Abstract
Background
Primary lymphoma of bone (PLB) is a rare disease, comprising a malignant lymphoid infiltrate of bone. The goal of this study was to identify socioeconomic, demographic, and anatomic factors as prognostic indicators of survival for this disease using the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
The SEER database was used to identify a study population of 692 patients diagnosed with PLB in the United States from 1989 to 2003. Survival was analyzed using the Kaplan–Meier method, with effects of potential prognostic factors on survival analyzed using the log-rank test. Multivariable analysis was performed by Cox proportional hazards regression.
Results
The overall 5-year survival rate was 49.6%, with a 10-year survival rate of 30.2%. Median overall survival was 4.9 years (95% CI: 3.9, 6.1). In multivariable analysis, age (p<0.0001), marital status (p=0.006), and appendicular vs. axial tumor location (p=0.004) were found to be independent predictors of survival.
Conclusions
This population-based study of PLB identified age, marital status, and tumor location as independent indicators of prognosis. This finding supports the clinical suspicion that an appendicular tumor location confers a better prognosis than an axial tumor location.
Keywords: Lymphoma, Bone, SEER, Socioeconomic
1. Introduction
The rare disease now known as primary lymphoma of bone (PLB) was initially described by Oberlin [1]. Parker and Jackson later described PLB as a distinct entity [2], which is understood to be a malignant lymphoid infiltrate of bone [3]. While this may include cortical or soft tissue invasion, the diagnosis generally excludes lymph node or distant visceral involvement to be considered a primary lymphoma of bone [4]. This definition has been contended throughout the literature, with some authors permitting lymph node involvement [5–7], though most authors recognize the disease as lymphoma localized only to bone upon diagnosis.
Primary lymphoma of bone affects 1.7/1,000,000 patients in the US [8]. Previous studies have suggested PLB comprise 5% of all extranodal lymphomas [9], and 3% of all bone malignancies [10]. Due to the low incidence of PLB, most prior studies have been from a single-institution, and have had small sample sizes [10–15]. The Surveillance, Epidemiology, and End Results (SEER) database is a publically available database created for the purpose of collecting demographic, clinical, and outcome data for cancer patients in the United States. There remains scant literature published using this database to evaluate larger patient populations [8].
The purpose of this population-based study was to identify prognostic factors for survival in patients with PLB, in order to determine whether disparities in survival exist between demographic cohorts. In doing so, the current study aimed to determine the prognostic significance of different tumor-specific and anatomic considerations that may influence overall survival from this disease. The authors chose to limit this study to adult patients, because PLB in children is often considered and treated as a systemic disease [16].
2. Material and methods
The study population was selected from the National Cancer Institute’s SEER database. The SEER database collects data from 18 geographic registries, covering approximately 28% of the U.S. population [17]. The SEER⁎Stat software (Version 8.0.4; NCI; Bethesda, MD) was used to identify 997 adult patients diagnosed with primary lymphoma of bone during a 15-year period from 1989 to 2003. Histology was selected by using ICD-O-3 codes 9590/3, 9591/3, 9670/3, 19671/3, 9675/3, 9680/3, and 9684/3. Primary site was selected as C40.0, C40.1, C40.2, C40.3, C40.8, C40.9, C41.2, C41.3, C41.4, and C41.9. Exclusion criteria included lesions of the skull and face, T-cell lymphoma, and cases without follow up data, yielding a final study population of 692 patients.
Tumor location was dichotomized as either appendicular or axial. The scapula was considered to be part of the appendicular skeleton, while the pelvic bones were considered to be part of the axial skeleton. Marital status was categorized as single, married, or other (including separated, divorced or widowed). Rural–urban continuum code was collapsed into a binary variable: Metro county or non-metro county, using guidelines by SEER and the Economic Research Service [18,19]. SEER registry region was aggregated into regions (Northeast, South, Southwest, Midwest, and West). Race was categorized as White, Black or Asian/Other. Age was considered as a categorical variable (<30 years, 30–59 years, ≥60 years).
Statistical analysis was performed in SAS version 9.3 (SAS Institute, Cary, NC). The effects of categorical variables on survival were assessed by computing Kaplan–Meier product limit curves and compared using the log-rank test. The effects of continuous variables were analyzed using Cox proportional hazards regression. The Bonferroni method was applied when performing multiple comparisons. Factors that appeared to be significantly associated with survival in the univariate analysis were considered for inclusion in the final multivariable Cox proportional hazards regression model. A result was considered statistically significant with a p-value <0.05. Efron’s method was used to adjust for tied failure times.
Incidence rates were age-adjusted to the 2000 US standard population, with confidence intervals calculated using the Tiwari modification. Annual percentage change was calculated using the weighted least squares method.
3. Results
The final analysis included 692 patients, whose demographic and clinical characteristics are presented in Table 1. The majority of patients were white (89.0%), non-Hispanic (91.3%), and lived in metropolitan counties (87.4%). The majority of patients were over the age of 60 years (55.6%), and diffuse large B-cell lymphoma was the most common histologic classification (71.2%). The western region of the United States contributed the largest proportion of patients to the study population (56.5%).
Table 1.
Descriptive demographic and clinical statistics of the study population.
| Characteristic | Frequency | % Of total |
|---|---|---|
| Total number of patients | 692 | 100.0 |
| Age | ||
| <30 | 67 | 9.7 |
| 30 to 59 | 240 | 34.7 |
| 60 or greater | 385 | 55.6 |
| Sex | ||
| Male | 370 | 53.5 |
| Female | 322 | 46.5 |
| Race | ||
| White | 616 | 89.0 |
| Black | 45 | 6.5 |
| Asian, other and unknown | 31 | 4.5 |
| Ethnicity | ||
| Non-Spanish–Hispanic–Latino | 632 | 91.3 |
| Spanish–Hispanic–Latino | 60 | 8.7 |
| Marital status | ||
| Single | 122 | 18.2 |
| Married | 383 | 57.2 |
| Divorced, separated, widowed | 165 | 24.6 |
| Geographic region | ||
| Southeast | 54 | 7.8 |
| South | 42 | 6.1 |
| Midwest | 111 | 16.0 |
| Northeast | 94 | 13.6 |
| West | 391 | 56.5 |
| County | ||
| Non-metro county | 87 | 12.6 |
| Metro county | 605 | 87.4 |
| Tumor site | ||
| Axial | 389 | 56.2 |
| Appendicular | 303 | 43.8 |
| Histology | ||
| Malignant lymphoma, NOSa | 46 | 6.7 |
| Malignant lymphoma, non-Hodgkin, NOSa | 75 | 10.8 |
| Malignant lymphoma, small B lymphocytes, NOSa | 21 | 3.0 |
| Lymphoplasmacytic lymphoma (NHL b) | 11 | 1.6 |
| Malignant lymphoma, mixed small and large cell, diffuse | 22 | 3.2 |
| Diffuse large B-cell (NHLb) lymphoma | 493 | 71.2 |
| Malignant lymphoma, large B, diffuse, immunoblastic | 24 | 3.5 |
| Grade | ||
| B-cell; pre-B; B-precursor | 668 | 96.5 |
| Well differentiated; grade I | 6 | 0.9 |
| Moderately differentiated; grade II | 9 | 1.3 |
| Poorly differentiated; grade III | 5 | 0.7 |
| Undifferentiated; anaplastic; grade IV | 4 | 0.6 |
| Surgery | ||
| Yes | 177 | 25.8 |
| No | 510 | 74.2 |
| Radiation | ||
| Yes | 475 | 70.6 |
| No | 198 | 29.4 |
NOS, not otherwise specified.
NHL, non-Hodgkin lymphoma.
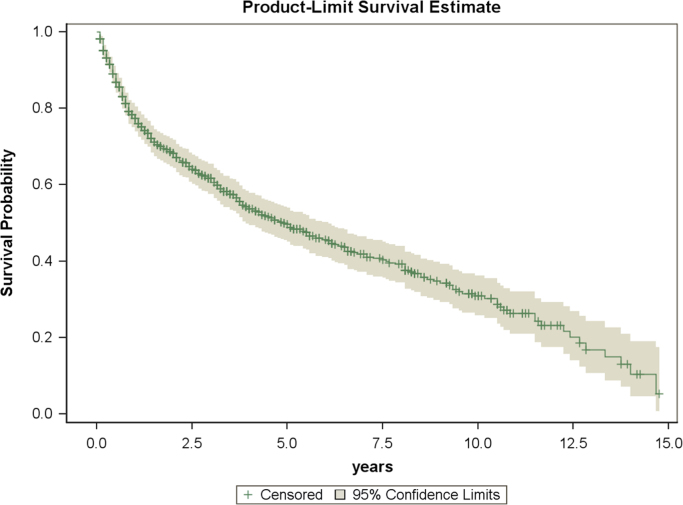
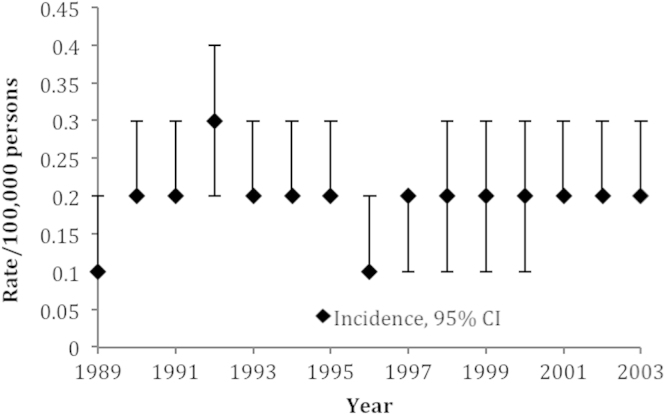
The estimated overall survival of patients for all patients in this study was 49.6% at 5-years, and 30.2% at 10-years (Fig. 1). The incidence of PLB during the 15-year study period ranged from 0.1/100,000 to 0.3/100,000 (Fig. 2). The annual percent change for this time period was non-significant, suggesting a stable incidence over the study period.
Fig. 1.
Kaplan–Meier plot of overall survival in study population.
Fig. 2.
Incidence of primary lymphoma of bone, age-adjusted to the 2000 US standard population.
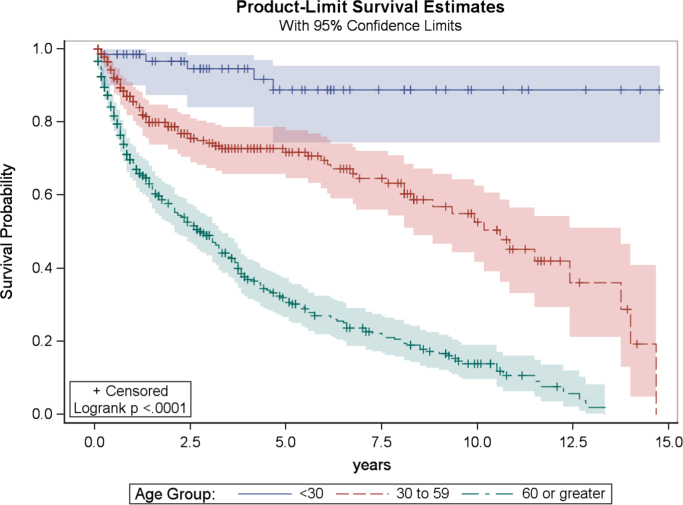
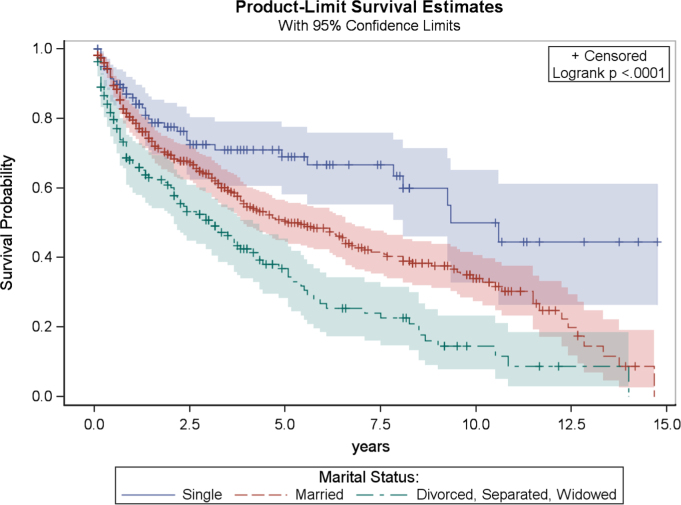
In univariate analysis, significant factors for overall survival included age (p<0.0001), marital status (p<0.0001), anatomic location of tumor (p<0.0001), geographic region (p=0.02), and tumor grade (p=0.01). After Bonferroni adjustment, tumor grade was no longer a significant prognostic indicator for overall survival. Furthermore, after Bonferroni adjustment for multiple comparisons, tumor grade and geographic region were not statistically significantly associated with overall survival. Univariate analysis results for categorical variables are presented in Table 2. Kaplan–Meier product limit curves are provided for age (Fig. 3), marital status (Fig. 4), and tumor location (Fig. 5).
Table 2.
Univariate analysis of effects on overall survival.
| Characteristic | 1-Year survival | 5-Year survival | 10-Year survival | p-Valuea |
|---|---|---|---|---|
| Overall | 77.4 | 49.6 | 30.2 | NAb |
| Age | <.0001⁎ | |||
| <30 | 98.5 | NA | NA | |
| 30–59 | 85.5 | 71.8 | 52.7 | |
| >60 | 68.8 | 30.6 | 13.9 | |
| Sex | .24 | |||
| Male | 78.0 | 49.5 | 35.6 | |
| Female | 76.7 | 49.8 | 24.5 | |
| Race | .09 | |||
| White | 77.2 | 48.2 | 29.4 | |
| Black | 82.8 | 62.2 | NA | |
| Other | 72.8 | 63.4 | 50.7 | |
| Ethnicity | .40 | |||
| Non-Hispanic | 78.2 | 50.0 | 30.7 | |
| Hispanic | 65.3 | 45.9 | 17.8 | |
| Marital status | <.0001⁎ | |||
| Married | 79.5 | 50.5 | 33.9 | |
| Single | 86.0 | 69.0 | 50.0 | |
| Divorced, separated, widowed | 66.6 | 34.3 | 11.6 | |
| Geographic region | .02⁎ | |||
| Northeast | 77.4 | 50.3 | NA | |
| Southeast | 73.1 | 8.1 | 0 | |
| Midwest | 73.8 | 46.3 | 26.1 | |
| West | 77.6 | 54.0 | 34.9 | |
| South | 78.8 | 41.7 | 34.2 | |
| Tumor location | <.0001⁎ | |||
| Appendicular | 85.6 | 58.4 | 33.7 | |
| Axial | 71.1 | 43.1 | 28.4 | |
| Histology | 1.0 | |||
| Malignant lymphoma, NOSc | 78.0 | 46.7 | 32.4 | |
| Malignant lymphoma, non-Hodgkin, NOSc | 73.2 | 39.2 | NA | |
| Malignant lymphoma, small B lymphocytes, NOSc | 69.7 | 25.7 | NA | |
| Lymphoplasmacytic lymphoma (NHLd) | 81.8 | 61.4 | NA | |
| Malignant lymphoma, mixed small and large cell, diffuse | 80.6 | 65.1 | 18.3 | |
| Diffuse large B-cell (NHLd) lymphoma | 77.9 | 50.4 | 31.5 | |
| Malignant lymphoma, large B, diffuse, immunoblastic | 66.7 | 51.9 | 28.8 | |
| Grade | .24 | |||
| B-cell; pre-B; B-precursor | 77.6 | 50.4 | 31.1 | |
| Grade I | 66.7 | NA | NA | |
| Grade II | 77.8 | 38.9 | NA | |
| Grade III | 60.0 | NA | NA | |
| Grade IV | NA | NA | NA | |
| Surgery | 0.19 | |||
| Yes | 78.6 | 42.1 | 27.4 | |
| No | 77.1 | 52.8 | 32.3 | |
| Radiation | 0.79 | |||
| No | 73.2 | 50.0 | 29.5 | |
| Yes | 80.3 | 49.2 | 29.9 | |
p Values are results of the log-rank test, with Bonferroni adjustments made for multiple comparisons.
NA, not available.
NOS, not otherwise specified.
NHL, non-Hodgkin lymphoma
Indicates result is statistically significant on a 0.05 level of significance.
Fig. 3.
Kaplan–Meier plot of overall survival related to age at diagnosis.
Fig. 4.
Kaplan–Meier plot of overall survival related to marital status.
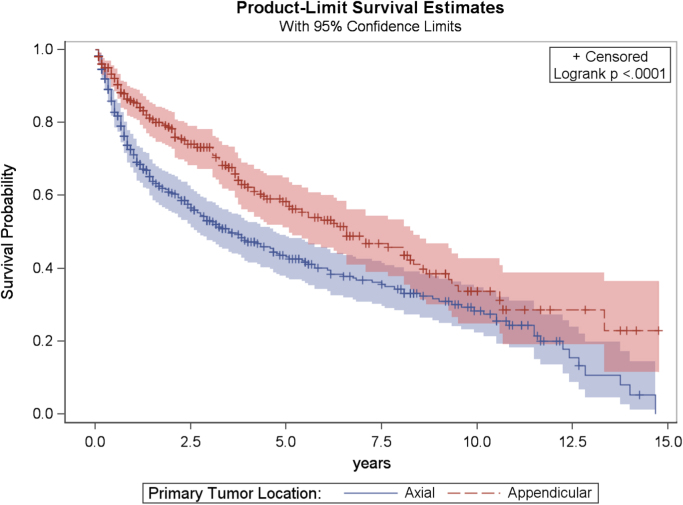
Fig. 5.
Kaplan–Meier plot of overall survival related to primary tumor location.
The final multivariable model demonstrated that age (p<0.0001), marital status (p=0.02), and appendicular or axial tumor location (p=0.004) remained significant independent prognostic variables for overall survival (Table 3). A survival advantage was demonstrated for younger patients. The mortality rate for PLB patients in the 30–59 age group is estimated to be 4.4 times that for those patients in the <30 age group, after adjusting for marital status and tumor location (CI: 1.7–11.2; p=0.002). Furthermore, patients aged 60 or older are estimated to have a mortality rate 12.8 times that for <30 year-old patients, after adjusting for tumor location and marital status (CI: 5.1–32.3; p<0.0001), though this finding is potentially influenced by medical comorbidities.
Table 3.
Multivariable analysis results.
| Variable | Hazard ratio | 95% CIa | p-Value |
|---|---|---|---|
| Tumor location | |||
| Appendicular | Reference group | ||
| Axial | 1.41 | 1.12–1.77 | 0.0035⁎ |
| Marital status | |||
| Married | Reference group | ||
| D, S, Wb | 1.41 | 1.11–1.79 | 0.0055⁎ |
| Single | 1.08 | 0.75–1.56 | 0.6889 |
| Age | |||
| <30 | Reference group | ||
| 30 to 59 | 4.39 | 1.72–11.19 | 0.0019⁎ |
| 60 or greater | 12.81 | 5.08–32.29 | <.0001⁎ |
CI, confidence interval.
D, divorced; S, separated; W, widowed.
Indicates statistically significant on a p<0.05 level.
The multivariable Cox regression model also showed that patients who were divorced, separated, or widowed had a significantly worse outcome than patients who were married or single; the mortality rate for Divorced, Separated or Widowed PLB patients was estimated to be 1.4 times that for married patients, after adjusting for tumor location and age (CI: 1.1–1.8; p=0.006). The hazard ratio for comparing single with married patients was non-significant, however (p=0.69).
Anatomical location of the primary tumor was also a significant factor in overall survival. Patients with an appendicular lesion had significantly better prognosis than patients with a primary axial lesion (Fig. 6). The mortality rate for PLB patients with axial-located disease is estimated to be 1.4 times that for those patients with appendicular-located disease, after adjusting for marital status and age (CI: 1.2–1.8; p=0.004).
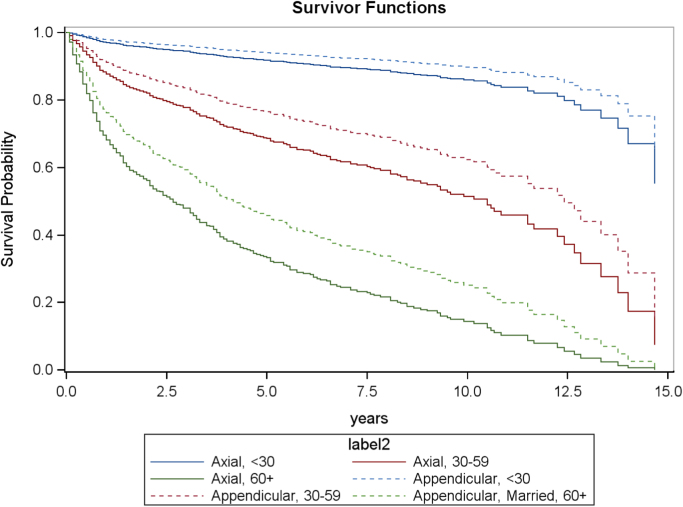
Fig. 6.
Multivariate adjusted survival curve comparing tumor location from 1989 to 2003 among married patients, stratified by age.
4. Discussion
The current study presents a population-based analysis of the effects of demographic factors on overall survival from primary lymphoma of bone. In order to account for the various clinical indicators, this study utilized the large patient population to better analyze the relative prognostic significance of patient- and tumor-related predictors of overall survival from the disease. To our knowledge, this is one of the largest studies of patients with primary lymphoma of bone published in the English language medical literature. Among the strengths of a large, population-based study is the inclusion of a larger collection of patients than would be feasible for a single institution study of such a rare disease, as well as the ability to sample a cross-section of the United States’ population.
Single institution studies have been published on individual chemotherapy regimens, as well as the individual prognostic potentials of several laboratory values (i.e. Lactate Dehydrogenase) [10,15]. However, the SEER database does not include data on chemotherapy [17], thus the relative effect of chemotherapy treatment regimens could not be evaluated in our analysis. We addressed the concern over evolving chemotherapy protocols by limiting the study to a 15-year period. While the authors recognize that significant improvements in imaging, staging, and clinical care have been made over the study period, there was no significant change in the incidence or overall survival over the course of this study, thereby supporting a stable clinical period for analysis. Limiting the years of the study also helped control for the changes in classification and categorization of the disease, while allowing for long-term follow-up for overall survival.
We found that appendicular tumor location is an independent predictor of survival in multivariable analysis when compared with axial location. While this has been suggested by previous authors, other relatively large studies investigating PLB have not yielded significant prognostic importance for overall survival, when considering confounding variables [8,15]. Age at time of diagnosis was also an independent predictor of survival, which has been consistently demonstrated in prior analyses [8,13,14]. Age over 60 years has previously been shown to influence both overall and disease-specific survival, so these findings are not entirely related to mortality from unrelated conditions. However, the ability to endure chemotherapy due to age and comorbidities may plan a significant role in considering treatment options.
In the current study, multivariable analysis demonstrated that divorced, separated, or widowed patients have a significantly poorer prognosis than married patients, independent of age. There was insufficient data available from the SEER database to elucidate the cause of this finding. However, using the SEER database, Aizer et al., also demonstrated that marital status was an independent predictor of survival following oncologic diagnoses [20]. They reported that the survival benefit associated with marriage was larger than the published survival benefit of chemotherapy for prostate, breast, colorectal, esophageal and head/neck cancers. Marital status was associated with lower stage at presentation in that study [20], suggesting that these findings may be due to lead time bias of earlier detection in married patients, rather than a true reflection of improved mortality.
5. Conclusions
In conclusion, there is evidence to suggest that socioeconomic factors, as well as clinical factors, contribute to the overall survival in patients with primary lymphoma of bone. Younger age, appendicular tumor location, and being married appear to be good prognostic factors for survival among patients with PLB.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Contributor Information
Andrew J. Jacobs, Email: ajacob24@pride.hofstra.edu.
Ryan Michels, Email: rmichels@nshs.edu.
Joanna Stein, Email: jstein4@nshs.edu.
Adam S. Levin, Email: alevin25@jhmi.edu.
References
- 1.Oberlin C. Les reticulosarcomes at les reticuloenotheliosarcomes de la moelle osseuse sarcomes dEwing. Bull Assoc Fr Etud Cancer. 1928;17:259–296. [Google Scholar]
- 2.Parker J, Jackson H. Primary reticulum cell sarcoma of bone. Surg Gynecol Obstet. 1939;68:45–53. [Google Scholar]
- 3.Pettit CK. Primary lymphoma of bone. A B-cell neoplasm with a high frequency of multilobated cells. Am J Surg Pathol. 1990;14(4):329–334. [PubMed] [Google Scholar]
- 4.Boston HC., Jr. Malignant lymphoma (so-called reticulum cell sarcoma) of bone. Cancer. 1974;34(4):1131–1137. doi: 10.1002/1097-0142(197410)34:4<1131::aid-cncr2820340424>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Ferreri AJ. Therapeutic management with adriamycin-containing chemotherapy and radiotherapy of monostotic and polyostotic primary non-Hodgkin’s lymphoma of bone in adults. Cancer Invest. 1998;16(8):554–561. doi: 10.3109/07357909809032885. [DOI] [PubMed] [Google Scholar]
- 6.Shoji H, Miller TR. Primary reticulum cell sarcoma of bone. Significance of clinical features upon the prognosis. Cancer. 1971;28(5):1234–1244. doi: 10.1002/1097-0142(1971)28:5<1234::aid-cncr2820280522>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Glotzbecker MP. Primary non-Hodgkin’s lymphoma of bone in children. J Bone Joint Surg Am. 2006;88(3):583–594. doi: 10.2106/JBJS.D.01967. [DOI] [PubMed] [Google Scholar]
- 8.Jawad MU. Primary lymphoma of bone in adult patients. Cancer. 2010;116(4):871–879. doi: 10.1002/cncr.24828. [DOI] [PubMed] [Google Scholar]
- 9.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29(1):252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Beal K, Allen L, Yahalom J. Primary bone lymphoma: treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer. 2006;106(12):2652–2656. doi: 10.1002/cncr.21930. [DOI] [PubMed] [Google Scholar]
- 11.Durr HR. Malignant lymphoma of bone. Arch Orthop Trauma Surg. 2002;122(1):10–16. doi: 10.1007/s004020100316. [DOI] [PubMed] [Google Scholar]
- 12.Ford DR. Primary bone lymphoma--treatment and outcome. Clin Oncol (R Coll Radiol) 2007;19(1):50–55. doi: 10.1016/j.clon.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Horsman JM. Primary bone lymphoma: a retrospective analysis. Int J Oncol. 2006;28(6):1571–1575. doi: 10.3892/ijo.28.6.1571. [DOI] [PubMed] [Google Scholar]
- 14.Lewis VO. Oncologic outcomes of primary lymphoma of bone in adults. Clin Orthop Relat Res. 2003;(415):90–97. doi: 10.1097/01.blo.0000093901.12372.ad. [DOI] [PubMed] [Google Scholar]
- 15.Demircay E. Malignant lymphoma of bone: a review of 119 patients. Clin Orthop Relat Res. 2013;471(8):2684–2690. doi: 10.1007/s11999-013-2991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doll C. Primary B-cell lymphoma of bone in children. Eur J Pediatr. 2001;160(4):239–242. doi: 10.1007/s004310000711. [DOI] [PubMed] [Google Scholar]
- 17.Surveillance Epidemiology and End Results. [accessed: July 30, 2013]; available from 〈http://seer.cancer.gov/〉; 2012.
- 18.Rural Classifications. [accessed: July 30, 2013]; Available from: 〈http://www.ers.usda.gov/topics/rural-economy-population/rural-classifications.aspx〉; 2013.
- 19.Rural-Urban Continuum Codes. [accessed: July 30, 2013]; Available from: 〈http://seer.cancer.gov/seerstat/variables/countyattribs/ruralurban.html〉; 2012.
- 20.Aizer AA. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]