Abstract
Gorham–Stout disease is a rare disease characterized by anarchic lymphovascular proliferation causing resorption of bone sometimes leading to disastrous complications. Bone tissue is progressively replaced by angiomatic and lymphangiomatic tissue and finally by fibrous tissue. This disease is known to be ubiquitous and of complex etiology.
We present a case of Gorham–Stout disease of the proximal fibula invading the proximal tibia and soft tissues of the popliteal space that was successfully treated with radiotherapy and zoledronic acid.
Keywords: Gorham–Stout disease, Pseudotumor of bone, Bone resorption, Bisphosphonates, Bone radiotherapy
1. Introduction
Gorham–Stout Disease (GSD), also known as ‘massive osteolysis’ and ‘vanishing bone disease’, is a rare bone condition characterized by spontaneous, idiopathic, and progressive proliferation of thin-walled vascular and lymphatic vessels replacing bone and marrow space by fibrous connective tissue. It leads to bone destruction which may sometimes be followed by new bone production [1]. In 1838 Jackson described the first case of a patient with a “boneless arm” [2]. Clinical and anatomopathological features were described by Gorham and Stout in 1955. They showed that this disease leads to progressive massive osteolysis by invasion of bone by lymphangiomatic tissue [3]. GSD mostly occurs in young adults; mean age is 25 years. Male and female are equally affected, without inheritance pattern or race predilection [4]. Clinical symptoms vary based on the location of bone involvement. This disease is most often regional and may involve several bones around a joint. Evolution may differ from one patient to another, GSD can be benign with a tendency to self-limitation or even spontaneous regression but it can also be very disabling [3]. Known to be ubiquitous, it frequently affects the skull and maxillofacial bones, ribs, cervical vertebrae, shoulder and pelvic girdle bones [5,6]. The affected bone(s) weaken(s) progressively, and pain and spontaneous fractures are the most common clinical features besides swelling and progressive deformity of the affected extremity. When localized at a lower extremity, limb length discrepancy and axial deformation may lead to gait abnormalities and major limping can occur [7]. Depending on location, it may also lead to neurological complications and paralysis (in case of vertebrae involvement), respiratory insufficiency and sometimes to death [8].
X-rays commonly show a typical licked candy stick appearance, based on concentric bone resorption. Common blood tests are usually normal, but bone alkaline phosphatase (BAP) may be elevated if a fracture occurred [8]. GSD is a diagnosis of exclusion. Diseases responsible of bone osteolysis such as infections, inflammatory or endocrine disorders, intraosseous malignancies or metastases should be considered [1].
Radiographic and anatomopathological characteristics are used as pathognomonic features for the diagnosis of GSD [1]. Treatments include surgery, radiation and medical therapy. Surgery is reserved for severe cases, e.g. when pathological fractures need to be fixed or when en bloc resection is required. Reconstruction is achieved with bone grafts or alloplastic prostheses if instability occurs. Interventions are often quite invasive. Recurrence rate is close to 20% [1]. Radiotherapy is usually chosen in order to reverse the progression of the lymphangiomatosis and to treat lesions that are not surgically resectable, at least without major consequences. Post-operative radiotherapy is required when the lesion could not be resected in one piece [9]. There are no “Food and Drug Administration” (FDA) approved therapies for the treatment of GSD. Several drugs have nevertheless been tried, including bisphosphonates (etidronate, clodronate, pamidronate and zoledronic acid) [10–14], interferon alpha-2b [14,15], anti-VEGF-A antibody, bevacizumab, propranolol [16], low molecular weight heparin, steroids, vitamin D and calcitonin [17,18]. However the experience with these compounds, including bisphosphonates, is extremely limited. We found only one report of a patient treated with zoledronic acid [11]. On the opposite, the use of zoledronic acid, the most powerful bisphosphonate, has become an integral component of cancer treatment in patients with metastatic bone disease. The drugs markedly delay and decrease the occurrence of skeletal-related events [19].
Etiology and mechanism of bone resorption in GSD remain poorly understood [3,7,8,20]. In a recent review the potential role of endothelial cells, macrophages, osteoclasts and osteoblasts is discussed. Active lymphangiomatosis and haemangiomatosis may be triggered by secretion of VEGF through activation of receptors of lymphatic and blood endothelial cells. Patients presenting GSD have high VEGF-A and -C blood levels whereas anti-lymphagiogenic factors levels (VEGFR2, TGF-beta, IFN-gamma, etc.) are reported to be low, maybe contributing to uncontrolled growth of lymphatic vessels in the affected bones [21]. Osteoclastic activity may vary according to the phase of the disease. On the one hand, osteoclast differenciation is stimulated by macrophages, VEGF-A, -C, -D and IL-6. On the other hand, high levels of TNF-alpha (produced by macrophages) inhibit osteoblastogenesis and promote osteoclastogenesis. Inhibition of osteoprotegerin and enhanced production of RANKL contribute to stimulate bone resorption. Bone homeostasis appears to be unaffected in other parts of the skeleton [21–22].
Bone is in a constant state of remodeling. The functions of osteoblasts and osteoclasts are well balanced to maintain bone homeostasis [23]. Bone diseases such as bone metastases that alter this equilibrium in favor of osteoclasts can induce loss of structural integrity of the skeleton. In this situation osteoclasts resorb bone by secreting proteases that dissolve the matrix and acids that release bone mineral into the extracellular space. In GSD the situation is more complex because of replacement of the bone by a fibrous tissue [23].
Nevertheless, considering the existence of a high osteoclastic activity, use of combination of radiotherapy and bisphosponate has been tried in a few patients. Bisphosphonates have been chosen because of their anti-osteoclastic but also anti-angiogenic activities. Side effects of zoledronic acid are usually mild and, although renal function has to be checked before each infusion, the risk of osteonecrosis of the jaw is not negligible at high doses [23–24].
2. Case report
We report on a 28-year-old male without any past medical history. After a minor work accident (professional mechanic) he noticed significant pain in his left knee. The pain increased over time and a radiograph was taken. It showed minor bone resorption at the neck of the left proximal fibula (Fig. 1). The patient was treated by minor pain medications. Despite 6 months of symptomatic care, pain did not resolve and limping appeared. Another X-ray was taken that showed a concentric shrinkage of almost all proximal fibula, a typical “licked candy stick” appearance. This image led to the radiological presumption of bone tumor. Bone scan showed increased Technetium uptake in the left tibial plateau extending to both the left tibial tuberosity and the left proximal fibula (Fig. 2). Computed-tomography scanner (CT-scan) showed bone resorption in those areas. Around the tibial metaphysis there was an area of massive osteolysis that looked like a pathological fracture. Magnetic resonance imaging (MRI) emphasized involvement of the left fibula and tibia by exuberant disorganized vessels also invading soft tissue of the popliteal space (Fig. 3). Biopsy was taken and histology described a diffuse lymphangioma, showing irregular vascular channels lined by a single epithelial layer, which were embedded in a fibrous stroma also containing residual bone trabeculae (Fig. 4). Endothelial cells did not display any atypia. They expressed podoplanin (D240), which confirmed their lymphatic differentiation. Radiologic imaging combined with histopathology confirmed the diagnosis of Gorham–Stout disease.
Fig. 1.
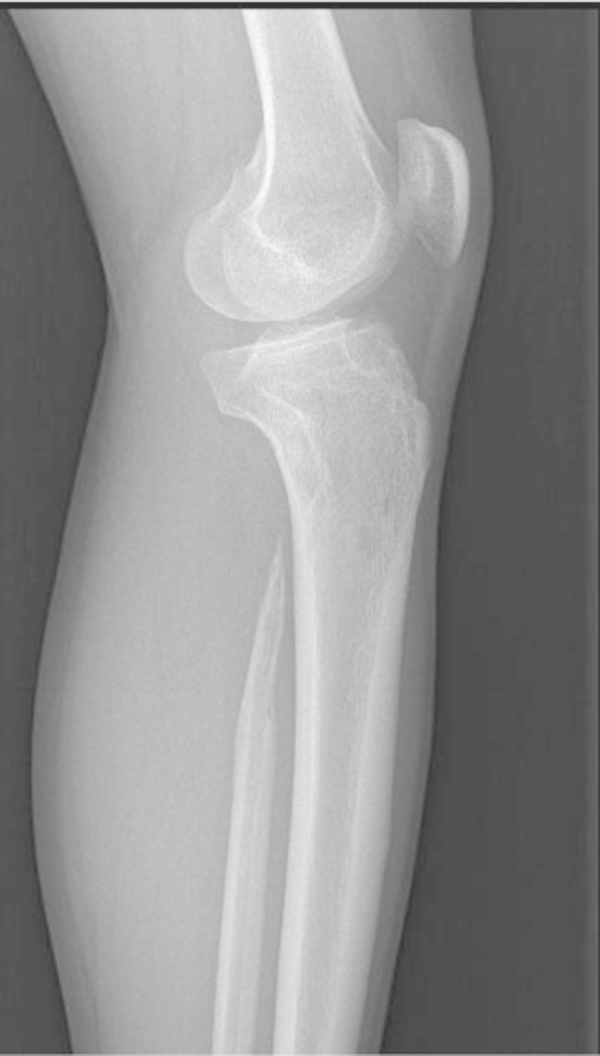
X-ray shows complete disappearance of the proximal part of fibula and a typical licked candy stick appearance.
Fig. 2.
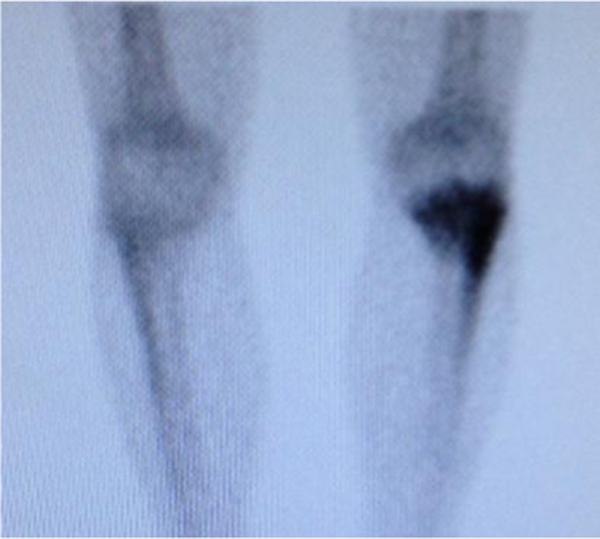
Bone scan – hypercaptation of the left proximal fibula and tibial plateau.
Fig. 3.
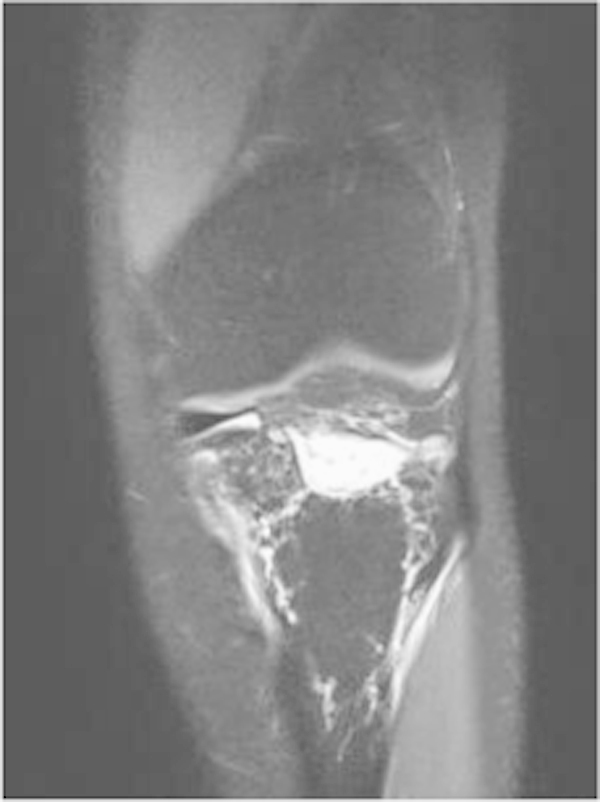
MRI shows lymphangiomatosis infiltrating left popliteal space, fibula and tibia.
Fig. 4.
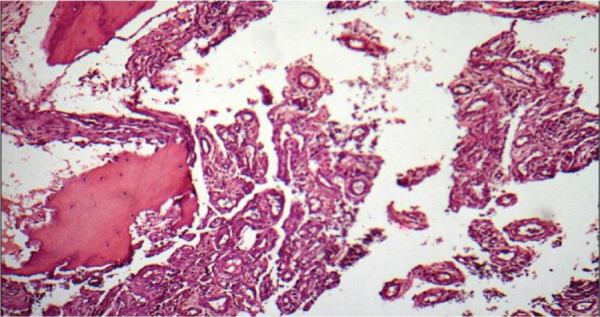
Bone biopsy of left proximal part of fibula, showing diffuse lymphangiomatosis.
The patient was first treated by radiotherapy with a total dose of 40 Gy given in 2 Gy fractions, once a day for 20 days. Thereafter, intravenous zoledronic acid (Zometa® – 4 mg) was administered once every 2 months during 24 months. The patient also received adequate supplementation of vitamin D and calcium. No surgical treatment was considered because of the low aggressive behavior and the necessity for extensive surgery given the location.
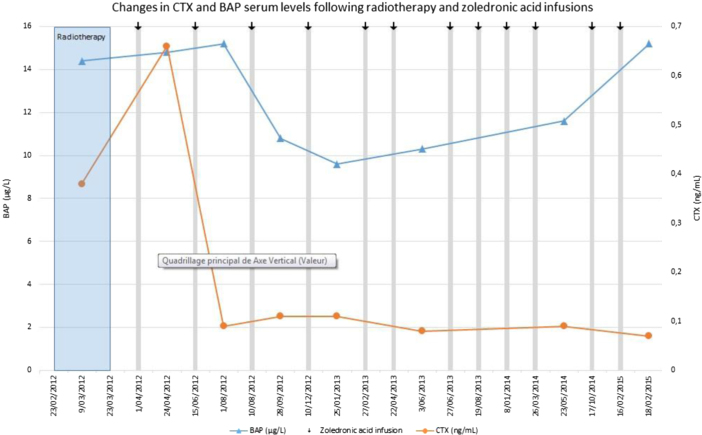
We monitored the biological responses to zoledronic acid through the measurement of bone alkaline phosphatase (BAP) and C-terminal telopeptide (CTX), a specific marker of bone resorption. Both markers rapidly decreased after initiation of bisphosphonate therapy (Fig. 5). We also closely monitored serum levels of calcium (Ca), creatinine, 25(OH)-vitamin D and parathyroid hormone (PTH). There was no side effect of zoledronic acid therapy and serum levels of creatinine, Ca and PTH remained normal throughout therapy (data not shown).
Fig. 5.
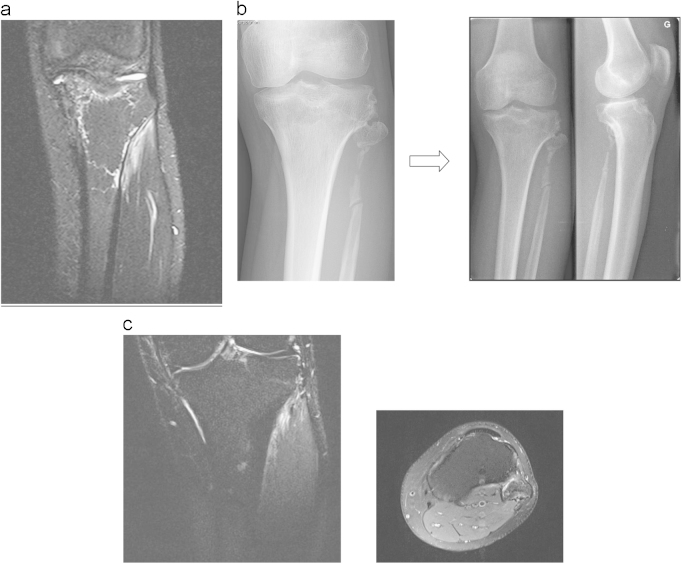
At thirty months after the end of the medical treatment, the patient’s situation improved markedly. Pain disappeared and was only present after longstanding solicitation. MRI showed major reduction of the bone invading lymphangioma (Fig. 6a). On radiographs slight bone regeneration was observed and remaining bone surfaces seemed stable (Fig. 6b, c).
Fig. 6.
(a) 9 months after radiotherapy and zoledronic acid – MRI shows reduction of lymphangiomatous invasion of the left tibia and popliteal space. (b) 24 and 30 months after radiotherapy and zoledronic acid – X-rays show a slight bone remineralization. (c) 30 months after radiotherapy and zoledronic acid – MRI shows disappearing of lymphangiomatosis around the left tibial plateau but a persistent invasion of the head of the left fibula.
3. Discussion
The invasion of the fibula and part of the tibia is a rare localization of GSD. Generally these lesions are found around the skull and maxillofacial bones, shoulder and pelvic girdle bones but quite rarely around the distal parts of the extremities [1]. In our case report, trauma was considered by the patient as a trigger for the development of GSD, but trauma has never been described as a risk factor for its development [2,3].
Radiation therapy (RT) is suggested by many authors as the most effective treatment of GSD [1,10,25]. Its role is to stop endothelial cell proliferation and therefore avoid progression of bone resorption. Dunbar et al. reported that treatment by radiotherapy (dose ranging from 40 to 45 Gy) leads to a good clinical outcome and few long-term complications. However, this study only included 4 patients [9]. A German team reported 10 cases of massive osteolysis treated by radiotherapy. Indications of RT alone were surgically unresectable lesions [1]. Postoperative-RT may also be given as an adjunct to surgery. Patients who were treated with doses over 36 Gy had a tendency to disease stabilization and less than 5% showed slight to moderate remineralization [1]. General outcomes were in favor of disease stabilization, allowing to conclude that radiotherapy (doses ranging from 30 to 45 Gy) might prevent osteolysis progression in up to 80% of cases [1]. In our patient, despite radiotherapy at efficient doses, bone resorption, estimated by serum CTX levels, was still elevated, arguing for persistent tumor-induced osteolysis. Additional treatment with the bisphosphonate zoledronic acid showed a rapid and persistent suppression of CTX levels and a marked decrease in BAP levels (Fig. 5). Such changes are similar to what is observed after bisphosphonate therapy in patients with metastatic bone disease [26,27]. For such patients, 4 mg of zoledronic acid is administered every 4 weeks during at least 2 years [23]. In the absence of guidelines, we chose to administer the classical dose of 4 mg of zoledronic acid once every 2 months since the bone lesion was unique. This reduced dosing should also be less likely to induce side effects such as osteonecrosis of the jaw [28].
Along the same line, there is no information in the literature on the optimal RT schedule in such cases. Doses ranging from 40 to 45 Gy should be adequate and we chose to give 2 Gy daily. This schedule was based on clinical and radiological evolution of the patient. RT limits natural evolution of the disease but has not been shown to favor bone regeneration. The presence of osteoclasts in the affected bones in active GSD justifies the use of bisphosphonates. Intravenous bisphosphonates have been extensively used in cancer patients with tumor bone disease and they are now part of the routine management of patients with bone metastases [23,26]. They markedly inhibit osteoclastic activity and decrease the frequency of skeletal-related events. As judged by the suppression of CTX levels in our patient, osteoclast activity was markedly inhibited and it is likely that zoledronic acid contributed to partial bone regeneration [13,29] (Fig. 5). In parallel to the marked clinical improvement, X-Rays and MRI suggested indeed new bone formation. Such bone regeneration is extremely rare in GSD. A bone biopsy after treatment would have probably confirmed decreased osteoclast activity and the progressive replacement of lymphagiomatic and fibrous tissue by a new bone formation but it was not performed because it would not have influenced our therapeutic attitude.
4. Conclusion
We report a well-documented case of Gorham–Stout disease in the proximal aspect of fibula invading proximal tibia and the popliteal space. Clinical outcome was favorable after a conservative therapeutical approach including radiotherapy and bisphosphonates. Striking features of this report are the thorough description on sequential MRIs, the histopathological aspect and the close follow-up of bone metabolism during therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.Heyd R., Micke O., Surholt C., Berger B., Martini C., Füller J. Radiation therapy for Gorham–Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81:179–185. doi: 10.1016/j.ijrobp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 2.J.B.S. Jackson. A boneless arm. Boston Med Surg J. 1838;18:368–369. [Google Scholar]
- 3.Gorham L.W., Stout A.P. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37:985–1004. [PubMed] [Google Scholar]
- 4.Patel D.V. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3:65–74. doi: 10.3121/cmr.3.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinée P., Tanyü M.O., Hauenstein K.H., Sigmund G., Stöver B., Adler C.P. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18:985–989. doi: 10.1097/00004728-199411000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Szabo C., Habre W. Gorham syndrome: anaesthetic management. Anaesthesia. 2000;55:157–159. doi: 10.1046/j.1365-2044.2000.055002157.x. [DOI] [PubMed] [Google Scholar]
- 7.Thompson J.S., Schurman D.J. Massive osteolysis. Clin Orthop Relat Res. 1974;103:206–211. [PubMed] [Google Scholar]
- 8.Ross J.L., Schinella R., Shenkman L. Massive osteolysis: an unusual cause of bone destruction. Am J Med. 1978;65:367–372. doi: 10.1016/0002-9343(78)90834-3. [DOI] [PubMed] [Google Scholar]
- 9.Dunbar S.F., Rosenberg A., Mankin H., Rosenthal D., Suit H.D. Gorham’s massive osteolysis: the role of radiation therapy and a review of the literature. Int J Radiat Oncol Biol Phys. 1993;26:491–497. doi: 10.1016/0360-3016(93)90968-2. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann G., Pfeil A., Böttcher J., Kaiser W.A., Füller J., Hein G. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129:967–972. doi: 10.1007/s00402-008-0742-3. [DOI] [PubMed] [Google Scholar]
- 11.Avelar R.L., Martins VB, Antunes A.A., Neto P.J., Andrade E.S. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Ped Otorhinolaryngol. 2010;74:319–322. doi: 10.1016/j.ijporl.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Tong A.C.K., Leung T.M., Cheung P.T. Management of massive osteolysis of the mandible: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:238–241. doi: 10.1016/j.tripleo.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 13.Hammer F., Ken W., Wesselmann U., Hofbauer L.C., Delling G., Allolio B. Gorham–Stout disease-stabilization during bisphosphonate treatment. J Bone Miner Res. 2005;20:350–353. doi: 10.1359/JBMR.041113. [DOI] [PubMed] [Google Scholar]
- 14.Hagberg H., Lamberg K., Aström G. Alpha-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350:1822–1823. doi: 10.1016/S0140-6736(05)63639-2. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi A., Ogawa C., Kanazawa T., Watanabe H., Suzuki M., Suzuki N. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham–Stout syndrome. J Pediatr Surg. 2005;40:47–50. doi: 10.1016/j.jpedsurg.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Ozeki M., Fukao T., Kondo N. Propanolol for intractable diffuse lymphangiomatosis. N Engl J Med. 2011;364:1380–1382. doi: 10.1056/NEJMc1013217. [DOI] [PubMed] [Google Scholar]
- 17.Branco F., Da Silva Horta J. Notes on a rare case of essential osteolysis. J Bone Joint Surg. 1958;40:519–527. doi: 10.1302/0301-620X.40B3.519. [DOI] [PubMed] [Google Scholar]
- 18.Holroyd I., Dillon M., Roberts G.J. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:125–129. doi: 10.1016/s1079-2104(00)80027-x. [DOI] [PubMed] [Google Scholar]
- 19.Aapro M., Abrahamsson P.A., Body J.J., Coleman R.E., Colomer R., Costa L. Guidance on the use of bisphophonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19:420–432. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 20.Heyden G., Kindblom L.G., Nielsen J.M. Disappearing bone disease: a clinical and histological study. J Bone Jt Surg. 1977;59:57–61. [PubMed] [Google Scholar]
- 21.Dellinger M.T., Garg N., Olsen B.R. Viewpoints on vessels and vanishing bones in Gorham–Stout disease. Bone. 2014;63:47–52. doi: 10.1016/j.bone.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Roodman G.D. Biology of osteoclast activation in cancer. J Clin Oncol. 2001:19. doi: 10.1200/JCO.2001.19.15.3562. [DOI] [PubMed] [Google Scholar]
- 23.Coleman R., Body J.J., Aapro M., Hadji P., Herrstaedt J. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2014;25:124–137. doi: 10.1093/annonc/mdu103. [DOI] [PubMed] [Google Scholar]
- 24.Body J.J. Breast cancer: bisphophonate therapy for metastatic done disease. Clin Cancer Res. 2006;12:6258–6263. doi: 10.1158/1078-0432.CCR-06-0840. [DOI] [PubMed] [Google Scholar]
- 25.Duffy B.M., Manon R, Patel R.R., Welsh J.S. A case of Gorham’s disease with chylothorax treated curatively with radiation therapy. Clin Med Res. 2005;3:83–86. doi: 10.3121/cmr.3.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Body J.J., Lichinitser M., Tjulandin S., Garnero P., Bergström B. Oral ibandronate is as active as intravenous zoledronic acid for reducing bone turnover markers in women with breast cancer and bone metastases. Ann Oncol. 2007;18:1165–1171. doi: 10.1093/annonc/mdm119. [DOI] [PubMed] [Google Scholar]
- 27.Coleman R., Costa L., Saad F., Cook R., Hadji P., Terpos E. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol/Hematol. 2011;80:411–432. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Rizzoli R., Body J.J., Brandi M.L., Cannata-Andia J., Chappard D., El Maghraoui A. Cancer-associated bone disease. Osteoporos Int. 2013;24:2929–2953. doi: 10.1007/s00198-013-2530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devlin R.D., Bone H.G., 3rd, Roodman G.D. Interleukin-6: a potential mediator of the massive osteolysis in patient with Gorham–Stout disease. J Clin Endocrinol Metab. 1996;81:1893–1897. doi: 10.1210/jcem.81.5.8626854. [DOI] [PubMed] [Google Scholar]