Abstract
BACKGROUND
Strategic vibration of musculotendinous regions of a limb elicits illusionary sensations of movement. As a rehabilitation technique, this ‘kinesthetic illusion’ has demonstrated beneficial results for numerous sensory-motor disorders. However, literature shows little consistency in the vibration parameters or body positioning used, and their effects have yet to be comprehensively investigated.
OBJECTIVE
To characterize the effects of the vibration amplitude, frequency, and limb position on the kinesthetic illusion.
METHODS
Movement illusions were induced in 12 participants’ biceps and triceps. The effect of amplitude (0.1 to 0.5 mm), frequency (70 to 110 Hz), and two limb positions were quantified on the strength of illusion (SOI), range of motion (ROM) and velocity.
RESULTS
Amplitude significantly affected the illusionary SOI, ROM and velocity in the biceps and triceps (p < 0.05). Increasing amplitude resulted in an increase of all three output variables. Limb position showed an effect on illusionary velocity in the biceps as well as ROM and velocity in the triceps (p < 0.05). Frequency demonstrated no statistical effect.
CONCLUSIONS
Amplitude demonstrated the most profound impact on the kinesthetic illusion in the experimental ranges tested. This work may help guide clinicians and researchers in selecting appropriate vibratory parameters and body positions to consistently elicit and manipulate the kinesthetic illusion.
Keywords: Kinesthetic illusion, vibration illusion, movement illusion, factorial design
1. Introduction
The kinesthetic illusion is a physiological phenomenon by which the introduction of vibration to a musculotendinous region of a limb will induce sensations that the limb is moving although it remains stationary. In fact these sensations can be so strong that participants have reported experiencing joints bending beyond physiological limits [1], or experienced illusionary distortion of objects or body parts in contact with the stimulated limb [2]. First published in 1972, Goodwin et al., reported participants experiencing sensations of elbow extension with the introduction of vibration to their distal biceps tendon; and elbow flexion with vibration of the distal triceps tendon [3]. They hypothesized that this phenomenon is a result of the vibrational stimulus exciting muscle spindle receptors. These afferent sensory organs, located in the belly of the muscle, are primarily responsible for detecting changes in muscle length. Most literature reports focusing vibration on the muscle tendon or musculotendinous regions [4,5] as strong vibration of these locations will induce small rapid cyclical changes in muscle length. When introduced at appropriate frequencies, this rapid physical stretching of the muscle produces a powerful excitatory response in muscle spindle activity [6]. Since first being published in 1972, these movement illusions have been incorporated in numerous rehabilitative and research applications.
The kinesthetic illusion elicits sensations of limb movement without dependence on a patient’s motor abilities. This has shown particular utility in research applications and treatment of those affected by neuromuscular disorders. For example, Rinderknecht et al., found that the kinesthetic illusion paired with virtual reality enhances the positive effects of motor imagery therapies in patients with upper limb paralysis following stroke [7]. The aim of their work was to induce and support plastic processes in affected brain regions to promote the ability to perform basic gestures like grasping. They suggest that virtual reality to visualize movement of a patients paralyzed hand, couple with illusionary sensations of movement, may provide a feasible rehabilitative technique for improved hand motorcontrol [7,8]. In children with cerebral palsy, Redon-Zouiteni et al. found that proprioceptive stimulation, through tendon vibration, resulted in improved upper body posture [9]. The kinesthetic illusion has also shown promise in the treatment of patients with spasticity. Krueger-Beck et al. found that the induction of movement illusions in a particular muscle group can reduce the level of involuntary activity [10]. Further applications of the kinesthetic illusion can be found in literature on the research and treatment of those suffering from complex-regional pain syndrome [11], treatment of lower back pain [12] and dystonia or essential tremor [13–16], among others [17–20].
However, the effective use of the kinesthetic illusion in a rehabilitative or research setting is fundamentally dependent on understanding how to introduce vibration such that this phenomenon can be consistently elicited and manipulated. The vibratory stimulus is most often presented to the participants in a sinusoidal waveform, with some exceptions [21–23]. Therefore this stimulus can be defined by two parameters, frequency and amplitude. However, the effects that frequency and amplitude have on experienced movement sensations are not always clear in previous reports. Early research by Roll et al., attempted to evaluate the effect of vibration frequency on the perceived movement velocity. By systematically manipulating frequency, they determined that perceived velocity increased when vibratory frequency was increased from 10 to 70 Hz. A further frequency increase in the range of 80 to 120 Hz resulted in a reduction of perceived velocity [21]. However, this study did not investigate amplitude effects, and allowed amplitude to vary between 0.2 to 0.5 mm. As this variation was not statistically blocked, it may have functioned as a confounding variable in their findings. Regardless, this work has led to the suggestion that 80 Hz produces the “optimal” illusion [5]. Yet, Clark argued that amplitude has a strong influence on the kinesthetic illusion and that decreasing amplitude results in a decreased velocity of the movement illusion [24]. Little follow up research has been conducted to investigate these claims; and arguably, amplitude effects have yet to be studied thoroughly.
As a result, literature shows little consistency in the vibratory values used to achieve the kinesthetic illusion. The original work performed by Goodwin et al. was successful in eliciting movement sensations using a hand held vibrator producing a frequency of 100 Hz and 1 mm amplitude (neutral-to-peak) [3]. Subsequent literature has reported eliciting the kinesthetic illusion with frequencies ranging from 10 Hz [21,25] to 160 Hz [26]. Studies not specifically focusing on the effects of low frequency have conducted testing with values as low as 60 Hz [27] and a high as 160 Hz [26]. The second parameter defining sinusoidal vibration is amplitude. In previous literature, this parameter also varies widely ranging from 0.2 mm [21,23,28] to 6 mm [29] (neutral-to-peak); although most often, 0.2 mm through 1 mm (neutral to peak). It may also be noted that, numerous authors neglect to define the vibration amplitude used in their studies [2,22,24,30–33]. As sinusoidal vibration is defined by both frequency and amplitude, it is difficult to state the importance of one variable without fully defining the other. A comprehensive study of the effects of vibratory parameters would require systematic manipulation of both frequency and amplitude. Incorporation of both parameters would allow assessment of individual effects, and would identify if frequency and amplitude interact with each other or hold a co-dependent relationship.
Beyond amplitude and frequency, the experimental setup an investigator or clinician chooses may also impact the perceived movement illusions. It has been shown that the position of a limb and state of muscle relaxation may impact movement sensations. Craske et al. have shown that limb positions which increase stretch in a muscle may make the limb more sensitive to perceptions of movement [1]. McCloskey demonstrated that contraction and fatigue of a muscle reduced perceived movement velocity [34]. It has also been reported that when participants are able to view their stimulated limb they will experience either no illusion or significantly reduced motion and velocity of illusion [35–38]. Furthermore, tactile feedback [39,40] as well as movement of the contralateral arm [37,41] may also reduce illusionary movement sensations.
Given the inconsistency of vibratory parameters found in the literature, it becomes a challenging task for a clinician or researcher to select the optimal vibratory parameters for a given experimental setup, and to fully understand the impact their choices may have on the resulting movement illusions. Therefore, this study aims to more comprehensively investigate the vibratory parameters affecting the kinesthetic illusion. Specifically, the effects of three fundamentally important independent variables: amplitude, frequency, and arm position, are quantified in relation to the strength of illusion (SOI), ROM and perceived velocity of the illusion. Consistent with past literature, it is hypothesized that all three independent variables will affect the kinesthetic illusion. The analysis performed will quantify the degree to which each variable affects the illusion, thereby facilitating future choice of parameters to most consistently elicit and manipulate the kinesthetic illusion.
2. Methods
Twelve able-bodied participants were recruited (9 male, 3 female; mean age: 24 SD 1.7 years). All participants reported right hand dominance, and no current (or previous) neurological or muscular conditions that may affect experimental results. Informed consent was obtained prior to participation; ethics was approved through our institute’s review board.
2.1. Experimental setup
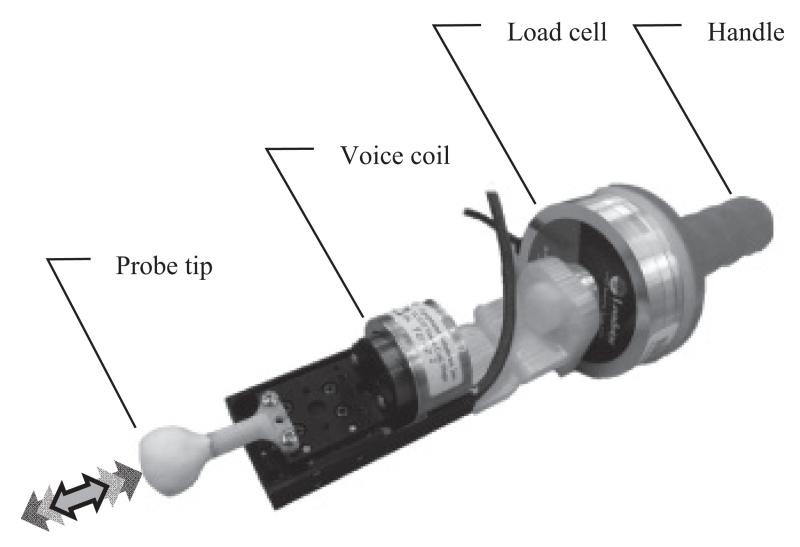
Vibration was introduced to the participants using a hand held [3] voice coil system (VCS1010, Equip-Solutions, Sunnyvale, USA) attached to a flat faced probe tip (1.8 cm diameter) (Fig. 1). The probe tip was depressed perpendicularly into the tissue of each participant with approximately 2.5 to 4 Newtons force as measured by an inline load cell (iLoad Pro, Loadstar Sensors, Fremont, USA). Video and audio footage of participant trials was digitally recorded (Pro9000, Logitech, Morges, Switzerland).
Fig. 1.
Hand held voice coil system.
Participants were seated in front of a table with moveable arm supports. The supports were configured to achieve the arm positions described below in the ‘Testing Procedure’ section. Specific care was given to minimize contact of the participants forearm with the table or support structure thus minimizing tactile feedback. Further consideration was given to position the limb such that relaxation of the tested muscle group was promoted. Both factors have been shown to influence the kinesthetic illusion [1,34].
2.2. Testing procedure
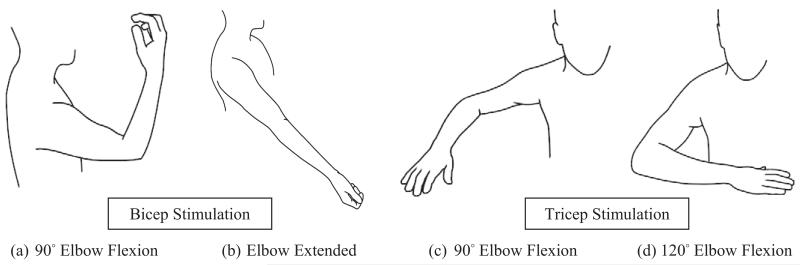
Vibration testing was conducted on each participant’s dominant-side (right) biceps and triceps. For each muscle group, the participant’s arm was tested in two elbow joint positions. These positions were selected to induce a state of muscle stretch in one position and relative slack in the next. For the biceps, stretch was achieved by fully extending the elbow and relative slack by positioning the elbow at approximately 90° flexion. For the triceps, stretch and relative slack were achieved by positioning the elbow to approximately 120° and 90° elbow flexion, respectively (Fig. 2). Joint angles were verified using a goniometer prior to testing. The order that each muscle group and position was tested in was selected at random.
Fig. 2.
Arm positions for the application of vibratory stimulus. Bicep stimulation provided at two positions. (a) approximately 90° flexion (relative muscle slack); (b) elbow fully extended (muscle stretch). Tricep stimulation provided at two positions; (c) approximately 90° flexion (relative muscle slack); (d) approximately 120° flexion (relative muscle stretch).
2.2.1. Initial testing
Prior to varying the parameters of amplitude and frequency, it was necessary to identify a location, that when vibrated, consistently elicited the kinesthetic illusion.
Before testing, participants were informed that they may experience a variety of sensations and one of these sensations may, or may not be, movement. Specific details such as when one may expect to feel movement, and at which joint or in which direction, were withheld. The participants’ vision was occluded and they were asked to report “any sensations beyond simple vibration”. Vibration with parameters shown effective at eliciting the kinesthetic illusion in prior pilot testing (90 Hz and 0.5 mm amplitude) was systematically introduced to various locations of the participant’s distal musculotendinous tissue. Each location was tested for approximately 20 seconds prior to moving to the next. If after 5 minutes of continuous testing a participant failed to experience movement sensations, they would be provided with the information “some participants report feeling movement in their elbow”. Testing would then continue for another 5 minutes. If the participant again failed to experience movement illusions, they would be seeded with the information, “participants often report sensations that their elbow is flexing (or extending)”. Testing would then continue till the participant experienced movement illusions or until 10 additional minutes passed at which point testing would be discontinued if no illusion was induced.
Once a participant reported sensations consistent with the kinesthetic illusion, probing of the surrounding tissue was conducted to precisely identify a location most consistently producing a strong kinesthetic sensation. The participant would be asked to compare stimulus locations in close proximity, with the investigator prompting, “Which one gives the strongest sensation of movement, number one or number two?” Vibration would be applied to location one or location two simultaneous with the investigator’s verbal cue. This was continued until a location consistently producing a stronger illusion than the surrounding tissue was identified. The final stimulus location was marked on the participant’s skin with a felt-tipped marker. This initial testing procedure was repeated for each muscle group in each arm position (as described in the experimental setup section).
2.2.2. Vibration parameter testing
To evaluate the effects of amplitude, frequency and muscle stretch (elbow joint position) on the kinesthetic illusion, a full factorial design was used. The manipulated variables, amplitude and frequency were introduced at three levels (0.1, 0.3, 0.5 mm neutral to peak and 70, 90 and 110 Hz, respectively). The third manipulated variable, muscle stretch, was introduced according to the elbow joint positions described in Fig. 2. In total each muscle site would be exposed to 18 unique combinations of amplitude, frequency and joint position. These combinations were randomly presented to each muscle for 20 seconds, at the corresponding location determined in the initial testing.
Following each combination, three output variables were quantified to characterize the induced kinesthetic illusion: strength of illusion (SOI), illusionary range of motion (ROM), and illusionary velocity. SOI was quantified on a 5 point Likert Scale. The participant was prompted, “We want you to describe the realism of the illusion. How strong or convincing was the illusion that your arm was moving?” A score of zero would be assigned to the absence of an illusion, and integers from one to five would represent: Not at all, Slightly, Somewhat, Very, and Extremely, respectively. ROM was quantified by asking the participant to manipulate a two-dimensional sagittal arm model to indicate the range they felt their joint moving, and then measuring the angular change in elbow position. Similar memory and recall methods have been used in previous literature [3,21,34]. Finally illusionary velocity was quantified by having the participants manipulate the two dimensional model “at the same velocity they felt their arm moving”. The time duration to complete each movement was taken from digital video footage. Velocity was calculated as the ROM divided by the movement duration.
2.2.3. Data treatment and analysis
To address the possible subjectivity and inter-participant error introduced as a result of manipulating the 2D sagittal model; ROM and velocity results were normalized. For example, the largest ROM value occurring in a specific muscle group of a participant’s would be identified. The remaining ROM values for that participant’s muscle group would then be normalized (divided) by the corresponding maximum ROM value. This procedure was repeated for each muscle group of each participant individually. Therefore ROM and velocity results fell between zero and one. A value of zero represents the absence of an illusion and therefore no motion or no velocity, and one representing the largest value experienced by the participant’s muscle group.
ANOVAs were perform to evaluate the significance of the three manipulate variables (amplitude, frequency, muscle stretch) on SOI, ROM and velocity independently. Correspondingly three ANOVAs were performed for each muscle group. Each ANOVA evaluated both main effects and two-way interactions effects with p < 0.05 assumed significant.
To characterize the nature of the relationships between significant manipulated variables and corresponding output variables, mean plots were utilized. From the ANOVA results, a mean plot was created for each manipulated variables having a significant effect on one of the measured output variables. Finally correlation matrices were created to quantify the linear-dependence of the three output variables (SOI, ROM, and velocity).
3. Results
The initial testing was performed to identify locations on a participant’s limb, that when vibrated, consistently elicited the kinesthetic illusion. However, it was found that only four of twelve participants were able to experience movement sensations while uninformed of the specifics of the kinesthetic illusion. After five minutes of testing, 8 participants were seeded with further information intending to lead them to experience the illusion. An additional 3 participants described sensations consistent with vibration induced movement illusions following this information. However, five participants still failed to experience the illusion after ten minutes of testing. At this stage, information was provided explicitly describing the kinesthetic illusion. These five participants all described consistent movement sensation shortly thereafter (Table 1).
Table 1.
Time intervals for participants to first experience the illusion
| Testing time (minutes) |
Information provided | Participants first experiencing illusion |
|---|---|---|
| 0–5 | None (Participants Uninformed) | 4 |
| 6–10 | “Some participants report feeling movement in their elbow.” | 3 |
| 11–15 | “Participants often report sensations that their elbow is flexing (or extending).” | 5 |
Conditions for participants to first experience the kinesthetic illusion, categorized by time interval and the corresponding number of participants to first experience during each interval
ANOVAs were conducted to identify variables having a significant effect on SOI, ROM or perceived velocity. In the biceps, amplitude was found to have a significant effect on all three output measures, SOI, ROM and velocity (p < 0.05). It was also shown that the perceived velocity was affected by the initial arm position of participants. No interaction effects were shown to be significant (Table 2a).
Table 2.
ANOVA results
| P-value |
||||
|---|---|---|---|---|
| SOI | ROM | Velocity | ||
| a. Bicep results | ||||
| Main effects | Amplitude | < 0.010* | < 0.010* | < 0.010* |
| Frequency | 0.700 | 0.886 | 0.969 | |
| Position | 0.366 | 0.120 | 0.012* | |
| Interaction effects | Position/Amplitude | 0.648 | 0.710 | 0.467 |
| Position/Frequency | 0.917 | 0.482 | 0.924 | |
| Amplitude/Frequency | 0.915 | 0.990 | 0.863 | |
| b. Triceps results | ||||
| Main effects | Amplitude | < 0.010* | < 0.010* | < 0.010* |
| Frequency | 0.404 | 0.537 | 0.936 | |
| Position | 0.611 | 0.009* | 0.033* | |
| Interaction effects | Position/Amplitude | 0.669 | 0.535 | 0.767 |
| Position/Frequency | 0.596 | 0.440 | 0.860 | |
| Amplitude/Frequency | 0.777 | 0.504 | 0.902 | |
P-values for both main effects and interaction effects shown.
Indicates statistically significant values (p < 0.05).
Similarly in the triceps, amplitude was found to have a significant effect on all three output measures, SOI, ROM and velocity (p < 0.05). It was also shown that the ROM and perceived velocity was affected by the initial arm position of participants. No interaction effects were shown significant (Table 2b).
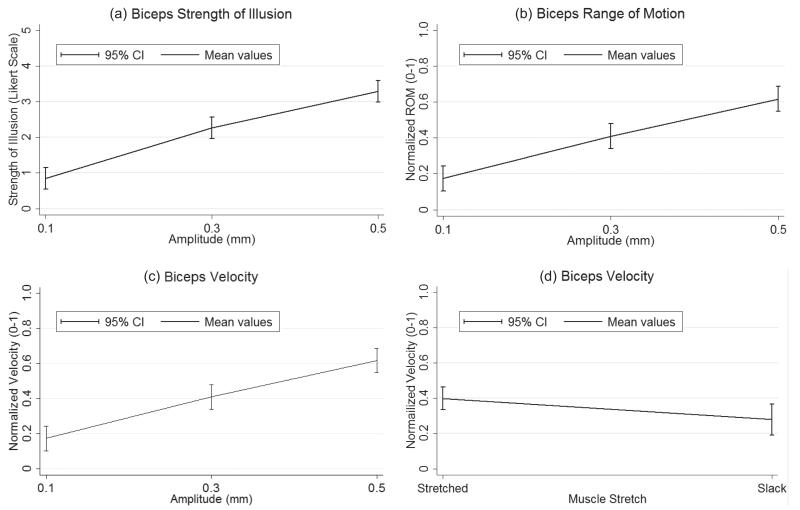
Mean plots were created to characterise the nature of the relationship between significant variables and corresponding output variables. In the biceps, as amplitude was increased SOI, ROM and perceived velocity were also found to increase (Figs 3a–c). At 0.5 mm amplitude the mean plots predict the average participant will experience the strongest, largest and fastest illusion when compared to 0.1 and 0.3 mm amplitude. Furthermore, from the ANOVA results, muscle stretch (joint position) was also determined to have a significant effect on perceived velocity. It can be seen that in joint positions creating more muscle stretch, perceived velocity also increased such that the fastest illusion can be predicted to occur when the elbow is fully extended (Fig. 3d).
Fig. 3.
Bicep mean plots of significant. Mean values and 95% confidence intervals (CI) for each significant manipulated variable plotted at the levels specified in the vibration parameter testing section above. (a) Strength of illusion as a function of amplitude; (b) Normalized range of motion as a function of amplitude; (c) Normalized velocity as a function of amplitude; (d) Normalized velocity as a function of muscle stretch.
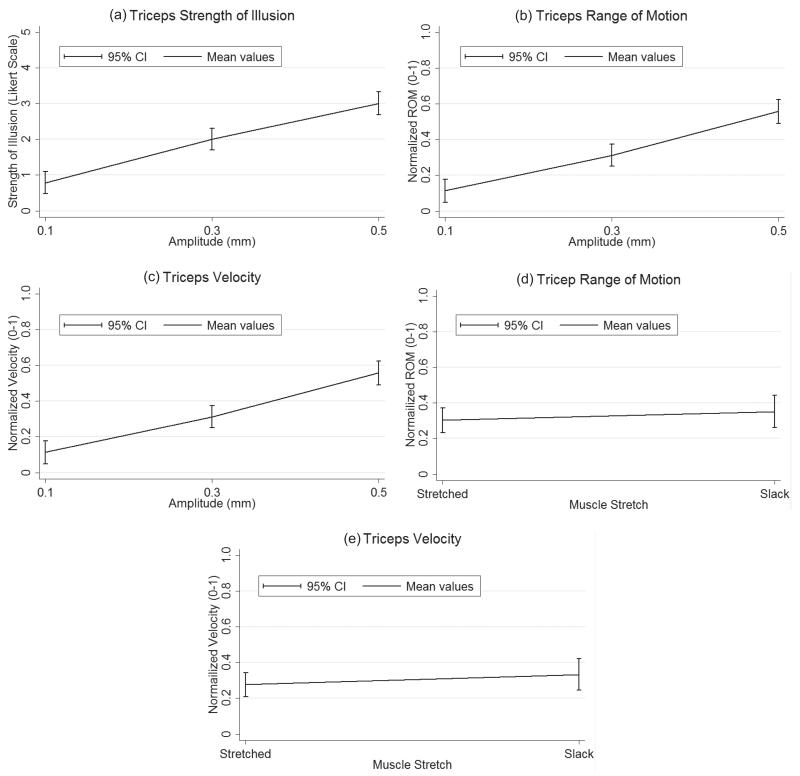
Similarly, in the triceps, as amplitude increased SOI, ROM and perceived velocity also increased (Figs 4a–c). Again, at 0.5 mm amplitude the mean plots predict the average participant will experience the strongest, largest and fastest illusion when compared to 0.1 and 0.3 mm amplitude Furthermore, from the ANOVA results, muscle stretch (joint position) was also determined to have a significant effect on ROM and perceived velocity. However, inverse to the bicep finding, it can be seen that in joint positions creating less muscle stretch, ROM and perceived velocity increased (Figs 4d–e).
Fig. 4.
Tricep mean plots of significant. Mean values and 95% confidence intervals (CI) for each significant manipulated variable plotted at the levels specified in the vibration parameter testing section above. (a) Strength of illusion as a function of amplitude; (b) Normalized range of motion as a function of amplitude; c. Normalized velocity as a function of amplitude; (d) Normalized range of motion as a function of muscle stretch; (e) Normalized velocity as a function of muscle stretch.
Finally it can be seen in the correlation matrices that the three output variables (SOI, ROM and received velocity) all strongly correlate with each other. In the biceps correlation coefficients range from 0.749 through 0.871 and in the tricep values coefficients range from 0.711 to 0.859 (Table 3).
Table 3.
Output variables correlation coefficients matrix
| Correlation (R) |
||||
|---|---|---|---|---|
| SOI | ROM | Velocity | ||
| Biceps | SOI | 1.000 | 0.806 | 0.749 |
| ROM | 1.000 | 0.871 | ||
| Velocity | 1.000 | |||
| Triceps | SOI | 1.000 | 0.763 | 0.711 |
| ROM | 1.000 | 0.859 | ||
| Velocity | 1.000 | |||
Pearson correlation coefficients (R) for the output variables strength of illusion (SOI), range of motion (ROM) and perceived velocity. Table divided by bicep and tricep results.
4. Discussion
When vibration of certain amplitude and frequency ranges is introduced to musculotendinous regions of a limb, illusionary sensation that the limb is moving may occur. Throughout literature, this kinesthetic illusion has been used in the research and rehabilitation of numerous affected populations [7–20]. Early work to understand this illusion suggests that the vibrational parameters amplitude and frequency may affect the velocity and ROM of these illusions [21,24]. However, little agreement exists when characterizing the extent and nature to which frequency and amplitude play a role. Beyond vibration parameters, research suggests that physiological factors such as joint position (or muscle stretch) [1], and visual feedback [35–37], amoung others [34,37,39–41,41], also play a role. Ultimately, this lack of agreement on vibration parameter effects, and abundance of information addressing physiological confounding factors, may present an obstacle to researchers and clinicians wanting to utilize the kinesthetic illusion in a laboratory or clinic.
With the goal of addressing this limitation, this study examined the effects of manipulating three fundamentally important variables (amplitude, frequency, and limb position) on the illusionary traits SOI, ROM and perceived velocity. It was found that amplitude was the one vibratory parameter that had the most prominent effect on the experience of the kinesthetic illusion.
In prior literature, it is rarely reported if participants were informed of the kinesthetic illusion prior to testing and the duration of time necessary to first experience the illusion. Our data suggests that movement illusions are not necessarily experienced immediately in first time participants. Only 4 out of our 12 participants experienced these sensations during the first 5 minutes of testing in the absence of explicit information describing the kinesthetic illusion (Table 1). There are a few possible explanations for these results. The first is the tonic vibration reflex. This is a natural reflex that results in the contraction of a muscle with sustained vibration [42]. As contraction of the vibrated muscle has been shown to weaken or abolish the kinesthetic illusion [34], it is possible these two physiological phenomena may have competed [3]. Some participants had to be repeatedly asked to relax their vibrated muscle and resist the impulse to contract. Anecdotally some participants demonstrated a higher sensitivity to the tonic vibration reflex and consequently took longer to first experience the kinesthetic illusion. A second explanation may lie in how each participant interpreted the sensations experienced. It was common for participants to have difficulty articulating the sensations or to first describe the sensations as “strange”. Although providing these participants with small amounts of information may have lead them to the kinesthetic illusion, this process may have also helped them form a clearer mental imagery of how to interpret the sensations they were experiencing. However, once participants began to experience the kinesthetic illusion, regardless of how much or how little information was initially provided, the subsequent description of illusionary movement and response to altering vibration parameters was very consistent across participants. Therefore, using the kinesthetic illusion in rehabilitative applications may require a degree of participant training, especially in populations with limited sensory capacity. In research applications, investigators must be aware that achieving illusionary movements may require time and a strategy to reveal enough information without biasing results. Regardless of the applications, eliciting the kinesthetic illusion may require more than the simple introduction of vibration to muscles or tendons.
In past studies vibratory frequency has been more often manipulated, and its effects generally more studied, than amplitude. However, from our factorial analysis, we found that amplitude significantly affected the SOI, ROM and perceived velocity of illusions in both the bicep and tricep groups; whereas frequency was found to have no significant effect. This suggests that in the experimental ranges examined (0.1–0.5 mm and 70–110 Hz) amplitude was the vibratory parameter ultimately governing the kinesthetic illusion. According to Roll et al., a decline in perceived velocity should have been present from 80 through 120 Hz [21]. However, our data suggest that the effects of amplitude so greatly outweighed any frequency phenomenon, that it was neither statistically distinguishable nor was it experienced by the participant group.
The amplitude mean plots in both the biceps and triceps show that increasing the amplitude in the range of 0.1 to 0.5 mm resulted in a corresponding increase in all three output variables (SOI, ROM, perceived velocity) (Figs 3 and 4a–c). Therefore, if a researcher or clinician wishes to manipulate the SOI, ROM, or velocity, experienced by an individual, this can be achieved through manipulation of vibrational amplitude. However it must be noted that this relationships can only be expected in the experimental amplitude range (0.1 mm to 0.5 mm). The mean plots did not show signs of ‘levelling-off’ or ‘plateauing’. Therefore it cannot be concluded that the strongest, largest or fastest illusion will occur at 0.5 mm, as it may occur beyond this amplitude value. Consequently, further work may be warranted to address the amplitude intervals in which the kinesthetic illusion can occur, and values inducing maximum illusions of SOI, ROM or velocity.
The mean plots (Figs 3 and 4) and correlation matrices (Table 3) also illustrated a dependency between output variables. In both the biceps and triceps strong correlation was found between all three outputs (SOI, ROM, Perceived velocity). Ultimately this suggests that these variables cannot be uncoupled and manipulated independently. For example a clinician or researcher wishing to increase the velocity of movement will achieve this by increasing the amplitude. Inherently this amplitude increase will also increase the amount of movement and strength of illusion the client experiences. As a result of this dependency it does not appear that the illusion can be elicited such that one of the output variables is low while the other remains high. As an example, it would not be possible to elicit a very strong illusion with large ROM, but feel as though it is moving with a slow velocity. The implications of this relationship suggest that researcher or clinician must be willing to achieve a balance of these three variables while designing experiments or therapeutic intervention using the kinesthetic illusion.
In may also be noted that muscle stretch (arm position) was found to have a significant impact on the velocity of illusion in the biceps, as well as ROM and velocity in the triceps. When evaluating their corresponding mean plots, the slope of the muscle stretch graphs is notably less than that of the corresponding amplitude effects graphs (Figs 3 and 4). Therefore, the conclusion can be drawn that amplitude has a more prominent effect on the kinesthetic illusion than that of muscle stretch. However, clinicians and researchers should be aware that altering initial body posture prior to testing may have the potential to influence the experienced illusion.
4.1. Limitations
This study was conducted on able-bodied individuals to understand this kinesthetic illusion as it may apply to rehabilitation and research applications. Therefore the results and analysis performed may not directly extrapolate to the selection of vibratory parameters for populations with sensory motor impairment; the nature of the experienced illusion may vary across injury type and individual. Furthermore this study was conducted within specific experimental ranges (0.1–0.5 mm amplitude, 70–110 Hz Frequency, 2 arm positions). As a result the findings and suggestions discussed are limited to illusionary movements elicited with-in these constraints.
Acknowledgements
The authors would like to thank Dr. Ming Chan, Katherine Edwards and Mina Fahmy for their support and assistance in this work. This work was supported by NIH grant R01NS081710-02.
Footnotes
Conflict of interest
The authors report no conflicts of interest.
References
- [1].Craske B. Perception of impossible limb positions induced by tendon vibration. Science. 1977;196(4285):71–73. doi: 10.1126/science.841342. [DOI] [PubMed] [Google Scholar]
- [2].Lackner JR. Some proprioceptive influences on the perceptual representation of body shape and orientation. Brain. 1988;111(2):281–297. doi: 10.1093/brain/111.2.281. [DOI] [PubMed] [Google Scholar]
- [3].Goodwin GM, McCloskey DI, Matthews PBC. The contribution of muscle afferents kinaesthesia shown by vibration induced illusions of movement and by the effects of paralyzing joint afferents. J Physiol (Lond) 1972;536:635–647. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- [4].Proske U, Gandevia SC. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92(4):1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- [5].Jones LA. Motor Illusions: What Do They Reveal About Proprioception? Psychol Bull. 1988;103(1):72–86. doi: 10.1037/0033-2909.103.1.72. [DOI] [PubMed] [Google Scholar]
- [6].Kandel E. Principles of neural science. 5th ed. McGraw-Hill; New York: 2013. p. Part V. [Google Scholar]
- [7].Rinderknecht MD, Kim Y, Santos-Carreras L, Bleuler H, Gassert R. Combined tendon vibration and virtual reality for post-stroke hand rehabilitation; IEEE World Haptics Conference; 2013.pp. 277–282. [Google Scholar]
- [8].Rinderknecht MD. Device for a novel hand and wrist rehabilitation strategy for stroke patients based on illusory movements induced by tendon vibration; 16th IEEE Mediterranean Electrotechnical Conference (MELECON); 2012.pp. 926–931. [Google Scholar]
- [9].Redon-Zouiteni C, Roll JP, Lacert P. Proprioceptive postural reprogrammation in childhood cerebral palsy Validation of tendon vibration as a therapeutic tool. Motricité cérébrale. 1994;15(2):57. [Google Scholar]
- [10].Krueger-Beck E, Nogueira-Neto GN, Nohama P. Vibrational stimulus in spasticity – A perspective of treatment. Revista Neurociencias. 2010;18(4):523–530. [Google Scholar]
- [11].Gay A, Parratte S, Salazard B, Guinard D, Pham T, Legré R, et al. Proprioceptive feedback enhancement induced by vibratory stimulation in complex regional pain syndrome type I: An open comparative pilot study in 11 patients. Joint Bone Spine. 2007;74(5):461–466. doi: 10.1016/j.jbspin.2006.10.010. [DOI] [PubMed] [Google Scholar]
- [12].Willigenburg NW, Kingma I, Hoozemans MJM, van Dieën JH. Precision control of trunk movement in low back pain patients. Human Movement Science. 2013;32(1):228–239. doi: 10.1016/j.humov.2012.12.007. [DOI] [PubMed] [Google Scholar]
- [13].Frima N, Nasir J, Grünewald RA. Abnormal vibration-induced illusion of movement in idiopathic focal dystonia: An endophenotypic marker? Movement Disorders. 2008;23(3):373–377. doi: 10.1002/mds.21838. [DOI] [PubMed] [Google Scholar]
- [14].Frima N, Grünewald RA. Abnormal vibration induced illusion of movement in essential tremor: Evidence for abnormal muscle spindle afferent function. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(1):55–57. doi: 10.1136/jnnp.2004.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frima N, Rome SM, Grünewald RA. The effect of fatigue on abnormal vibration induced illusion of movement in idiopathic focal dystonia. Journal of Neurology Neurosurgery and Psychiatry. 2003;74(8):1154–1156. doi: 10.1136/jnnp.74.8.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rome S, Grünewald RA. Abnormal perception of vibration-induced illusion of movement in dystonia. Neurology. 1999;53(8):1794–1800. doi: 10.1212/wnl.53.8.1794. [DOI] [PubMed] [Google Scholar]
- [17].Han J, Jung J, Lee J, Kim E. Effects of vibration stimuli on the knee joint reposition error of elderly women. Journal of Physical Therapy Science. 2013;25(1):93–95. [Google Scholar]
- [18].Roll R, Kavounoudias A, Albert F, Legré R, Gay A, Fabre B, et al. Illusory movements prevent cortical disruption caused by immobilization. Neuroimage. 2012;62(1):510–519. doi: 10.1016/j.neuroimage.2012.05.016. [DOI] [PubMed] [Google Scholar]
- [19].Quercia P, Demougeot L, Dos Santos M, Bonnetblanc F. Integration of proprioceptive signals and attentional capacity during postural control are impaired but subject to improvement in dyslexic children. Experimental Brain Research. 2011;209(4):599–608. doi: 10.1007/s00221-011-2593-3. [DOI] [PubMed] [Google Scholar]
- [20].Vaugoyeau M, Hakam H, Azulay J- Proprioceptive impairment and postural orientation control in Parkinson’s disease. Human Movement Science. 2011;30(2):405–414. doi: 10.1016/j.humov.2010.10.006. [DOI] [PubMed] [Google Scholar]
- [21].Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Experimental Brain Research. 1982;47(2):177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- [22].Gilhodes JC, Roll JP, Tardy-Gervet MF. Perceptual and motor effects of agonist-antagonist muscle vibration in man. Experimental Brain Research. 1986;61(2):395–402. doi: 10.1007/BF00239528. [DOI] [PubMed] [Google Scholar]
- [23].Roll JP, Vedel JP, Ribot E. Alteration of proprioceptice messages induced by tendon vibration in man: A microneurographic study. Experimental Brain Research. 1989;76(1):213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- [24].Clark FJ, Matthews PB, Muir RB. Effect of the amplitude of muscle vibration on the subjectively experienced illusion of movement [proceedings] J Physiol (Lond) 1979;296:14P–15P. [PubMed] [Google Scholar]
- [25].Meek SG, Jacobsen SC, Goulding PP. Extended physiologic taction: Design and evaluation of a proportional force feedback system. Journal of Rehabilitation Research and Development. 1989;26(3):53–62. [PubMed] [Google Scholar]
- [26].Eklund G. Position sense and state of contraction: The effects of vibration. J Neurol Neurosurg Psychiatr. 1972;35:606–611. [Google Scholar]
- [27].Verschueren SMP, Brumagne S, Swinnen SP, Cordo PJ. The effect of aging on dynamic position sense at the ankle. Behav Brain Res. 2002;136(2):593–603. doi: 10.1016/s0166-4328(02)00224-3. [DOI] [PubMed] [Google Scholar]
- [28].Calvin-Figuière S, Romaiguère P, Roll J- Relations between the directions of vibration-induced kinesthetic illusions and the pattern of activation of antagonist muscles. Brain Res. 2000;881(2):128–138. doi: 10.1016/s0006-8993(00)02604-4. [DOI] [PubMed] [Google Scholar]
- [29].Kito T, Hashimoto T, Yoneda T, Katamoto S, Naito E. Sensory processing during kinesthetic aftereffect following illusory hand movement elicited by tendon vibration. Brain Res. 2006;1114(1):75–84. doi: 10.1016/j.brainres.2006.07.062. [DOI] [PubMed] [Google Scholar]
- [30].White O, Proske U. Illusions of forearm displacement during vibration of elbow muscles in humans. Experimental Brain Research. 2009;192(1):113–120. doi: 10.1007/s00221-008-1561-z. [DOI] [PubMed] [Google Scholar]
- [31].Seizova-Cajic T, Smith JL, Taylor JL, Gandevia SC. Proprioceptive movement illusions due to prolonged stimulation: Reversals and aftereffects. PLoS ONE. 2007;2(10) doi: 10.1371/journal.pone.0001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lackner J. Human Sensory-Motor Adaptation to the Terrestrial Force Environment. Brain Mechanisms and Spatial Vision. 1985;21:175. [Google Scholar]
- [33].Capaday C, Cooke JD. The effects of muscle vibration on the attainment of intended final position during voluntary human arm movements. Experimental Brain Research. 1981;42(2):228–230. doi: 10.1007/BF00236912. [DOI] [PubMed] [Google Scholar]
- [34].McCloskey DI. Differences between the senses of movement and position shown by the effects of loading and vibration of muscles in man. Brain Res. 1973;61:119–131. doi: 10.1016/0006-8993(73)90521-0. [DOI] [PubMed] [Google Scholar]
- [35].Guerraz M, Provost S, Narison R, Brugnon A, Virolle S, Bresciani J- Integration of visual and proprioceptive afferents in kinesthesia. Neuroscience. 2012;223:258–268. doi: 10.1016/j.neuroscience.2012.07.059. [DOI] [PubMed] [Google Scholar]
- [36].Seizova-Cajic T, Azzi R. Conflict with vision diminishes proprioceptive adaptation to muscle vibration. Experimental Brain Research. 2011;211(2):169–175. doi: 10.1007/s00221-011-2663-6. [DOI] [PubMed] [Google Scholar]
- [37].Izumizaki M, Tsuge M, Akai L, Proske U, Homma I. The illusion of changed position and movement from vibrating one arm is altered by vision or movement of the other arm. J Physiol (Lond) 2010;588(15):2789–2800. doi: 10.1113/jphysiol.2010.192336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lackner JR, Taublieb AB. Influence of vision on vibration-induced illusions of limb movement. Exp Neurol. 1984;85(1):97–106. doi: 10.1016/0014-4886(84)90164-x. [DOI] [PubMed] [Google Scholar]
- [39].Blanchard C, Roll R, Roll J-, Kavounoudias A. Combined contribution of tactile and proprioceptive feedback to hand movement perception. Brain Res. 2011;1382:219–229. doi: 10.1016/j.brainres.2011.01.066. [DOI] [PubMed] [Google Scholar]
- [40].Rabin E, Gordon AM. Prior experience and current goals affect muscle-spindle and tactile integration. Experimental Brain Research. 2006;169(3):407–416. doi: 10.1007/s00221-005-0154-3. [DOI] [PubMed] [Google Scholar]
- [41].Lackner JR. Some influences of tonic vibration reflexes on the position sense of the contralateral limb. Exp Neurol. 1984;85(1):107–113. doi: 10.1016/0014-4886(84)90165-1. [DOI] [PubMed] [Google Scholar]
- [42].Eklund G, H K. Motor effects of vibratory muscle stimuli in man. Electroenceph Clin Neurophysiol. 1965;19:619. [Google Scholar]