in the epithelium and cell death in the stroma of the K5-Esr1KO vagina. BrdU and MKI67 immunostaining in control (A and C) and K5-Esr1KO (B and D) mouse vagina. Note BrdU-positive cells in the upper layer of the K5-Esr1KO mice (red arrowheads in B). BrdU-labeling indices were not altered between control and K5-Esr1KO in the OVX and E2-treated group (E). TUNEL assay indicates loss of ESR1 has not influenced cell apoptosis in the vaginal epithelium, but increase in vaginal stromal cell death in the E2-treated K5-Esr1KO mice (F–H). Black arrowheads in G indicate apoptotic cells in the stroma. Dotted lines indicate the basal layer of the epithelium. (Scale bar, 100 µm.)
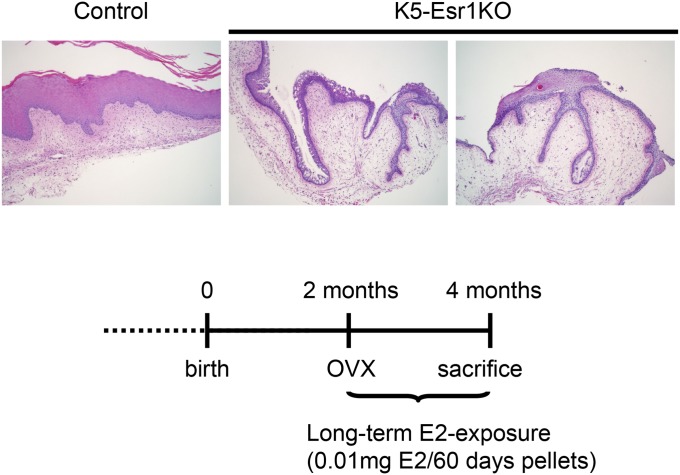
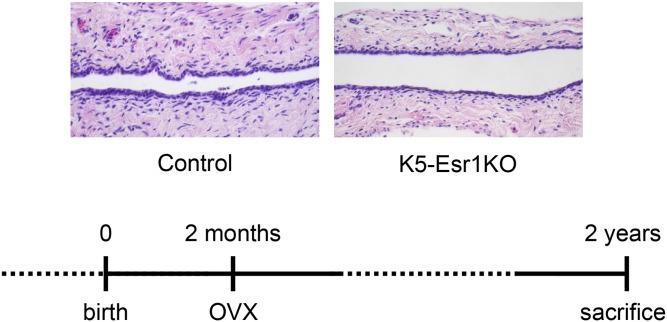
Taken together, these data indicate that epithelial cells in the K5-Esr1KO mouse vagina remain in an undifferentiated state and retain proliferative potency, even after moving out from the basal cell layer. We next tested whether prolonged E2 exposure could enhance cell proliferation and result in a cancerous lesion. We found that the epithelium did not exhibit keratinization, although some abnormal epithelial pathology, including down-growth into the stroma, was observed in the K5-Esr1KO mouse vagina 2 mo after implantation of an E2 pellet (Fig. S1). In the absence of estrogens, vaginal epithelial cells did not proliferate, and K5-Esr1KO mice exhibited atrophied vaginal epithelia, even in 2-y-old animals (Fig. S2).
Fig. S1.
Long-term E2 exposure induces invagination of epithelial cells in the vagina of K5-Esr1KO mice. n = 5. (Scale bar, 100 µm.)
Fig. S2.
HE staining of vaginae of 2-y-old OVX-control and OVX-K5-Esr1KO mice, as treated with experimental design. n = 5. (Scale bar, 100 µm.)
We also examined cell death in the K5-Esr1KO mouse vagina. Apoptotic indices in vaginal epithelial cells as measured by TUNEL staining did not differ between controls and K5-Esr1KO mice (Fig. 2 F–H). However, the apoptotic index was augmented in the stroma of estrogen-treated K5-Esr1KO mice compared with controls (Fig. 2 F–H), suggesting that epithelial ESR1 contributes to maintaining stromal cell survival.
Evaluation of E2-Induced Gene Expression in K5-Esr1KO Mouse Vagina.
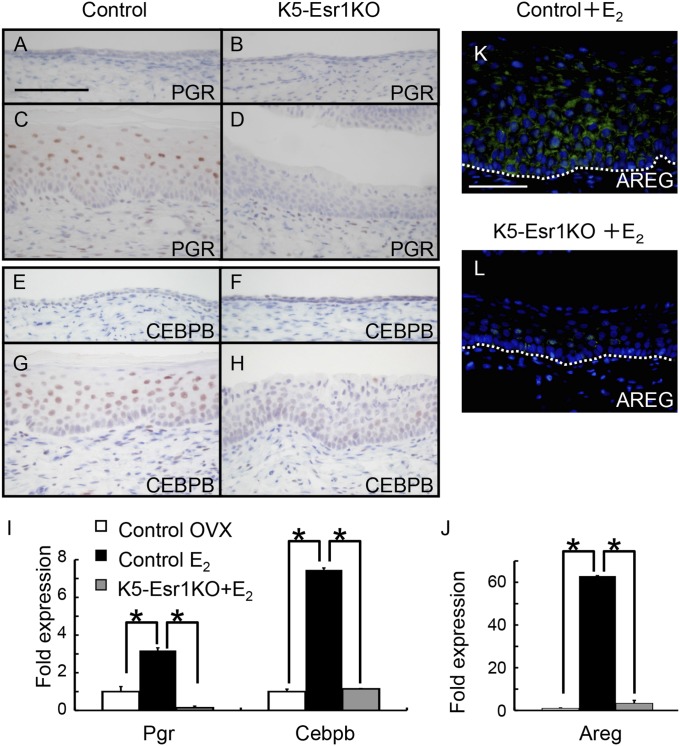
We next evaluated expression of progesterone receptor (PGR) and CCAAT/enhancer-binding protein β (CEBPB) (14). Both genes were induced by E2 administration in the control mouse vagina, and their proteins were localized in the epithelium (Fig. 3 A–I). Expression of these genes was not induced by E2 administration in the K5-Esr1KO mouse vagina. It has been reported that PGR-mediated signaling inhibits vaginal epithelial cell proliferation (21) and that CEBPB is indispensable for epidermal cell differentiation (22). Therefore, the failure of E2 to induce expression of these genes in the K5-Esr1KO mouse vagina may be associated with the observed phenotypes.
Fig. 3.
Expression patterns of cell differentiation markers in the mouse vagina. Immunohistochemistry of PGR (A–D) and CEBPB (E–H). Both proteins are detected in the suprabasal layer of differentiating vaginal epithelial cells of the control mice treated with E2 (C and G), but not in the K5-Esr1KO mice (B and H). Expression of Pgr, Cebpb, and Areg mRNAs failed to increase in the vagina of E2-treated K5-Esr1KO mice (I and J). AREG protein is detected in the vaginal epithelium in the control mice treated with E2 (K), but not in the K5-Esr1KO mice (L). (Scale bar, 100 µm.)
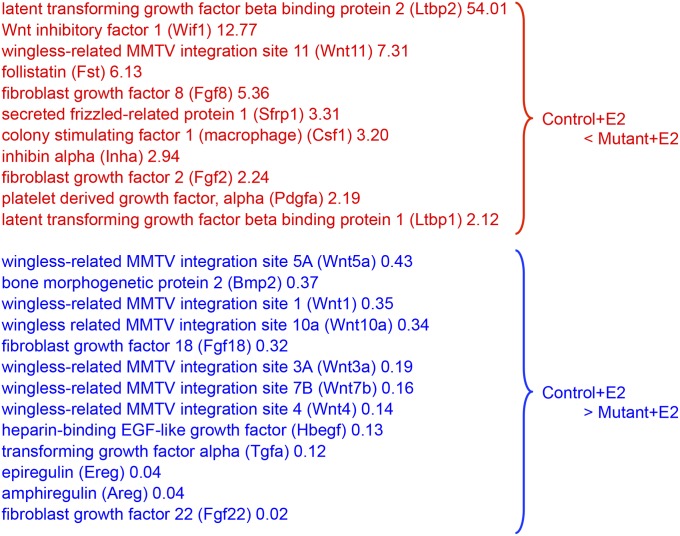
To further analyze genes involved in vaginal epithelial cell differentiation, we conducted DNA microarray analysis of vaginae from control and K5-Esr1KO mice treated with E2 (Dataset S1). We found that several secreted growth factors were differentially expressed (Fig. S3). Of those, we focused on the erbB ligand, amphiregulin (Areg), because erbB ligands have been implicated as effectors of ESR1 in mammary gland development (23). Areg is also involved in keratinocyte growth and is associated with psoriasis, a hyperproliferative skin disorder (24, 25). Expression of Areg was increased after E2 administration in the control mouse vagina, but its expression was persistently low in the K5-Esr1KO vagina (Fig. 3J). AREG was exclusively expressed in the vaginal epithelium in controls, but expression was not observed in the K5-Esr1KO mice (Fig. 3 K and L), suggesting an autocrine mechanism for epithelial ESR1 signaling.
Fig. S3.
Expression profiles for secreted growth factor genes from DNA microarray analysis.
Areg Is a Mediator of Vaginal Epithelial Cell Differentiation.
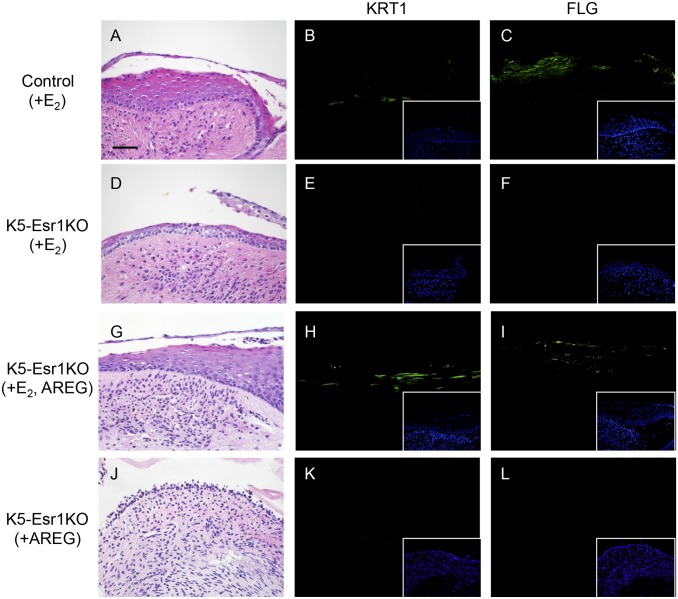
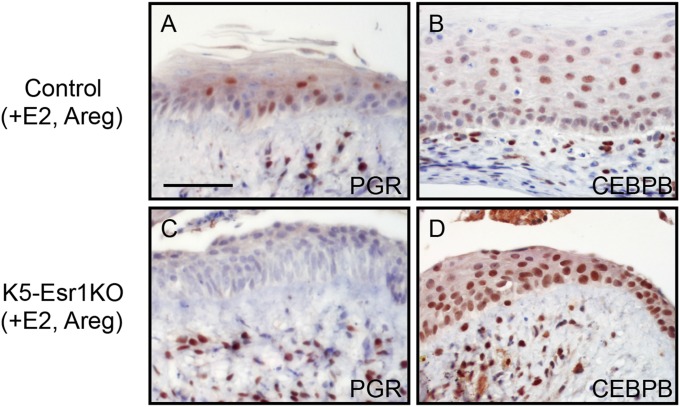
We next evaluated the effects of AREG on vaginal epithelial cell differentiation, using an organ culture system. Epithelial cells exhibited keratinized cell morphology and expressed KRT1 and FLG in organ-cultured vaginae from control mice treated with E2 (Fig. 4 A–C). In contrast, neither KRT1 nor FLG was expressed in vaginae from K5-Esr1KO mice treated with E2 (Fig. 4 D–F). However, the addition of AREG and E2 induced KRT1 and FLG expression, together with keratinized morphology (Fig. 4 G–I). In the absence of E2 supplementation, AREG alone could not induce KRT1 or FLG (Fig. 4 J–L), suggesting AREG could not bypass the whole effects of E2 in the mouse vagina. To investigate potential epistatic relationships among Areg, Pgr, and Cebpb, immunohistochemistry was performed using organ-cultured vaginae supplemented with E2 and AREG. Both PGR and CEBPB were expressed in the control mouse vagina (Fig. 5 A and B). PGR was not expressed, although CEBPB was induced, in the epithelial cells of K5-Esr1KO vaginae (Fig. 5 C and D). These data indicate that CEBPB is likely to be a downstream target of erbB signaling, rather than a direct target of epithelial ESR1.
Fig. 4.
AREG as a potential factor for vaginal epithelial cell differentiation. HE staining (A, D, G, and J) and immunohistochemistry for KRT1 (B, E, H, and K) and FLG (C, F, I, and L) in organ-cultured vagina. Organ-cultured vaginae from control mice treated with E2 express KRT1 and FLG (B and C), but the vaginae of K5-Esr1KO mice do not (E and F). Note that addition of AREG leads to KRT1 and FLG expression (H and I). (Insets) Counterstaining with Heckest. (Scale bar, 100 µm.)
Fig. 5.
AREG induces CEBPB in vagina of K5-Esr1KO mouse. Immunohistochemistry of PGR (A and C) and CEBPB (B and D) in organ-cultured vagina. Organ-cultured vaginae treated with E2 and AREG from control mice express PGR and CEBPB (A and B). In the K5-Esr1KO mouse vagina, addition of AREG fails to express PGR (C) but rescues CEBPB expression (D). (Scale bar, 100 µm.)
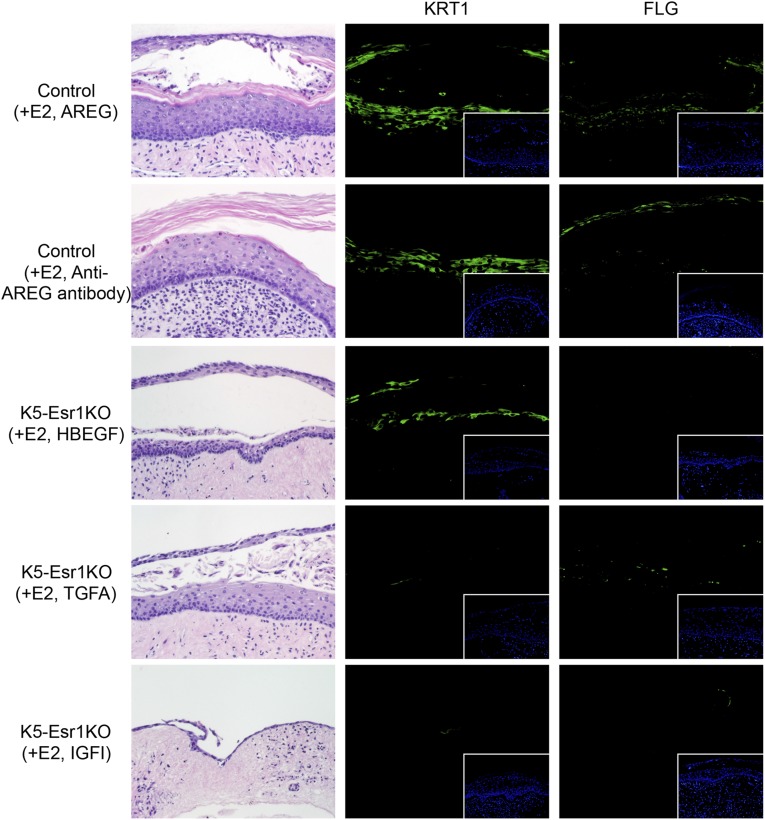
Organ-cultured vaginae from wild-type mice treated with E2 and anti-AREG antibody showed epithelial keratinization and expressed KRT1 and FLG (Fig. S4). These results suggest that the amphiregulin effects might be redundant. We further examined the selectivity of erbB ligands for vaginal epithelial cell differentiation, including heparin-binding EGF-like protein (HBEGF) and transforming growth factor α (TGFA). We also tested insulin-like growth factor-I (IGF1), which is assumed to be a stromal factor that activates epithelial cell proliferation (10, 26). HBEGF induced expression of KRT1, but not FLG (Fig. S4). TGFA had a minimal effect on KRT1 and FLG expression. IGF1 did not induce KRT1 or FLG expression, and the epithelium was atrophied (Fig. S4). Thus, although there is some redundancy among erbB ligands, AREG is the most active inducer of squamous cell differentiation in the mouse vaginal epithelium.
Fig. S4.
Organ-cultured vagina from K5-Esr1KO and control mice exposed to E2 with AREG, anti-AREG antibody, HBEGF, TGFA, or IGF1. HE staining and immunohistochemistry for KRT1 and FLG.
Discussion
Organ growth, development, and function are regulated by complex interactions among cells and tissues. Female reproductive organs are excellent models to analyze such interactions because epithelial cell proliferation and differentiation can be completely controlled by estrogen. Classical tissue recombination experiments have suggested that epithelial cell proliferation in female reproductive organs (e.g., uterus, vagina, and mammary gland) is mediated by stromal ESR1 in a paracrine manner (4–6). In contrast, genetic studies in mouse models have shown that epithelial ESR1 is required for cell proliferation in the mammary gland, but not in the uterus (11, 12). Therefore, to clarify and extend those observations, we conducted Esr1KO in the mouse vagina and demonstrated that vaginal epithelial cells can proliferate in the absence of epithelial ESR1 in vivo.
The vaginal epithelium is a stratified keratinized squamous type similar to that in the epidermis. Therefore, further cell and tissue interactions are required for coordinated and fine-tuned morphogenetic events. Stratified squamous epithelia are maintained by proliferation of basal keratinocytes and their subsequent coordinated cell cycle exit, migration into the suprabasal layers, and terminal differentiation. In wild-type mice, suprabasal cells exit the cell cycle and express the differentiation marker KRT1 and the terminal keratinized cell marker FLG in the upper layers. However, in the absence of epithelial ESR1, differentiation markers were not expressed, in accord with previous tissue recombination experiments (5, 14). We also found that vaginal epithelial cells in the K5-Esr1KO mouse continue to proliferate, even after basal layers are removed, indicating that the suprabasal cells remain undifferentiated and proliferative while failing to differentiate into keratinocytes. Therefore, we infer that epithelial ESR1 is indispensable for keratinocyte commitment and maintenance of epithelial homeostasis by controlling cell differentiation. This is in contrast to the role of epithelial ESR1 in the uterus, where epithelial ESR1 is involved in the maintenance of epithelial cell survival by preventing excessive apoptosis, likely as a result of inactivation of cell cycle progression-related genes (11, 27). Subsequently, loss of ESR1 results in enhanced apoptosis in the uterine epithelium (11); such apoptosis was not observed in the vaginal epithelium of K5-Esr1KO mice.
These results suggest that loss of ESR1 in the vaginal epithelium impaired the action of E2-mediated secondary factors mediating squamous differentiation. We investigated known estrogen target genes such as Pgr and Cebpb in the epithelia. Pgr regulation by estrogens has been well studied in the female reproductive tracts, using tissue recombination experiments that showed that Pgr expression is controlled by stromal ESR1 in the uterus and epithelial ESR1 in the vagina (28, 29). These results are consistent with genetic mouse models in both organs (current study and ref. 11). In general, progesterone-PGR signaling acts antagonistically to E2-induced effects (21, 30). Progesterone signaling that would ordinarily inhibit E2-induced epithelial cell proliferation was impaired in the uterine epithelium of the Esr1KO mouse (27). Thus, loss of epithelial PGR expression might contribute to dysregulation of the proliferative response to estrogen in the K5-Esr1KO mouse vagina.
CEBPB is another estrogen-regulated transcription factor in female reproductive organs (31, 32). CEBPB is a key regulator of proliferation and/or differentiation in multiple tissues, including squamous epithelia. Epidermal ablation of CEBPB leads to increased basal keratinocyte proliferation and severe defects in keratinocyte commitment and differentiation, despite some redundancy with CCAAT/enhancer-binding protein α (Cebpa) (22). CEBPB also plays a crucial role in restricting TRP63 expression to basal keratinocytes (22). These phenotypes coincide with the results shown here in the K5-Esr1KO mouse vagina and support an important role for CEBPB in squamous differentiation in the vagina.
ErbB signaling has long been implicated downstream of steroid hormone signaling in female reproductive organs (8, 9, 33). In the current study, we found that the erbB ligand, AREG, is expressed specifically in the epithelium, dependent on epithelial ESR1 expression. Organ culture experiments revealed that AREG can compensate for the loss of epithelial ESR1 on squamous differentiation with respect to KRT1, FLG, and CEBPB expression. Furthermore, AREG is the most active of the erbB ligands and growth factors we examined in rescuing the effects of Esr1 loss of function. Therefore, AREG is likely to be a key intrinsic factor required for squamous differentiation in the mouse vagina. This indirect signaling mechanism, mediated through secreted growth factors, enables the amplification and systematic coordination of estrogen stimulation within the target cells/tissues. In contrast, neither AREG nor other growth factors examined could alone induce epithelial cell growth and squamous differentiation. Hence, we propose that additional factors downstream of Esr1 in the epithelium and/or stroma are necessary for full activation of estrogen effects in the vagina and the coordinated crosstalk between growth factors and estrogens that is essential for female reproductive organ homeostasis. Intriguingly, the stromal cells exhibit increased cell death when epithelial ESR1 is lost. We hypothesize that secreted factors derived from the epithelium act on the stroma to protect against stromal cell death and are currently investigating the existence of these factors. The number of apoptotic cells was not altered by supplementation of growth factors examined in the current organ culture study.
Estrogen actions need to be tightly regulated, and it is conceivable that dysregulation of estrogen signaling results in reproductive disorders, including tumorigenesis. Squamous cell carcinoma is the most common invasive malignancy of the cervix, vagina, and vulva (34). Although much remains to be learned about the underlying molecular mechanisms, loss of ESR1 has been associated with squamous cell carcinoma progression and invasion (35). Although we showed dysregulated epithelial cell proliferation and differentiation in the K5-Esr1KO mouse vagina and found that prolonged estrogen exposure induced abnormal epithelial invagination into the stroma, cancerous lesions and indications of metastatic phenotypes (e.g., effects on basement membrane) were not observed. This suggested that loss of the epithelial ESR1 signal alone is not sufficient to cause squamous cell carcinoma. Therefore, other factors are likely to be involved in carcinogenesis and invasive phenotypes, and future studies are needed to further clarify tissue-specific roles of ESR1 for carcinogenesis, invasion, and metastasis. A different mouse background should also be tested, because the current mouse background (C57BL6) is genetically resistant to such cancerous lesions from estrogen treatment.
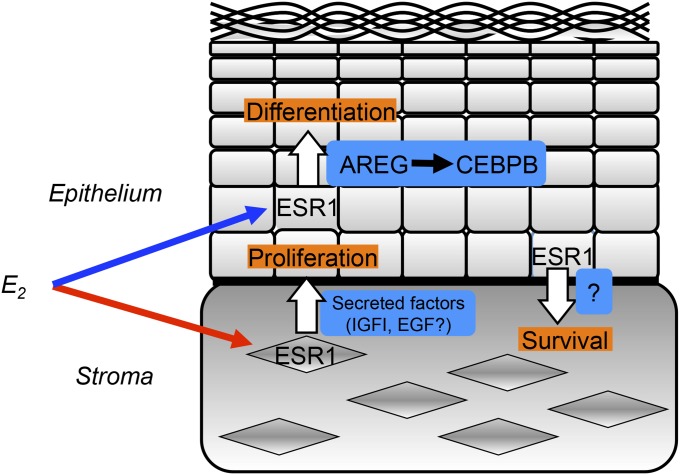
In summary, our mouse model demonstrated that the vaginal epithelium undergoes cell proliferation mediated by the stromal ESR1, whereas differentiation is promoted by epithelial ESR1 (Fig. 6). Loss of epithelial ESR1 leads to failure of commitment of cells to keratinized differentiation, resulting in aberrant epithelial cell proliferation under stimulation by stromal ESR1. Therefore, ESR1 is required in the vaginal epithelium for appropriate response to estrogens and tissue homeostasis. It is intriguing that ESR1 has unique roles and differentially mediates estrogen action in the epithelia of the mammary gland, uterus, and vagina. Overall, an improved understanding of estrogen action at the tissue level will shed light on the mechanisms of estrogen-mediated homeostasis and underlying disorders in female reproductive organs such as endometriosis, endometrial hyperplasia, or cancer.
Fig. 6.
A possible scheme depicting estrogen-induced cell proliferation and differentiation and its regulation of homeostasis in mouse vagina.
Materials and Methods
Mouse and Treatment.
C57BL/6J (CLEA), K5-Cre (36), Esr1-null, and Esr1-floxed mice (1) were maintained under 12 h light/12 h dark at 23–25 °C and fed laboratory chow (CA-1; CLEA) and tap water ad libitum. To obtain vaginal epithelial cell-specific Esr1KO mice (K5Cre/+;Esr1flox/-), K5Cre/+;Esr1+/− male were crossed with Esr1flox/flox female mice. For control mice, Cre-negative-Esr1flox/+ siblings were used. In most experiments, mice were OVX at 6 wk of age and killed at 8 wk of age. For examining effects of estrogen, a single daily injection of 0.1 µg E2 (Sigma) was given to OVX mice for 3 d; the mice were killed 24 h after the last injection. This timing is sufficient to allow induction of a stratified and fully keratinized epithelium in the mouse vagina. For a long treatment, OVX mice were implanted with E2 pellets (0.01 mg per pellet, 2 mo; Innovative Research of America) into s.c. tissue for 2 mo. All procedures and protocols were approved by the institutional animal care and use committee at the National Institute for Basic Biology.
Histology and Immunohistochemistry.
Hematoxylin/eosin staining was performed by a standard procedure. The significance of differences in the thickness of epithelia between control and K5-Esr1KO mice was assessed by Welch's t test. For immunohistochemistry, paraformaldehyde-fixed, paraffin-embedded sections were incubated with the following primary antibodies: ESR1, TRP63, PGR, CEBPB (Santa Cruz), KRT8 (Progen), KRT1, KRT14, FLG (Covance), MKI67 (Thermo Fisher Scientific), and AREG (R&D Systems). The sections were stained with the Vectastain ABC Kit (Vector Laboratories). Immunofluorescence analysis was performed with Alexa Fluor protein-conjugated secondary antibodies (Life Technologies) and counterstained with Hoechst 33342 (Sigma).
For BrdU immunostaining, mice were injected with BrdU (Sigma) at 100 mg/kg body weight. One hour after the injection, tissues were collected. BrdU-incorporated cells were detected with anti-BrdU antibody (1:20, Roche). TUNEL assay for the detection of apoptotic cells was performed with the in situ apoptosis detection kit (Roche). Statistical analyses were performed using Student's t test or Welch's t test, followed by F-test; differences with P < 0.05 were considered significant. Error bars represent the SEM. More than four (immunohistochemistry) or six (BrdU and TUNEL) animals were analyzed; representative pictures are shown.
Quantitative RT-PCR.
Total RNA (2 µg), isolated from each group using an RNeasy kit (Qiagen), was used in RT-PCR reactions carried out with SuperScript III reverse transcriptase and SYBR Green Master Mix (Life Technologies), according to manufacturer’s instructions. PCR conditions and sequences of some primer sets are given in a previous report (33). Others used are Pgr, TAT GAG AAC CCT TGA CGG TGT TG, CAG GGC CTG GCT CTC GTT A; Cebpb, CGG GTT TCG GGA CTT GAT GCA AT, CTA GAC AGT TAC ACG TGT GTT GCG. Relative RNA equivalents for each sample were obtained by standardization of ribosomal protein L8 levels. More than three pools of samples per group were run in triplicate to determine sample reproducibility. Error bars represent the SE, with all values represented as fold change compared with the control treatment group normalized to an average of 1.0. Statistical analysis was performed by ANOVA followed by Dunnett’s test; differences with P < 0.05 were considered significant. Error bars represent the SEM.
DNA Microarray.
The quality of Cyanine3-labeled cRNA was analyzed using the Agilent 2100 Bioanalyzer (Agilent). Cyanine3-labeled cRNA was prepared using the Quick Amp Labeling Kit and One-color RNA Spike-in kit (Agilent) and purified using the RNeasy Micro Kit (Qiagen) following the manufacturer’s protocol. The quality of cRNA was analyzed using the Agilent 2100 Bioanalyzer (Agilent). cRNA (1.65 µg) was hybridized to the 4 × 44K mouse gene expression microarray (G4122F), using standard Agilent protocols. After 17 h incubation with rotation, the microarrays were washed with Gene Expression Wash Buffer Kit (Agilent) according to the manufacturer’s protocols. DNA microarrays were scanned using a GenePix 4000B scanner (Molecular Devices) at 5 µm resolution. The signal intensity of the spots was digitized using the microarray imager software (Combimatrix Molecular Diagnostics). Gene expression and statistical analyses were performed using the Subio Platform (Subio Inc). Data files generated by the Agilent Feature Extraction Software were imported into Subio Platform. The digitized raw data were normalized by the mean value of all spots of signal intensity in each array. Signal intensities (gProcessedSignal) were normalized by 75th percentile and transformed into log ratios (base 2). We merged duplicates of each experimental condition and applied statistical analysis on the averages. Subsequently, these data were assessed by t test (P < 0.05) to identify differentially expressed genes between treatment groups.
Organ Culture.
Eight volumes of Cellmatrix type I-A (Nitta Gelatin) were mixed with 1 volume 10× Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture Ham’s F-12 (DMEM/F12; Sigma) and 1 volume 200 mM Hepes buffer containing 262 mM NaHCO3 and 0.05 N NaOH was added to the mixture. This cold gelatin mixture (200 µL) was poured into inner millicell cell culture inserts (Merck) placed into the well of a 24-well plate and allowed to gel at 37 °C. Vaginae of mice aged 3 wk were cut and placed into Hank's Balanced Salt Solution (Life Technologies). Tissues were washed three times in Hank's Balanced Salt Solution and mixed with fresh 200-µL cold gelation mixture. Tissues and gelation mixture were overlaid onto a base of gelled collagen in each cell culture insert and allowed to gel at 37 °C. Subsequently, 200 µL DMEM/F12 supplemented with 20% (vol/vol) charcoal/dextran-treated FBS (HyClone) was added to each well, and tissues were cultured at 37 °C in a humidified, 5% CO2/air atmosphere for 3 d. Recombinant AREG, HBEGF, TGFΑ (R&D systems), IGF1 (JRH Biosciences; 0.5 µg/µL), and anti-AREG antibody (5 µg/mL) were added to the medium from day 0 of culture. Tissues for each group were collected from at least 4 mice.
Supplementary Material
Acknowledgments
We thank Dr. J. Takeda (Osaka University) and Pierre Chambon (Institute for Genetics and Cellular and Molecular Biology) for providing K5-Cre and ERα-floxed mice, respectively. We also thank Dr. I. Hirakawa and Dr. H. Miyakawa (National Institute for Basic Biology) and Dr. T. Nakajima and Dr. T. Sato (Yokohama City University) for critical advice on the DNA microarray and organ culture. We are grateful to Dr. B. Blumberg (University of California at Irvine) for his critical readings of the manuscript. This study was supported by a Grant-in-Aid for Scientific Research B from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a Health Sciences Research Grant from the Ministry of Health, Labour and Welfare, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513550112/-/DCSupplemental.
References
- 1.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 2.Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 3.Lubahn DB, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha GR, et al. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J Mammary Gland Biol Neoplasia. 1997;2(4):393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan DL, et al. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology. 1998;139(10):4345–4352. doi: 10.1210/endo.139.10.6241. [DOI] [PubMed] [Google Scholar]
- 6.Cooke PS, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94(12):6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson KG, Sakai Y, Eitzman B, Steed T, McLachlan J. Exposure to diethylstilbestrol during a critical developmental period of the mouse reproductive tract leads to persistent induction of two estrogen-regulated genes. Cell Growth Differ. 1994;5(6):595–606. [PubMed] [Google Scholar]
- 8.Ignar-Trowbridge DM, et al. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci USA. 1992;89(10):4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson KG, Takahashi T, Bossert NL, Walmer DK, McLachlan JA. Epidermal growth factor replaces estrogen in the stimulation of female genital-tract growth and differentiation. Proc Natl Acad Sci USA. 1991;88(1):21–25. doi: 10.1073/pnas.88.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghahary A, Chakrabarti S, Murphy LJ. Localization of the sites of synthesis and action of insulin-like growth factor-I in the rat uterus. Mol Endocrinol. 1990;4(2):191–195. doi: 10.1210/mend-4-2-191. [DOI] [PubMed] [Google Scholar]
- 11.Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor α is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA. 2010;107(45):19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci USA. 2007;104(37):14718–14723. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller SO, Clark JA, Myers PH, Korach KS. Mammary gland development in adult mice requires epithelial and stromal estrogen receptor alpha. Endocrinology. 2002;143(6):2357–2365. doi: 10.1210/endo.143.6.8836. [DOI] [PubMed] [Google Scholar]
- 14.Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Müllerian) epithelial differentiation. Dev Biol. 2001;240(1):194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- 15.Miyagawa S, Sato M, Sudo T, Yamada G, Iguchi T. Unique roles of estrogen-dependent Pten control in epithelial cell homeostasis of mouse vagina. Oncogene. 2015;34(8):1035–1043. doi: 10.1038/onc.2014.62. [DOI] [PubMed] [Google Scholar]
- 16.Villacorte M, et al. β-Catenin signaling regulates Foxa2 expression during endometrial hyperplasia formation. Oncogene. 2013;32(29):3477–3482. doi: 10.1038/onc.2012.376. [DOI] [PubMed] [Google Scholar]
- 17.Gimenez-Conti IB, et al. Expression of keratins in mouse vaginal epithelium. Differentiation. 1994;56(3):143–151. [PubMed] [Google Scholar]
- 18.Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398(6729):714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 19.Mills AA, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398(6729):708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 20.Kurita T, Mills AA, Cunha GR. Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development. 2004;131(7):1639–1649. doi: 10.1242/dev.01038. [DOI] [PubMed] [Google Scholar]
- 21.Yoo YA, et al. Progesterone signaling inhibits cervical carcinogenesis in mice. Am J Pathol. 2013;183(5):1679–1687. doi: 10.1016/j.ajpath.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez RG, et al. C/EBPalpha and beta couple interfollicular keratinocyte proliferation arrest to commitment and terminal differentiation. Nat Cell Biol. 2009;11(10):1181–1190. doi: 10.1038/ncb1960. [DOI] [PubMed] [Google Scholar]
- 23.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci USA. 2007;104(13):5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoll SW, Johnson JL, Li Y, Rittié L, Elder JT. Amphiregulin carboxy-terminal domain is required for autocrine keratinocyte growth. J Invest Dermatol. 2010;130(8):2031–2040. doi: 10.1038/jid.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook PW, et al. Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Res. 1992;52(11):3224–3227. [PubMed] [Google Scholar]
- 26.Miyagawa S, et al. Persistent gene expression in mouse vagina exposed neonatally to diethylstilbestrol. J Mol Endocrinol. 2004;32(3):663–677. doi: 10.1677/jme.0.0320663. [DOI] [PubMed] [Google Scholar]
- 27.Winuthayanon W, Hewitt SC, Korach KS. Uterine epithelial cell estrogen receptor alpha-dependent and -independent genomic profiles that underlie estrogen responses in mice. Biol Reprod. 2014;91(5):110. doi: 10.1095/biolreprod.114.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurita T, Lee KJ, Cooke PS, Lydon JP, Cunha GR. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod. 2000;62(4):831–838. doi: 10.1095/biolreprod62.4.831. [DOI] [PubMed] [Google Scholar]
- 29.Kurita T, et al. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62(4):821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331(6019):912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantena SR, et al. C/EBPbeta is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci USA. 2006;103(6):1870–1875. doi: 10.1073/pnas.0507261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson GW, Johnson PF, Hennighausen L, Sterneck E. The C/EBPbeta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 1998;12(12):1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyagawa S, Katsu Y, Watanabe H, Iguchi T. Estrogen-independent activation of erbBs signaling and estrogen receptor alpha in the mouse vagina exposed neonatally to diethylstilbestrol. Oncogene. 2004;23(2):340–349. doi: 10.1038/sj.onc.1207207. [DOI] [PubMed] [Google Scholar]
- 34.Platz CE, Benda JA. Female genital tract cancer. Cancer. 1995;75(1) Suppl:270–294. doi: 10.1002/1097-0142(19950101)75:1+<270::aid-cncr2820751312>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 35.Zhai Y, et al. Loss of estrogen receptor 1 enhances cervical cancer invasion. Am J Pathol. 2010;177(2):884–895. doi: 10.2353/ajpath.2010.091166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarutani M, et al. Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proc Natl Acad Sci USA. 1997;94(14):7400–7405. doi: 10.1073/pnas.94.14.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.