Abstract
The 9th Bone and the Oncologist New Updates conference was held in Ottawa, Canada during 2014. This annual meeting focuses on innovative research into the mechanisms and consequences of treatment-induced and metastatic bone disease. Given the recent presentation of the Oxford overview's “Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: A meta-analysis of individual patient data from randomized trials” at the San Antonio Breast Cancer Symposium, a debate as to the pro's and con's of adjuvant bisphosphonate use in early stage breast cancer was undertaken. As bisphosphonate treatment in post-menopausal women appeared to demonstrate a similar magnitude of benefit to that of other commonly used adjuvant strategies the debate assessed whether or not there was sufficient data to incorporate adjuvant bisphosphonates into standard practice and if so, in which patient populations.
Keywords: Cancer, Bone health, Bone metastases, Bone-targeted agents
1. Introduction
The Bone and Oncologist New Updates (BONUS) meeting is an annual conference, based in Canada that focuses on new advances in the multidisciplinary management of cancer related bone disease. An important goal of the meeting has been to drive research collaboration within the attending audience of basic scientists and clinicians, but also to produce guidelines and recommendations to the broader audience of health care workers involved in the care of cancer patients. The meeting has previous produced a number of documents covering basic science [1], translational research [2–4], clinical research [5–10] and practice guidelines [11,12]. Each year a debate is held on a controversial bone-related topic and so for the 2014 BONUS meeting, the debate focused on the recently presented meta-analysis by the Early Breast Cancer Trialists Collaborative Group (EBCTCG) on the use of adjuvant bisphosphonates in early stage breast cancer [13].
Given the recognized effects of bisphosphonates in metastatic breast cancer [14,15] and that potential anticancer effects have been demonstrated in preclinical [16], translational [17], patients with cancer therapy-induced bone loss [18] and population based-studies [19,20] a number of clinical trials assessing bisphosphonates' effect on outcome in early stage breast cancer have been performed. Many of these trials presented conflicting results [21,24], but a consistent trend of beneficial effect on breast cancer recurrence was seen in older women. Of concern was that a useful clinical effect might have been missed because of trial design, end-points used, and under-powering of clinical trials for sub-group analyses [16]. Hence, an individual patient data meta analysis was performed and presented at the San Antonio Breast Cancer Symposium, December 2013 [13]. As this was considered by the BONUS meeting organizers an important, but potentially controversial topic, a debate format was used to best demonstrate contrasting views. While the original title for the debate was “This house believes that adjuvant bisphosphonates represent a gold-standard for post-menopausal women with higher risk breast cancer” the debaters asked if they could amend the title. In this commentary, we summarize the debates findings, and incorporate comments from the audience.
2. All women with invasive breast cancer over 50 should be offered a bisphosphonate
2.1. Presenter – Dr. Alexander Paterson
Multiple trials have studied bisphosphonates as a component of adjuvant systemic therapy for breast cancer (Table 1). An initial large trial of adjuvant clodronate conducted from 1989–1995 randomized 1069 patients with operable primary breast cancer to clodronate or placebo, given for two years. The primary endpoint of relapse in bone during the medication period showed a significant reduction in the occurrence of bone metastases in the clodronate arm, HR 0.44 (95% CI 0.22–0.86, p=0.016). However, for the entire period of follow up, the reduction in occurrence of bone metastases was non-significant (HR 0.77, 95% CI 0.56–1.08, p=0.127). The secondary endpoint of mortality also showed a significant reduction in the clodronate arm, HR 0.77 (95% CI 0.59–1.0, p=0.047) with an unplanned subgroup analysis of post-menopausal women showing a greater effect on mortality, with HR 0.61 (95% CI 0.42–0.88) [25].
Table 1.
Selected larger trials of adjuvant bisphosphonates in non-metastatic breast cancer.
| Trial | Agent | N | Duration bone agent | Outcome measure | Outcome | 95% CI | p | Summary |
|---|---|---|---|---|---|---|---|---|
| Powles (25) | Clodronate or placebo | 1069 | 2 years | Relapse in bone (during treatment) | HR=0.44 | 0.22–0.86 | 0.018 | Favors clodronate |
| Relapse in bone (follow up) | HR=0.77 | 0.56–1.08 | 0.127 | |||||
| Mortality (all) | HR=0.77 | 0.59 1.0 | 0.047 | Favors clodronate | ||||
| Mortality (Post menopausal subgroup) | HR=0.61 | 0.42–0.88 | Favors clodronate | |||||
| AZURE (23) | Zoledronic acid or standard care | 3360 | 5 years | DFS (all) | HR=0.94 | 0.82–1.06 | ||
| DFS (>5 years post menopausal) | HR=0.77 | 0.69–0.96 | Favors zoledronic acid | |||||
| NSABP B-34 (22) | Clodronate or placebo | 3311 | 3 years | DFS (all) | HR=0.91 | 0.78–1.07 | ||
| BMFI (all) | HR=0.77 | 0.55–1.07 | 0.12 | |||||
| BMFI (>50 years age at entry) | HR=0.62 | 0.4–0.95 | 0.022 | Favors clodronate | ||||
| OS (all) | HR=0.84 | 0.67–1.05 | 0.13 | |||||
| OS (>50 years age at entry) | HR=0.80 | 0.61–1.04 | 0.094 | Favors clodronate | ||||
| Meta-analysis (13) | Any bisphosphonate | 22,982 | Any duration | DR (all) | 22.3 versus 20.9% | 0.03 | ||
| DR (post menopausal) | 21.9 versus 18.4% | 0.0003 | Favors bone agent | |||||
| BC Mortality (all) | 8.7 versus 16.9% | 0.04 | ||||||
| BC Mortality (post menopausal) | 18.3 versus 15.2% | 0.004 | Favors bone agent |
More recently, the AZURE trial was a randomized open label trial of standard therapy versus standard therapy and zoledronic acid (given for five years) in 3360 women with stage II or III breast cancer. The primary endpoint of disease free survival (DFS) did not differ between the two groups (HR 0.94, 95% CI 0.82–1.06). However in a planned subgroup analysis, zoledronic acid improved DFS in women who were more than 5 years since menopause at trial entry [23]. A further similar trial, NSABP B34, randomized 3311 women with stage I–III breast cancer to oral clodronate or placebo for three years. Again DFS did not differ between groups at a median of 90.7 months follow up (HR 0.91, 95% CI 0.78–1.07) but in women greater than 50 years or older at study entry, clodronate showed benefits for recurrence free interval (HR 0.75, 95% CI 0.57–0.99, p=0.045), bone-metastases free interval (HR 0.62, 95% CI 0.40–0.95, p=0.027) and non-bone metastases free interval (HR 0.63, 95% CI 0.43–0.91, p=0.014). There was no benefit seen for overall survival between the two treatment arms although there was a numerical difference in deaths in women over 50 years of age, favoring the clodronate arm [22].
Given these and other conflicting results, an individual patient data meta-analysis was undertaken, with the results being presented at the San Antonio Breast Cancer Symposium, 2013 [13]. This analysis included 36 trials comprising 22982 patients, with primary outcomes of time to recurrence (TTR), time to first distant recurrence (TFDR) and breast cancer mortality. Planned subgroup analysis included menopausal status (if menopausal status was not documented, women >55 years of age were considered post-menopausal). Among all women the results showed no improvements in recurrence rate (26.5% no bisphosphonate versus 25.4% bisphosphonate, p=0.08), and a borderline improvement in distant recurrence rates (22.3% no bisphosphonate versus 20.9% bisphosphonate, p=0.03). Among the 11,036 post-menopausal women included in this analysis, significant improvements were seen in rates of distant recurrence (21.9% no bisphosphonate versus 18.4% bisphosphonate, p<0.001) and this difference was driven by a bisphosphonate-related reduction in bone recurrence (8.8% versus 5.9%, p<0.001). Ten-year breast cancer mortality was also significantly improved in post-menopausal women in the bisphosphonate arm with mortality rates of 18.3% in the no bisphosphonate group and 15.2% in the bisphosphonate group, p=0.004. This led the authors to conclude adjuvant bisphosphonates reduce bone metastases and improve survival in post-menopausal women, with a 34% reduction in risk of bone recurrence (p<0.001) and a 17% reduction in risk of breast cancer death (p=0.004). The effect was seen irrespective of bisphosphonate type. There were no effects in pre-menopausal women (in particular, no deleterious effects) and no effects on non-breast cancer deaths, contralateral breast cancer or local-regional recurrence.
In summary, this EBCCTG overview demonstrated a positive effect from bisphosphonates in a pre-determined sub-group analysis, resulting from consistent findings from multiple well-conducted trials. The finding is plausible, and the results support a modified vicious cycle hypothesis [26,27]. The exact mechanism of an enhanced anti-tumor effect of bisphosphonates in a low estrogen environment is uncertain, but certainly feasible [2,28,29]. One hypothesis may be that, by preventing enhanced bone destruction induced by the lack of estrogen, bisphosphonates interfere with the tumor-growth-supportive functions of bone-derived growth factors demonstrated in the vicious cycle hypothesis [26,28]. Alternately, low estrogen levels may not be the cause, other possibilities include increased levels of pro-inflammatory proteins leading to enhanced macrophage activity in the aging process reduced by bisphosphonates [30]. The magnitude of benefit of bisphosphonates is similar, if not greater than, other strategies that have been widely adopted in the breast cancer clinic. The absolute benefit in mortality for post menopausal women of 3.1% at 10 years compares to the estimated absolute benefit of anthracycline containing chemotherapy over CMF (cyclophosphamide, methotrexate and 5-fluorouracil) of 3% at 5 years [31] and taxanes in addition to anthracycline chemotherapy regimens, a 3.2% gain at 8 years [32].
Further discussion presented included a rational approach as to whom to offer bisphosphonate therapy to. Suggested populations included; patients with osteopenia or osteoporosis, patients on aromatase inhibitors (in whom the administration of bisphosphonates may ameliorate the accelerated loss of bone mineral density seen with aromatase inhibitors [33–35]), those with higher risk disease (by stage, grade and receptor status). The question of which bisphosphonate to use may be answered with the results of the SWOG 0307 trial, that will likely be presented in 2015 [36]. Interim toxicity and patient preference results of SWOG 0307 suggest clodronate may be the preferred bisphosphonate. After 3 years of therapy, rates of osteonecrosis of the jaw were lowest for clodronate (0.28%) compared with zoledronic acid (1.15%) and ibandronate (0.66%), p=0.003. Patient preference favored oral medications at trial initiation (76% versus 24%) and this preference changed little at the completion of therapy [37].
3. Adjuvant bisphosphonates DO NOT represent a gold-standard for post-menopausal women with higher risk breast cancer
3.1. Presenter – Dr. Eitan Amir
While the presentation of the Early Breast Cancer Trialists Collaborative Group (EBCTCG) results [13] raises many questions it is important to question the methodology of performing meta-analyses. The first point to consider is whether or not a meta-analysis of subgroups should be used to influence clinical decision making? Subgroup analysis have an increased probability of type I error (false positive) when the null hypothesis is true (negative trial). This leads to difficulty in interpretation. Hypotheses tested usually address an overall treatment effect in the study population, with no assumption of homogeneity of effect across subgroups. The direction, not magnitude, of the treatment effect is expected to be the same in subgroups. Stratification or regression techniques can be used to adjust the overall comparison for subgroups or covariates. However, subgroup analyses are generally of secondary interest and more appropriate for hypothesis generation for future studies. If data from the EBCTCG meta analysis will be used to change clinical practice, this will be the first time that (predominantly unstratified) subgroup analysis has been used for decision making. This is methodologically concerning as a precedence as there is a higher than desirable chance the findings are a false positive.
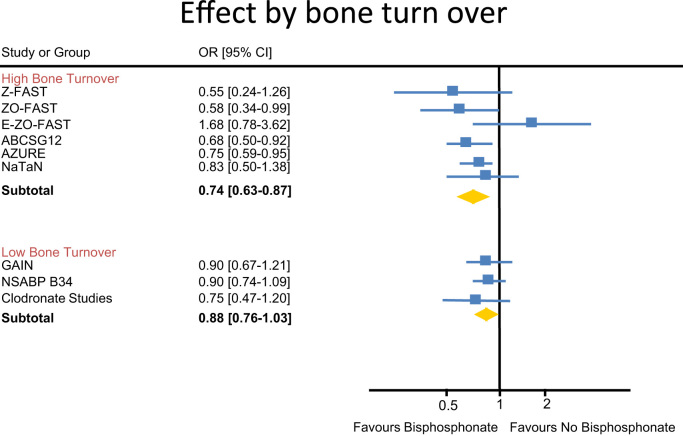
In addition to the validity of a sub group analysis changing clinical practice, the issue of patient selection was discussed. Do all post-menopausal women benefit from adjuvant bisphosphonates or can we identify a subgroup more likely to benefit? If adjuvant bisphosphonates predominantly reduce bone metastases; are patients with higher bone turn-over more likely to develop bone metastases? Therefore would adjuvant bisphosphonates predominantly be active in patients with higher bone turn-over? For example, if we use the use of an adjuvant aromatase inhibitor as a surrogate for high turnover [21,22,38–43] it appears that there is indeed greater benefit in this patient population (Fig. 1).
Fig. 1.
Effect of bisphosphonate in high versus low bone turnover populations.
In summary, the quality of data to support adjuvant bisphosphonates in post-menopausal women should not be considered as hypothesis testing, but rather hypothesis generating. Future clinical trials should focus on patients with higher bone turn-over as they appear to benefit most from adjuvant bisphosphonate therapy. Due to the above concerns, adjuvant bisphosphonates DO NOT represent a gold-standard for post-menopausal women with higher risk breast cancer.
3.2. Audience poll
Following the debate the audience were ask to vote on, “Are adjuvant bisphosphonates now standard of care of women with early stage breast cancer?” The majority of the audience voted no.
4. Discussion
While a debate can offer a light hearted means of assessing a particular topic it does provide an arena for opposing views to be presented. Sadly, it is unlikely that more definitive data will answer the question of whether adjuvant bisphosphonates are standard of care for women with early stage breast cancer. The D-CARE study [44] is evaluating the role of adjuvant denosumab and if positive would likely lead to denosumab being the standard of care. However, if positive in only a subgroup of patients, this will lead further confusion to the topic. Initial results for the primary outcome of bone metastases free survival are expected in 2016. The SWOG 0307 trial [36], comparing adjuvant clodronate with ibandronate and zoledronic acid, is expected to present results in 2015. While this trial may answer the question of which bisphosphonate is preferable, the absence of a placebo arm means that the question of this debate will remain unclear unless one arm is significantly superior to the others. In addition, the results of the SOFT [45] and TEXT [46] trials mean that increasing numbers of premenopausal women are going to undergo ovarian suppression, should these women also receive a bisphosphonate? To date we are unaware of the incorporation of adjuvant bisphosphonates into any practice guidelines but whether that changes when the EBCTCG meta-analysis is published in peer-reviewed format remains to be seen.
5. Conclusion
The BONUS conference brings together physicians, scientists and other professionals with an interest in bone health in cancer. This year's meeting demonstrated the ever changing role of bone targeted agents in management of patients with cancer. The EBCTCG meta-analysis of bisphosphonate use in early breast cancer provides evidence for the benefit of adjuvant bisphosphonate therapy in post-menopausal women in both reducing rates of disease recurrence and breast cancer mortality. Publication of the data is awaited. While compelling, caution in data interpretation must be exercised as should clinical judgment and consideration at an individual patient level.
Disclosures of potential conflicts of interest
AP is an author on previously published clodronate trials, ‘NSABP B-34’ and ‘Randomized, Placebo-Controlled Trial of Clodronate in Patients With Primary Operable Breast Cancer, Powles et al.’. AP is an author of the EBCTCG meta analysis of bisphosphonate use in early breast cancer (currently unpublished). AP and MC are co-investigators on the SWOG 0307 trial.
Honoraria received from Amgen (AP), Novartis (AP) and Roche Pharma (AP). Research funding from Amgen (AP, MC), Early Breast Cancer Clinical Trialists Group (AP), and National Institutes of Health US (AP).
References
- 1.Fralick M., Bouganim N., Kremer R., Kekre N., Robertson S., Vandermeer L. Histomorphometric and microarchitectural analyses using the 2 mm bone marrow trephine in metastatic breast cancer patients–preliminary results. J Bone Oncol. 2012;1(3):69–73. doi: 10.1016/j.jbo.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell K., Amir E., Paterson A., Josse R., Addison C., Kuchuk I. Does estrogen play a role in response to adjuvant bone-targeted therapies? J Bone Oncol. 2013;2(4):167–173. doi: 10.1016/j.jbo.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addison C.L., Pond G.R., Zhao H., Mazzarello S., Vandermeer L., Goldstein R. Effects of de-escalated bisphosphonate therapy on bone turnover biomarkers in breast cancer patients with bone metastases. SpringerPlus. 2014;3:577. doi: 10.1186/2193-1801-3-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.C.L. Addison, N. Bouganim, J. Hilton, L. Vandemeer, S. Dent, E. Amir, et al., A Phase II, multicenter trial evaluating the efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal related events, Breast Cancer Res Treat. 2014;144(3):615-24. 10.1007/s10549-014-2906-x. Epub 2014 Mar 18. [DOI] [PMC free article] [PubMed]
- 5.Hutton B., Addison C., Campbell K., Fergusson D., Mazarello S., Clemons M. A systematic review of dosing frequency with bone targeted agents for patients with bone metastases from breast cancer. J Bone Oncol. 2013;2:123–131. doi: 10.1016/j.jbo.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuchuk I., Hutton B., Moretto P., Ng T., Addison C.L., Clemons M. Incidence, consequences and treatment of bone metastases in breast cancer patients—experience from a single cancer centre. J Bone Oncol. 2013;2(4):137–144. doi: 10.1016/j.jbo.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutton B., Morretto P., Emmenegger U., Mazzarello S., Kuchuk I., Addison C.L. Bone-targeted agent use for bone metastases from breast cancer and prostate cancer: a patient survey. J Bone Oncol. 2013;2(3):105–109. doi: 10.1016/j.jbo.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutton B., Addison C., Mazzarello S., Joy A.A., Bouganim N., Fergusson D. De-escalated administration of bone-targeted agents in patients with breast and prostate cancer—a survey of Canadian oncologists. J Bone Oncol. 2013;2(2):77–83. doi: 10.1016/j.jbo.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons M.J., Cochrane B., Pond G.R., Califaretti N., Chia S.K., Dent R.A. Randomised, phase II, placebo-controlled, trial of fulvestrant plus vandetanib in postmenopausal women with bone only or bone predominant, hormone-receptor-positive metastatic breast cancer (MBC): the OCOG ZAMBONEY study. Breast Cancer Res Treat. 2014;146(1):153–162. doi: 10.1007/s10549-014-3015-6. [DOI] [PubMed] [Google Scholar]
- 10.Kuchuk I., Simos D., Addison C.L., Clemons M. A national portfolio of bone oncology trials—the Canadian experience in 2012. J Bone Oncol. 2012;1(3):95–100. doi: 10.1016/j.jbo.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuchuk I., Mazzarello S., Butterfield K., Appleton A., Addison C.L., Clemons M. Oral care and the use of bone-targeted agents in patients with metastatic cancers: A practical guide for dental surgeons and oncologists. J Bone Oncol. 2013;2(1):38–46. doi: 10.1016/j.jbo.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.S. Mazzarello, M. Clemons, I.D. Graham, C. Jacobs, Surviving surveys, J Oncol Pract (2014). (August 5), 001484 [DOI] [PubMed]
- 13.Coleman R, Gnant M, Paterson A, et al. Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: A meta-analysis of individual patient data from randomized trials. 2013 San Antonio Breast Cancer Symposium; 12 December 2013.
- 14.Van Poznak C.H., Temin S., Yee G.C., Janjan N.A., Barlow W.E., Biermann J.S. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncoly: Off J Am Soc Clin Oncol. 2011;29(9):1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso F., Harbeck N., Fallowfield L., Kyriakides S., Senkus E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol: Off J Eur Soc Med Oncol. 2012;23(Suppl 7):vii11–vii19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 16.Clemons M., Russell K., Costa L., Addison C.L. Adjuvant bisphosphonate treatment for breast cancer: why did something so elegant become so complicated? Breast Cancer Res Treat. 2012;134(2):453–457. doi: 10.1007/s10549-012-2077-6. [DOI] [PubMed] [Google Scholar]
- 17.Aft R., Naughton M., Trinkaus K., Watson M., Ylagan L., Chavez-MacGregor M. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 2010;11(5):421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amir E., Ocana A., Seruga B., Josse R., Clemons M. Medical oncology: zoledronic acid for breast cancer therapy-induced bone loss. Nat Rev Clin Oncol. 2010;7(4):187–188. doi: 10.1038/nrclinonc.2010.19. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Aharon I., Vidal L., Rizel S., Yerushalmi R., Shpilberg O., Sulkes A. Bisphosphonates in the adjuvant setting of breast cancer therapy--effect on survival: a systematic review and meta-analysis. PloS ONE. 2013;8(8):e70044. doi: 10.1371/journal.pone.0070044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter M.C., Holen I., Coleman R.E. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34(5):453–475. doi: 10.1016/j.ctrv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Gnant M., Mlineritsch B., Stoeger H., Luschin-Ebengreuth G., Heck D., Menzel C. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12(7):631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 22.Paterson A.H., Anderson S.J., Lembersky B.C., Fehrenbacher L., Falkson C.I., King K.M. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13(7):734–742. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman R., Cameron D., Dodwell D., Bell R., Wilson C., Rathbone E. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15(9):997–1006. doi: 10.1016/S1470-2045(14)70302-X. [DOI] [PubMed] [Google Scholar]
- 24.G. von Minckwitz, V. Möbus, A. Schneeweiss, J. Huober, C. Thomssen, M. Untch, et al., German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer, Clin Oncol 2013; 31(28):3531-9 [DOI] [PubMed]
- 25.Powles T., Paterson S., Kanis J.A., McCloskey E., Ashley S., Tidy A. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;20(15):3219–3224. doi: 10.1200/JCO.2002.11.080. [DOI] [PubMed] [Google Scholar]
- 26.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 27.Russell K., Clemons M., Costa L., Addison C.L. Adjuvant bisphosphonate treatment for breast cancer: Where are we heading and can the pre-clinical literature help us get there? J Bone Oncol. 2012;1(1):12–17. doi: 10.1016/j.jbo.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clezardin P. Mechanisms of action of bisphosphonates in oncology: a scientific concept evolving from antiresorptive to anticancer activities. BoneKEy Rep. 2013;2:267. doi: 10.1038/bonekey.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suriano R., Chaudhuri D., Johnson R.S., Lambers E., Ashok B.T., Kishore R. 17Beta-estradiol mobilizes bone marrow-derived endothelial progenitor cells to tumors. Cancer Res. 2008;68(15):6038–6042. doi: 10.1158/0008-5472.CAN-08-1009. [DOI] [PubMed] [Google Scholar]
- 30.Omoigui S. The Interleukin-6 inflammation pathway from cholesterol to aging – role of statins, bisphosphonates and plant polyphenols in aging and age-related diseases. Immun Ageing. 2007;4(1) doi: 10.1186/1742-4933-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Early Breast Cancer Trialists' Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists' Collaborative Group Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eastell R., Adams J.E., Coleman R.E., Howell A., Hannon R.A., Cuzick J. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol: Off J Am Soc Clinl Oncol. 2008;26(7):1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 34.Coleman R.E., Banks L.M., Girgis S.I., Kilburn L.S., Vrdoljak E., Fox J. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8(2):119–127. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 35.Perez E.A., Josse R.G., Pritchard K.I., Ingle J.N., Martino S., Findlay B.P. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol: Off J Am Soc Clin Oncol. 2006;24(22):3629–3635. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 36.S0307 Zoledronate, Clodronate, or Ibandronate in Treating Women Who Have Undergone Surgery for Stage I, Stage II, or Stage III Breast Cancer 2014 [cited 2014 29 December]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT00127205〉.
- 37.Gralow J, Barlow W, Paterson AHG, Lew D, Stopeck A, Hayes DF, et al., editor. SWOG S0307 phase III trial of bisphosphonates as adjuvant therapy in primary breast cancer: Comparison of toxicities and patient-stated preference for oral versus intravenous delivery. ASCO Annual Meeting. Chicago; 2014.
- 38.Brufsky A.M., Harker W.G., Beck J.T., Bosserman L., Vogel C., Seidler C. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118(5):1192–1201. doi: 10.1002/cncr.26313. [DOI] [PubMed] [Google Scholar]
- 39.Coleman R., de Boer R., Eidtmann H., Llombart A., Davidson N., Neven P. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol: Off J Eur Soc Med Oncol. 2013;24(2):398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 40.Llombart A., Frassoldati A., Paija O., Sleeboom H.P., Jerusalem G., Mebis J. Immediate Administration of Zoledronic Acid Reduces Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women With Early Breast Cancer: 12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer. 2012;12(1):40–48. doi: 10.1016/j.clbc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Coleman R.E., Marshall H., Cameron D., Dodwell D., Burkinshaw R., Keane M. Breast-Cancer Adjuvant Therapy with Zoledronic Acid. N Engl J Med. 2011;365(15):1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 42.von Minckwitz G, Rezai M, Eidtmann H, Tesch H, et al., (eds). Postneoadjuvant treatment with zoledronate in patients with tumor residuals after anthracyclines-taxane-based chemotherapy for primary breast cancer – The phase III NATANstudy (GBG 36/ABCSG XX). San Antonio Breast Cancer Symposium. San Antonio; 2013.
- 43.von Minckwitz G., Mobus V., Schneeweiss A., Huober J., Thomssen C., Untch M. German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31(28):3531–3539. doi: 10.1200/JCO.2012.47.2167. [DOI] [PubMed] [Google Scholar]
- 44.Study of Denosumab as Adjuvant Treatment for Women With High Risk Early Breast Cancer Receiving Neoadjuvant or Adjuvant Therapy (d-CARE) [cited 29.12.14]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT01077154〉.
- 45.P.A. Francis, M.M. Regan, G.F. Fleming, I. Lang, E. Ciruelos, M. Bellet, et al., Adjuvant ovarian suppression in premenopausal breast cancer, N Engl J Med 2015; 372:436-446 [DOI] [PMC free article] [PubMed]
- 46.Pagani O., Regan M.M., Walley B.A., Fleming G.F., Colleoni M., Lang I. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]