Abstract
Bone cancers are characterised by the development of tumour cells in bone sites, associated with a dysregulation of their environment. In the last two decades, numerous therapeutic strategies have been developed to target the cancer cells or tumour niche. As the crosstalk between these two entities is tightly controlled by the release of polypeptide mediators activating signalling pathways through several receptor tyrosine kinases (RTKs), RTK inhibitors have been designed. These inhibitors have shown exciting clinical impacts, such as imatinib mesylate, which has become a reference treatment for chronic myeloid leukaemia and gastrointestinal tumours. The present review gives an overview of the main molecular and functional characteristics of RTKs, and focuses on the clinical applications that are envisaged and already assessed for the treatment of bone sarcomas and bone metastases.
Keywords: Bone metastasis, Bone sarcoma, Receptor tyrosine kinase, Growth factor, Inhibitor, Therapy
1. Introduction
To be able to play their physiological role (intra- and inter-cellular signal transmission and adaptation to changes in the microenvironment), cells must be able to receive, integrate and respond to numerous extracellular messengers. These communications between cells and their environment are made possible through the attachment of molecules considered as messengers to their receptors, identified as effectors (cytokines, growth factors, etc). As proposed by Ehrlich in 1910, “to act, a substance must be fixed." These receptors are essentially located at the cell membrane, although there are also intra-cytoplasmic receptors such as steroid hormone that can be translocated into the nucleus to regulate expression of numerous genes. Membrane receptors possess (i) an extracellular hydrophilic domain, often glycosylated, which recognises the ligand; (ii) a hydrophobic trans-membrane domain that makes embedding possible within the lipid bilayer of the plasma membrane; and (iii) an intra-cytoplasmic domain dedicated to signal transduction within the cell. The binding of a ligand to its receptor is specific, reversible and involves a large number of low-energy bonds (hydrogen, ionic, hydrophobic, and Van der Waals). Thus, at equilibrium, the dissociation rate is equal to the rate of association. Among the receptors of cytokine/growth factors, six types of receptor have intrinsic enzymatic activity (kinase or phosphatase receptors, and guanylyl cyclase-coupled receptors) or not (the G protein-coupled receptors, the receptor-type “channel”, and cytokine receptors).
The guanylyl cyclase-coupled receptors include natriuretic peptide, nitric oxide, carbon monoxide and enterotoxin receptors. The binding of the ligand to the extracellular domain of its receptor leads to intracellular activation of the guanylate cyclase domain of the receptor chain, and synthesis of a cyclic GMP for activating the cAMP-dependent protein kinase environment [1]. The G protein-coupled receptors are characterised by seven transmembrane domains. The trimeric G proteins located on the cytoplasmic side of the cell membrane transduce and amplify cell signalling through the production of cyclic AMP. The chemokine receptors are included in this family environment [2]. The ion channel linked receptors are ligand-dependent ion channels and their opening or closing activities are associated with the nature of the ligand. These receptors can be ionotropic or metabotropic. In the first case, the receptor is actually the pore, and opens following a conformational change made possible by the ligand binding. On the contrary, in the case of metabotropic receptors, ligand-stimulated receptors activate a ligand-independent channel through the intracellular effector environment [3]. Cytokine receptors can be divided into four groups: (i) receptors with an immunoglobulin-like ectodomain (IL-1α/β, IL-18); (ii) the trimeric members of the TNF receptor superfamily (which include, for instance, RANK, TRAIL receptors and TNF receptors-α/β); (iii), class I-cytokine receptors (or haematopoietin receptors) environment [4]; and (iv) class II-cytokine receptors (or interferon and IL-10 receptors) [5]. Class I/II- cytokine receptors have oligomeric structures, where a specific α-chain warrants specific ligand recognition, while one or two channels (β/γ) are used for signal transduction. For instance, the receptors of interleukins (IL) 2, 4, 7, 9 and 15 consist in a specific chain to the cytokine, and the shared IL-2 γ-receptor chain, IL-2 and IL-34 also share a β-receptor chain environment [6]. Similarly, the IL-6 cytokine family (IL-6, IL-11, CNTF, OSM and LIF) shares the gp130 receptor chain environment [7]. Among the cytokine receptor families, some are characterised by intrinsic kinase activity and consequently by their ability for autophosphorylation. They form the receptor tyrosine kinase (RTK) family.
All these receptors tightly control tissue homeostasis, and any dysregulation of these ligand–receptor systems (mutations, overexpression, etc.) disturbs cell communication and leads to pathological situations. Bone formation and bone remodelling are then controlled by a large panel of cytokines and growth factors regulating the dialogue between osteoblasts, osteoclasts and their environment [8]. It has been recognised that cancer cells (bone sarcomas and metastatic cells originating from carcinomas) dysregulate the balance between osteoblasts and osteoclasts, activate osteoclastogenesis and then stimulate bone resorption. Consequently, activated osteoclasts resorb the extracellular bone matrix and release numerous growth factors entrapped in the organic matrix, which stimulate in turn the proliferation of cancer cells. Based on these observations, numerous chemical drugs have been developed to specifically target the various receptor tyrosine kinases activated by mutations, or by the ligands present in the tumour microenvironment. The present review summarises the classification, structure and mechanism, and focuses on the targeting of action of the receptor tyrosine kinases. Their use in the treatment of bone cancers (bone sarcomas and bone metastases) is described and discussed.
2. The receptor tyrosine kinase (RTK) family
2.1. Classification and structure of RTKs
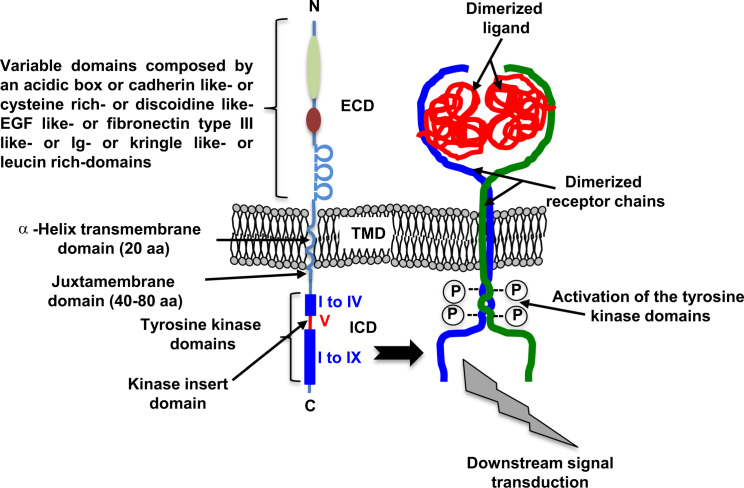
Protein kinases are key enzymes in the regulation of various cellular processes that catalyse the transfer of a phosphate group from ATP to a hydroxyl group of a serine or a threonine. Among the 90 identified genes encoding proteins with tyrosine kinase activity, 58 encode receptors divided into 20 subfamilies [9,10] (Table 1). Of these subfamilies, EGFR/ErbB (class I), the receptor for insulin (class II), for PDGF (Class III), for FGF (class IV), for VEGF (class V) and HGF (MET, Class VI) are strongly associated with oncological diseases. These RTKs are characterised by a single trans-membrane domain and a glycosylated N-terminal extracellular domain with a high number of disulfide bonds. This extracellular domain is involved in the dimerisation process of the receptors, and consequently in ligand recognition (Fig. 1). The composition of these domains (immunoglobulin domains, rich in leucine, lysine and cystein, fibronectin type III domain, etc.) depends on the classes of RTKs and then defines the specificity of the ligands. The RTKs are inserted into the cell membrane thanks to an α-helix trans-membrane domain composed of 20 amino acids. The trans-membrane domain plays a key role in the formation and stabilisation of the dimer of the receptor chains. In the lipid environment of the cell membrane, the α-helices are non-covalently oligomerised [11] (Fig. 1). This type of process makes it possible to pre-dimerise the RTKs in the cell membrane capable of interacting with the corresponding ligand [12].
Table 1.
Classification and characteristics of the human RTKs.
| Class | Family name | Members | Molecular characteristics of the extracellular domains |
|---|---|---|---|
| I | EGFR | EGFR, ERBB2, ERBB3, ERBB4 | 2 cysteine-rich domains |
| II | Insulin R | INSR IGFR | 2 chains α and β, with one cysteine-rich and 2 FNIII domains |
| III | PDGFR | PDGFRα, PDGFRβ, M-CSFR, KIT, FLT3L | 5 Ig-like domains |
| IV | VEGFR | VEGFR1, VEGFR2, VEGFR3 | 7 Ig-like domains |
| V | FGFR | FGFR1, FGFR2, FGFR3, FGFR4 | 3 Ig-like domains, 1 acidic box |
| VI | CCK | CCK4 | 7 Ig-like domains |
| VII | NGFR | TRKA, TRKB, TRKC | 2 Ig-like domains, rich leucin domains |
| VIII | HGFR | MET, RON | 1 transmenbrane α chain linked with one extracellular β chain |
| IX | EPHR | EPHA1 to 6, EPHB1 to 6 | 1 Ig-like, 1 cysteine-rich and 2 FNIII-like domains |
| X | AXL | AXL, MER, TYRO3 | 2 Ig-line, 2 FNIII-like domains |
| XI | TIE | TIE, TEK | 2 Ig-like, 1 EGF, and 3 FNIII-like domains |
| XII | RYK | RYK | 1 transmenbrane β chain linked with one extracellular α chain |
| XIII | DDR | DDR1, DDR2 | 1 discoidin-like domain |
| XIV | RET | RET | 1 cadherin-like domain |
| XV | ROS | ROS | 6 FNIII-like domains |
| XVI | LTK | LTK, ALK | 1 cysteine-rich domain |
| XVII | ROR | ROR1, ROR2 | 1 Ig-domain, 1 cysteine-rich domain and one kringle-like domains |
| XVIII | MUSK | MUSK | 4 Ig-like and 1 cysteine-rich domains |
| XIX | LMR | AATYK1, AATYK2, AATYK3 | A short extracellular domain |
| XX | Undetermined | RTK106 | A short receptor chain with a short extracellular domain |
EGFR: epidermal growth factor receptor; InsR: insulin receptor; PDGFR: platelet-derived growth factor receptor; VEGFR: vascular endothelial growth factor receptor; FGFR: fibroblast growth factor receptor; CCK: colon carcinoma kinase; NGFR, nerve growth factor receptor; HGFR: hepatocyte growth factor receptor; EphR: ephrin receptor; Axl: from the Greek word anex-elekto, or uncontrolled, a Tyro3 protein tyrosine kinase; TIE: tyrosine kinase receptor in endothelial cells; RYK: receptor related to tyrosine kinases; DDR: discoidin domain receptor; Ret: rearranged during transfection; ROS: RPTK expressed in some epithelial cell types; LTK: leukocyte tyrosine kinase; ROR: receptor orphan; MuSK: muscle-specific kinase; LMR: Lemur; Ig: immunoglobulin; FN: fibronectin. (From Blume-Jensen and Hunter [10]).
Fig. 1.
General organisation of the molecular domains that make up the RTKs. RTKs are characterised by the dimerisation of two receptor chains with an N-terminal (N) extracellular domain (ECM), and a C-terminal (C) intracellular domain (ICD). The extracellular domain is implicated in the recognition of the dimeric ligands and the formation of the receptor chain dimerisation process. The extracellular domain is associated with ligand recognition and is composed of various domains depending on the RTK class. The transmembrane-domain is composed of an α-helix chain, which contributes to the stabilisation of the dimeric receptor chains. The binding of a dimeric ligand (in red) to the extracellular domains of the receptor chains strengthens the stabilisation of the receptor chains, which are auto-phosphorylated through their tyrosine kinase domains and then transduced in specific downstream signalling pathways.
The cytoplasmic domain harbours a specific domain with tyrosine kinase activity that is involved in the catalysis of the ATP-dependent phosphorylation of receptor chains. It includes two domains: a juxtamembrane region composed of 40–80 amino acids corresponding to the tyrosine kinase domain and a carboxy-terminal region. The tyrosine kinase domain is composed of 12 subdomains organised into two lobes, connected by the kinase insert domain (subdomain V) (Fig. 1). The tyrosine kinase domain includes an activation loop, whose orientation (and phosphorylation) determines the active or inactive state of the kinase domain. The ATP required for kinase activity is housed between the two lobes. The small lobe (named lobe N, for N-terminal, subdomains I–IV), composed of β-sheets and one α helix, binds, stabilises and orients the ATP previously complexed with Mg2+ ions. The large lobe (named C, for C-terminal, subdomains VI–IX) is mainly composed of α helices, and plays a part in the chelation of ATP by Mg2+ ATP. It then binds the protein substrate containing the tyrosine target and catalyses the transfer of the phosphate group from the ATP to the receptor chains [13]. The size of the tyrosine kinase domain is relatively constant between the different RTKs. On the contrary, the size and content of the juxta- and C-terminal domains vary considerably between the RTK families, conferring the specificity of intracellular signals. For instance, the intracellular domain of PDGFRβ has 552 amino acids and the intracellular domain of EGFR has 542 amino acids, while the FGFR1 shows 425 and TrkA only 356 amino acid residues. The number of tyrosine residues (phosphorylable or not) and their distribution vary significantly between the RTKs. Thus, 27 tyrosine residues are detected for the PDGFRβ (of which 19 can be phosphorylated) and only 11 tyrosines can be detected in TrkA (with 6 phoshorylable tyrosines) [16] . However, a pair of tyrosine residues phosphorylated after RTK activation is found in the activation loop and is required for the functionality of the receptor. The activation of these tyrosine residues stabilises the “open” conformation of the activation loop and both lobes, and also allows the ATP and peptidic substrate environment to bind [13]. An additional, third tyrosine amino acid (located in a close upstream domain) participates in the conformational change of the activation loop. All the mutations on these tyrosine residues result in inactivation of the receptor chains. EGFR is an exception in the RTK families and it has only one tyrosine residue at this position, which is not essential for receptor chain activation and function.
2.2. General mechanism of action
It is admitted that the binding of a dimeric ligand to its receptor chains increases the proximity or/and stabilises the receptor chains that will be then auto-phosphorylated through their kinase domains (a process called trans-phosphorylation). This non-covalent dimerisation is associated with conformational changes that lead to the activation of the cytoplasmic kinase domains of the receptors. In most cases, one of the two receptor chains will trans-phosphorylate specific cytoplasmic tyrosines from other monomeric chain environment [14]. In some cases, the constitutive form of the RTKs is a dimer such as insulin receptors. In addition, some ligands such as EGF are monomeric, and their binding to their receptor induces a conformational change that shifts the intra-molecular loop and exposes a binding domain in the receptor that results in its dimerisation environment [15]. In others, the dimerisation of the ligand is required to activate the receptor chain (i.e., the NGF–TrkA system environment [16]).
In the absence of the ligand, the activation loop self-regulates activation of the receptor because its “closed” conformation inhibits catalytic activity (cis-inhibition). Dimerisation of the RTK chains following ligand binding induces the rotation of the N- and C-lobes, as well as the major axis of the protein. The activation loop, which is masked by its tyrosine residues, the ATP binding site, moves to enable ATP binding and the autophosphorylation of tyrosine residues located on the opposite receptor chain. The trans phosphorylation of key tyrosine residues located in the activation loop stabilises the “open” conformation, and breaks the binding between these tyrosines and the binding sites to the protein substrates, making it possible to access the C lobe, then activating its kinase activity. In addition, other tyrosine residues are phosphorylated by protein kinases previously recruited on the phosphorylated tyrosines of the RTK environment [17]. Several molecular “brakes” in kinase activity have been developed to limit phosphorylation levels. These molecular domains are located in the activation loop, in the juxtamembrane domain (KIT, PDGFR) or in the C-terminal domain (i.e., Tie2). In the last two cases, these molecular repressions will be removed by cis-phosphorylation of the RTKs during the ligand binding-induced conformational changes [18]. Phosphorylation of the catalytic domain of the RTKs activates and increases the activity of the kinase domain, whereas the non-catalytic domains create various anchoring sites for cytoplasmic targets involved in intracellular signal transduction. These tyrosines are mostly located on the juxta-membrane and C-terminal domains, and at the insert kinase domain residues, allowing the binding, activation and phosphorylation of numerous cytoplasmic proteins that will then relay the signal towards various intracellular activation pathways. These proteins have SH2 or PTB domains that recognise tyrosine phosphorylated receptor chains, and have intrinsic enzymatic activity, such as Src or PLCγ, or serve as adapter proteins for recruiting other enzymes, such as Grb2 linked to the MAPK activation pathway. The proteins recruited by their SH2 domains are named “adapter”, while those that bind directly to the receptor chains or to the Grb2 adaptative protein are called “anchoring proteins”. Adaptive and anchoring proteins can bind to similar phosphorylated tyrosine residues or to several tyrosine residues from the same receptor chains. Thus, Gab1 binds to tyrosine1068 and tyrosine1086 of EGFR. Insulin and FGF receptors bind to a protein assembly that can be phosphorylated and used as adaptive proteins [19].
2.3. RTKs and activated signalling pathways
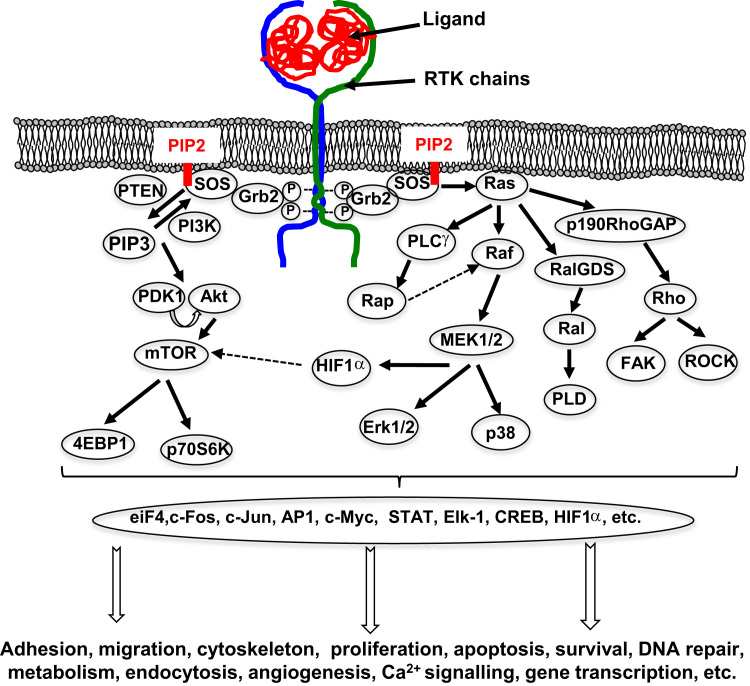
RTKs are considered as protein platforms, or the starting point for many cellular signalling pathways by recruiting enzymatic effectors (PLCγ, PI3K, Src, etc.) either directly on to their intra-cytoplasmic domain, or indirectly through adapter proteins (Grb2, Shc, etc.), forming complexes capable of activating intracellular enzymes (Ras, etc.) (Fig. 2). RTK downstream signalling pathways are mainly MAPK, PI3K, Src, and other signalling pathways involving PLCγ, JAK / STAT, etc. While the early stages of signal transduction following the activation of RTKs is based mainly on tyrosine phosphorylation, signal propagation associates various phosphorylations on serine/threonine residues in the majority of cellular processes, as well as other processes such as ubiquitination, glycosylation or acetylation [20].
Fig. 2.
Main signalling pathways activated by the ligand-induced RTK auto-phosphorylations. The phosphorylation cascades initiated by the RTK phosphorylations lead to the activation of numerous transcription factors, which consequently control the regulation of many physiological processes.
The MAPK pathway plays a part in controlling cell proliferation, cell death or differentiation, and migration, as well as promoting angiogenesis. The MAPK signalling cascade is divided into four major pathways used by RTKs and leading to ERK1/2 activation (Fig. 2). After activation of the RTKs by their ligand, the adaptive protein Grb2 binds by its SH2 domains, the phosphorylated tyrosine residues of the receptor chains and the adaptive protein SOS by their SH3 domain, which is bound to the PIP2 membrane. This binding allows the activation of Ras, a small G protein, via SOS, a GEF protein exchanging the GDP for a GTP. In fact, Ras oscillates between its active and inactive state, thus acting as a “switch” for intracellular effector molecules. Once activated, Ras allows phosphorylated signal transduction through recruitment and phosphorylation of Raf kinases A, B or C (or MAP3K) [21]. Activated Raf phosphorylates MEK1 and MEK2 (or MAP2K1/2) on serine218/serine222 and serine222/serine226 residues of their activation loop, and activated MEK1/2 itself catalyses the phosphorylation of Erk1 and Erk2 (or MAPK1/2) on their threonine202/185 and tyrosine204/187 residues. Phosphorylated Erk1/2 will be then translocated to the nucleus to activate transcription factors that will regulate the transcription of genes involved in the survival and growth of the cells, or activate cytosolic proteins, such as RSK1/2, which target cytoplasmic effectors or will finally be translocated into the nucleus to act as a transcription factor [22].
The targets of these transcription factors are transcriptional regulators such as STAT, Elk-1, CREB or H3 histone that activate transcription of early genes. Of these early genes, c-Fos, c-Jun or c-Myc stimulate the expression of other genes such as cyclin D1 or CDK6, which control progression in the G1 phase and G1/S transition. When RTK activation, and therefore that of Erk1/2, is maintained, expression of the previous proteins is stabilised as c-Fos, which is phosphorylated on threonine residues by its RSK1/2 and Erk1/2, and forms the complex AP-1 with c-Jun, which also activates the transcription of target genes (Fig. 2). The MAPK pathway also activates three additional pathways: p38, JNK and ERK5. In the first pathway, p38α/β/γ/δ are activated by a MAP2K such as MKK3 or MKK6, previously activated by a MAP3K such as TAK1, and consequently, p38 induces the transcription of various genes involved in cell proliferation, angiogenesis, inflammation and the production of immunomodulatory cytokines. In the JNK pathway, the TAK1-, MEKK1-, or MLK-MAP3Ks activate the MAP2K4 or MAP2K7, which activates JNK1, 2 or 3, for instance, and lead to the control of cell apoptosis or the development of the immune system [23]. In the ERK5 pathway, WNK1 activates MEKK2 and 3, which phosphorylate MEK5, leading to ERK5 activation. The translocation of ERK5 into the nucleus regulates cell proliferation and survival by activating the transcription of cyclin D1, for example, allowing G1/S transition in the cell cycle in the same way as Erk1/2. ERK5 also has more specific substrates, such as the MEF2 transcription factor family, the pro-apoptotic protein BAD, connexin 43, etc. [24].
The PI3K/Akt/mTOR pathway controls cell cycle progression, the cell survival/cell apoptosis balance. Its activation facilitates cell proliferation and migration, the metabolism of glucose, etc. PI3K is a “lipid” kinase that phosphorylates membrane lipids via its catalytic p110 subunit (α, β or δ) once recruited by its two SH2 domains from the p85 regulatory subunit on activated RTKs. PIP2 then forms phosphatidylinositol 3,4,5-triphosphate (PIP3) by transferring a phosphate group, and Akt (PKB, for Protein Kinase B) and PDK-1 then bind to the membrane, where the PDK-1 is activated by PIP3 phosphorylates Akt (Fig. 2). Activated Akt becomes an activation crossroad for many proteins, allowing cells to survive by inhibiting, ubiquitinating and degrading pro-apoptotic proteins such as BAD and p53, and by inducing the expression of anti-apoptotics such as Bcl-2 or Akt. In addition, Akt also induces cell proliferation by activating various cyclins and by inhibiting several cell cycle repressors such as p21 or p27. Akt also allows the transcription of pro-angiogenic genes such as VEGF and HIF-1α, which are involved in numerous oncological processes. In addition, Akt inhibits the glucose metabolism by suppressing GSK3, and regulates the lipid metabolism through mTOR activation [25].
The role of the Src pathway in signal transmission within the cell was demonstrated for the first time in fibroblasts stimulated with PDGF [26]. Src, Fyn and Yes belong to the Src family, activated by RTKs, and are associated with numerous other kinases such as Ras, PI3K, PLCγ or FAKs. The members of the Src family therefore have redundant functions in the intracellular signalling pathways described below. Src family members are recruited on RTKs (EGFR, FGFR, IGFR, MCSF-R, HGFR, etc.) after their activation and transmit mitogen signals inducing DNA synthesis, cell survival, cytoskeleton rearrangements, cell adhesion and motility, but also control receptor turnover [27]. Src family members can bind phosphorylated residues by their SH2 domains, resulting in kinase activity after conformational modifications. This activation is very complex and requires the recruitment of Ras and Ral GTPases. Several studies have shown that SFKs may regulate activation of RTKs directly by phosphorylating tyrosine residues such as tyrosine845, tyrosine1101 and EGFR [28]. c-Src can be recruited within membrane complexes formed by integrins, and then phosphorylate these RTKs [29]. Furthermore, the Shp2 protein tyrosine phosphatase also plays a key role in this activation by blocking the activities of negative regulators (Csk for instance) [30].
PLCγ and JAK/STAT are additional signalling pathways associated with RTK activation. Various RTKs can bind through their phosphorylated tyrosine residue, the SH2 domains of STAT transcription factors, as demonstrated for MET and STAT3. The activation of these transcription factors results in their dimerisation and translocation into the nucleus to activate specific target genes [31].
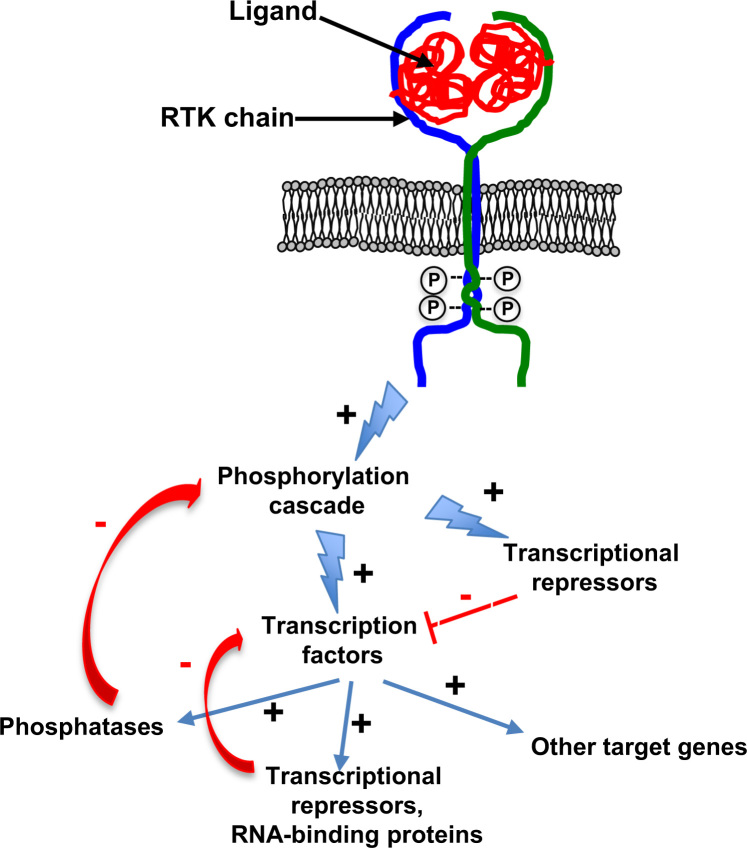
2.4. Feedback loops controlling RTK activation
RTK activities are tightly controlled by numerous positive or negative molecular feedback loops that prolong the auto-activation of the receptors and signal amplitude, by inducing the production of the ligand for instance. Such feedback loops are essential for stabilising the RTK system [32]. These controls include proteins already present within the cell that are mobilised on activation of RTKs and/or subjected to post-translational modifications for immediately regulating the signal induced (early negative feedback) (Fig. 3). They also associate the synthesis of response elements (late negative feedback) such as IEGs early or DEGS late genes that regulate the activity of AP-1, c-Myc, p53 or the MAPKs. Thus, Erk1/2, a downstream protagonist of the MAPK pathway, directly inhibits (early negative feedback) the phosphorylation of the effector proteins by inhibiting the kinase activity of upstream enzymes (RAF and MEK) [33]. In addition, the translocation of Erk1/2 into the core may also activate the expression of transcriptional repressors, such as phosphatases (e.g., DSPs) to inhibit MAPK activity (negative feedback late) [34].
Fig. 3.
The negative feedback loops regulating RTK activation. The window of time required for inducing mRNA and protein synthesis after RTK activation is between 15 and 90 min. These mechanisms are tightly regulated by negative feedback loops. Indeed, the phosphorylation cascade induced by RTK activation leads to the activation of numerous transcription factors and simultaneously their repressors. The translocation of the various transcription factors can also induce the expression of transcriptional repressors or phosphases, which in turn can repress the corresponding transcription factors and/or the upstream kinase activites. +: activation; –: repression.
By decreasing the amplitude of the signals generated and the stimulation of cellular activity, adapter proteins such as kinases, phosphatases and ubiquitin ligases located in the cytoplasm are the first early negative regulators of RTK activities [35]. The signal generated is then attenuated, based on the ubiquitination of RTKs by the E3 ubiquitin ligase c-CBL for instance, which leads to the endocytosis of the receptors and their degradation in the lysosomal compartment [36]. After activation by the ligand, the RTK is effectively clustered in clathrin-rich membrane regions and then internalised in clathrin-dependent endocytic vesicles to reduce the induced signal [37].
3. RTKs in oncology
3.1. RTK mutations and carcinogenesis
RTKs are involved in numerous pathological disorders, especially in oncology. Around 30% of RTKs are mutated or overexpressed in various human cancers (MET, KIT, FLT3, etc.) [38]. Oncogenic mutations or gene duplications in the juxtamembrane region of KIT and FLT3 result in constitutive activation of these receptors in the absence of their ligand, and are consequently directly linked to the carcinogenesis process [39]. Duplications in the juxtamembrane region of FLT3 are responsible, for instance, for the constitutive activation of the receptor in 15–30% of cases of acute myeloid leukaemia [40] and in 65% of gastrointestinal stromal tumours (GISTs) [41]. Autocrine stimulation or overexpression of EGFR was also associated with many solid tumours. Thus, EGFR/ErbB-1 and ErbB-2 are overexpressed in lung [42], breast [43,44] and prostate [45,46] cancer, and their expression is linked to marked aggressiveness and poor prognosis. Such observations have strengthened the therapeutic development of RTK inhibitors in the last three decades.
3.2. RTK inhibitors and bone cancers
3.2.1. RTK inhibitors target the bone tumour niche
Primary malignant bone tumours (bone sarcomas) and bone metastases (from breast, prostate carcinomas, etc.) are characterised by their ability to dysregulate their micro-environment and especially the balance between bone apposition and bone resorption. Osteoblasts [8,45–51] and osteoclasts [8,52–54] express numerous RTKs and are then cellular targets of the corresponding ligands released in the cancer micro-environment. Based on these observations, the impact of RTK inhibitors has been assessed in bone remodelling. Recently, Bao et al., using broad kinase inhibitor screening applied to the mouse MC3T3-E1 osteoprogenitor cell line, identified two families of inhibitor affecting cell survival differentially [55]. The first family included pro-osteoblastic drugs such as lapatinib (EGFR/HER2 inhibitor), erlotinib (EGFR inhibitor) and sunitinib (FLT3/PDGFR/VEGFR/CSF-1R inhibitor), which stimulated osteoblastic proliferation. In contrast, the second family grouped together seven kinase inhibitors (GSK1838705A, PF-04691502, masitinib targeting KIT or XL880 targeting MET and VEGFR), which inhibited osteoblast viability in a dose- and time-dependent manner. Nilotinib and CEP-751 may be added to the second family. Nilotinib potently inhibited osteoblast proliferation [56]. While nilotinib inhibits numerous RTKs (KIT, EPHA3, EPHA8, DDR1, DDR2, PDGFRB), its effects may be associated with the inhibition of PDGFR [65]. Pinski et al. demonstrated that proliferation induced apoptosis, but not quiescent human osteoblasts after treatment with CEP-751, a trk receptor tyrosine kinase inhibitor [57]. Similarly, inhibiting IGF1R also led to the inhibition of proliferation and induction of apoptosis of osteoblasts [58]. Nevertheless, these RTK inhibitors, due to their multiple targeting, exert very complex effects and can exert dual activities on bone cells. Imatinib mesylate (Gleevec), which targets a broad range of tyrosine kinase proteins, including bcr/abl, c-kit, cFMS and the PDFGR among others, is able to inhibit osteoblast proliferation and also to activate their activities through the inhibition of PDGFRβ activity [59]. Gobin et al. confirmed recently this dual activity depending on the doses of inhibitor used. Low doses of imatinib mesylate increased the in vitro mineralisation process, and high doses of the drug markedly affected mineral deposits [60].
RTKs are also expressed by osteoclast precursors and mature osteoclasts, and numerous studies have shown that RTK inhibitors strongly affect osteoclastogenesis and bone resorption. Imatinib mesylate decreases osteoclastogenesis, and increases mature osteoclast apoptosis through the inhibition of cFMS signalling [61]. Sorafenib, an RET, and VEGFR inhibitors similarly target osteoclasts [62]. Dasatinib abolishes osteoclast formation in vitro by inhibiting cFMS activation, and increases osteoblast activities by repressing PDGFR signalling [63]. In addition, these authors demonstrated that the administration of dasatinib in animals resulted in dysregulated bone remodelling in favour of an increase in bone formation, which may be associated with the inhibition of osteoclast activity [63]. In 2012, Garcia-Gomez et al. confirmed the anabolic and anti-catabolic effects of dasatinib [64]. Overall, these works revealed that bone cells are potential targets for RTK inhibitors, and that using RTK inhibitors in an oncological bone context will have an impact on the bone tumour niche.
3.2.2. RTK inhibitors as therapeutic drugs for bone sarcomas
Bone sarcomas derive from the mesoderm, and sarcoma cells originate from mesenchymal stem cells [65]. Osteosarcoma and Ewing’s sarcomas are the two main types of bone sarcoma diagnosed in children and young adults. The peak of incidence for both tumours is at puberty, suggesting that there is a strong link with bone growth and the numerous growth factors, hormones and cytokines released during this period. In this context, RTK inhibitors assessed on bone cells were also assessed in bone sarcomas (Table 2) [66,67]. Recently, Rettew et al. identified several RTKs by using a phosphoproteomic approach and demonstrated that Axl, EphB2, FGFR2, IGF-1R and Ret more specifically controlled the behaviour of human osteosarcoma cells in vitro from a functional point of view [68]. PDGFR was also identified as a therapeutic target in osteosarcoma, and selective inhibition of PDGFR activation led to apoptosis of osteosarcoma cells in vitro [69]. These data were confirmed by a phospho-receptor tyrosine kinase array kit, which identified seven receptors (PDFGFRβ, Axl, RYK, EGFR, EphA2, 10, and IGF1R) as molecular targets for imatinib mesylate [60]. In this study, the authors showed that imatinib mesylate induced anti-proliferatives in pre-clinical models of osteosarcoma, and that of the seven modulated RTKs, PDGFRα appeared as the main target of the drug. Similar observations were made in Ewing’s sarcoma [70]. Unfortunately, clinical investigations demonstrated only low or no efficacy in children with relapse bone sarcomas, even in patients selected for tumour expression of KIT or PDGFRα [71–73] (Table 2). Dasatinib and Sunitinib were used in phase I clinical trials and defined the doses usable in a paediatric context [77,79]. Although no objective responses were observed, four patients with sarcomas were in a stable condition [79]. Complementary investigations are needed to evaluate the therapeutic efficacy of dasatinib and sunitinib in sarcomas. Pazotinib, targeting VEGFR, PDGFR and c-KIT, and sorafenib, targeting RET and VEGFR, had interesting benefits in paediatric sarcomas [71,54,85] (Table 2).
Table 2.
Main RTK inhibitors assessed in bone sarcomas.
| RTK inhibitor | Molecular targets | Investigations, Patients, doses | References |
|---|---|---|---|
| Imatinib mesylate (Gleevec) | PDFGR, c-KIT | Pre-clinical in vitro and in vivo assessment | [60,69] |
| Phase II, 189 sarcoma patients (13 ES, 27 OS), 100–300 mg/day of gleevec, orally twice a day according the body-surface area | [71] | ||
| Phase II, 7 ES, 400 mg of gleevec, orally twice daily prescribed with a cycle length of 28 days. | [72] | ||
| Phase II, 70 patients, 12 ES, 26 OS, 440 mg/m2/day of gleevec | [73] | ||
| Pre-clinical in vitro assessment (drug combinations) | [74] | ||
| Dasatinib | Src (inhibition of RTK-transduced signalling pathways) c-KIT, EPHA2, PDGF-β | Pre-clinical in vitro assessment | [75] |
| Pre-clinical in vivo assessment | [76] | ||
| Phase I, 39 patients (2 ES, 1 OS) of 50, 65, 85, and 110 mg/m2/dose of dasatinib, administered orally twice daily for 28 days | [77] | ||
| Sunitinib | FLT3, PDGFR, VEGFR, cFMS | Pre-clinical in vitro and in vivo assessment | [78] |
| Phase I, 33 patients (2 ES, 2 OS), from 15 and 20 mg/m2/days of sunitinib with dose escalation | [79] | ||
| Pazotinib | VEGFR, PDGFR, c-KIT | Pre-clinical in vitro and in vivo assessment | [80] |
| Phase I, 51 patients (3 ES, 4 OS) (tablet formulation), pazotinib administered once daily in 28-day cycles at four dose levels (275–600 mg/m2); powder suspension initiated at 50% of the maximum-tolerated dose for the intact tablet | [81] | ||
| Pre-clinical in vitro and in vivo assessment (combination with Topotecan) | [82] | ||
| Sorafenib | RET, VEGFR | Pre-clinical in vitro and in vivo assessment | [83] |
| Phase I, 11 patients (2 OS), from 90 mg/m2 to 110 mg/m2 of sorafenib twice daily | [84] | ||
| Phase II, 35 OS, 400 mg of sorafenib twice daily until progression or unacceptable toxicity | [85] | ||
OS: osteosarcoma; ES: Ewing’s sarcoma.
Protein assays have identified new RTKs with potential therapeutic benefits. Axl, a TAM (Tyro3, Axl and Mer) receptor tyrosine kinase, is thus expressed in most osteosarcomas [86] and a correlation was found between its expression and the clinical outcome [87,88]. In addition, Fleuren et al. demonstrated that high Axl expression correlated with worse overall survival compared to Ewing’s sarcoma patients with lower expression [89] similar to MET [90]. The MET inhibitor (PF-2341066) then appeared efficient in a xenograft model of osteosarcoma [91]. EphA2 was the most abundant surface protein on cancer cells and may be involved in the pathogenesis of osteosarcoma by modulating bone remodelling and the communications between tumour cells and their environment [92–94]. Recently, Kuijjer et al. provided an in vitro rationale for using IGR1R inhibitors in osteosarcoma [95]. However, IGF1R mRNA expression, cell surface expression, copy number, and mutation status were not associated with tumour responsiveness to anti-IGF1R targeting [96]. EGFR are expressed by osteosarcoma cells, but gefitinib and BIBW2992 targeting the receptors were not effective on osteosarcoma cells, so the question of EGFR targeting remains open [97]. Similarly, HER-2 is expressed by osteosarcoma cells but its prognostic relevance is still controversial [98] and the results for the patients treated were limited [99]. A randomised study of patients with HER2-positive osteosarcoma would be of major interest for better understanding the role of HER-2 in the pathogenesis of bone sarcomas, and for evaluating their therapeutic value. EphA10 and RYK are two other RTKs expressed by osteosarcoma cells and represent other therapeutic opportunities [100,101].
Overall, these data revealed the potential therapeutic interest for targeting RTKs in bone sarcomas. Clinical investigations must nevertheless be adapted to the expression/mutation/activation state of RTKs, which is the prerequisite for patient enrolment.
3.2.3. RTK inhibitors: therapeutic benefits for bone metastases
As with bone sarcomas, bone metastastic cells, from breast or prostate carcinoma for instance, dysregulate local bone remodelling and the associated TRKs/growth factors, which in turn facilitate tumour development [102]. Consequently, numerous TRKs and their ligands have been associated with the pathogenesis of carcinomas and their capacity to form bone metastases. Many investigations at the pre-clinical and clinical levels have thus been developed in the last 10 years (Table 3). Unfortunately, whilst most of the drugs developed had interesting anti-cancer effects on the primary tumours or/and the establishment of bone metastases, the results of the clinical trials were often disappointing. Imatinib mesylate for instance, which is very efficient in soft tissue sarcomas, had no palliative or clinical activity in metastatic castration-resistant prostate cancer [105]. Combining it with bisphosphonates and docetaxel did not improve overall survival and brings into question the value of PDGFR inhibition with taxane chemotherapy in prostate cancer bone metastases [105–107]. Similarly, phase III clinical trials did not confirm the combination of dasatinib (which targets c-KIT, EPHA2, PDGFR) and docetaxel in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (Table 3). Sunitinib initially appeared promising in metastatic castration-resistant prostate cancer [116], however, the phase III clinical trial did not significantly prolong the overall survival of patients after failure of a docetaxel-based regimen [117]. Sorafenib was developed to target RET and VEGFR [121] and has a moderate activity as a second-line treatment for metastatic castration-resistant prostate cancer [123]. HGFR (c-MET) and its ligand HGF control numerous cellular signalling cascades that direct cell growth, proliferation, survival, and motility, and also regulate the epithelial–mesenchymal transition (EMT) with a strong impact on the development of metastases. Cabozantinib was specifically developed to inhibit the downstream signalling pathways transduced by c-MET and VGEFR [125–132]. Cabozantinib is currently approved by the U.S. Food and Drug Administration for the treatment of progressive, metastatic medullary thyroid cancer. The clinical evaluation demonstrated in phase II clinical trials that the use of this drug appeared clinically relevant in castration-resistant prostate cancer patients, as it improved bone scans and bone biomarkers, and reduced both soft tissue lesions and the number of circulating tumour cells [134,135]. The phase III COMET-II trials indicated that cabozantinib has not fulfilled the promise reported in the phase II trials (Exelixis announcement: http://www.exelixis.com/investors-media/press-releases). Indeed, 50% of patients in the cabozantinib arm reported a pain response, compared to 17% of patients in the control arm receiving mitoxantrone/prednisone. This difference in pain response between the arms was not statistically significant. Tivantinib, another c-MET inhibitor, has shown promising therapeutic value in pre-clinical models [137,138]. Erlotinib has moderate clinical effect as a single-agent in chemotherapy-naïve castration-resistant cancer [143] and its combination with docetaxel did not show any added therapeutic value [144]. Genitinib, lapatinib and vandetanib alone or in combination with other drugs failed to show significant therapeutic activity compared with the conventional drugs in breast and prostate cancers (Table 3). Dovotinib is a recently developed multi-RTK inhibitor (FGFR, VEGFR) that has shown interesting pre-clinical activity in metastatic castration-resistant prostate cancer: anti-angiogenic activity, anti-tumour activity and clinical activity in 34 patients with bone metastases [160]. However, its combination with histone deacetylase inhibitor did not show any additional value [161]. Clinical trials are required to confirm its therapeutic value.
Table 3.
Main RTK inhibitors assessed in carcinoma and associated-bone metastases.
| RTK inhibitor | Molecular targets | Investigations, patients, doses | References |
|---|---|---|---|
| Imatinib mesylate (Gleevec) | PDFGR, c-KIT | Pre-clinical in vivo assessment | [103,104] |
| Phase I, 28 patients (MeCRPC) 400 mg/day of gleevec, combination with zoledronic acid | [105] | ||
| Phase I, 21 patients (MeCRPC) 600 mg/day of gleevec, combination with docetaxel | [106] | ||
| Phase II, 144 patients (MeCRPC) docetaxel combined with 600 mg/kg/day of gleevec or placebo | [107] | ||
| Dasatinib | Src (inhibition of RTK-transduced signalling pathways) c-KIT, EPHA2, PDGFR-β | Pre-clinical in vivo assessment | [108–110] |
| Phase I, 16 patients with solid tumours, 100 mg of dasatinib, increased by increments of 50 mg up to a maximum dose of 250 mg for 4 weeks | [111] | ||
| Phase II, 47 patients (MeCRPC), 700 or 70 mg/day | [112,113] | ||
| Phase III, 1522 patients (MeCRPC), 100 mg/day of dasatinib combined with docetaxel | [114] | ||
| Sunitinib | FLT3, PDGFR, VEGFR, cFMS | Pre-clinical in vivo assessment | [115] |
| Phase II, 36 patients (MeCRPC), 50 mg/day of sunitinib 4-weeks on followed by 2-weeks off per cycle up to a maximum of eight cycles prior docetaxel | [116] | ||
| Phase III, 873 patients (docetaxel-refractory MeCPRC), 37.5 mg/day of sunitinib with or without prednisolone | [117] | ||
| Phase II, 60 patients (Her-2+ advanced breast carcinoma) 37.5 mg/day of sunitinib combined with trastuzumab | [118] | ||
| 223 patients (clear-cell renal cell carcinoma with bone metastases), 50 mg/day, 4 weeks on, 2 weeks off | [119] | ||
| 209 patients (renal clear carcinoma, 76 with bone metastases) 50 mg/day, in 6-week cycles (4 weeks on, 2 weeks off) combined with bisphosphonates | [120] | ||
| Sorafenib | RET, VEGFR | Pre-clinical in vivo assessment | [121] |
| Phase II, 22 patients (MeAIPC), 400 mg/day of sorafenib in 28-day cycles | [122,123] | ||
| Case report, bone metastases bilateral carcinoma, 400 mg/day of sorafenib | [124] | ||
| Cabozantinib | c-MET, VEGFR2 | Pre-clinical in vitro assessment | [125–129] |
| Pre-clinical in vivo assessment | [130–132] | ||
| Phase I, 11 patients (MeCRPC), 60, 40 or 20 mg of cabozantinib | [133] | ||
| Phase II, 144 patients (MeCRPC), 40 or 100 mg/day of sorafenib until disease progression or unacceptable toxicity | [134] | ||
| Phase II, 171 patients (CRPC), 100 mg/day of cabozantinib vs placebo | [135] | ||
| Phase II, 65 patients (MeCRPC) 100 mg/day or 40 mg/day of cabozantinib. | [136] | ||
| Tivantinib | c-MET | Pre-clinical in vivo assessment | [137,138] |
| Cediranib | VEGFR | Pre-clinical in vivo assessment | [139] |
| Phase I, 26 patients (hormone refractory prostate cancer), escalating doses of 1–30 mg/day of cediranib | [140] | ||
| Vatalanib | VEGFR | Pre-clinical in vivo assessment | [141] |
| Erlotinib | EGFR | Pre-clinical in vivo assessment | [142] |
| Phase I, 29 patients (MeCRPC), 150 mg of erlotinib daily until disease progression | [143] | ||
| Phase II, 22 patients (AIPC), docetaxel 60 mg/m2 IV on day 1 and erlotinib 150 mg/day (days 1–21) | [144] | ||
| Gefinilib | EGFR | Pre-clinical in vivo assessment | [145–147] |
| Phase II, 38 patients (MeCRPC), 500 mg/day of gefitinib | [148] | ||
| Phase II, 82 patients (hormone-refractory prostate cancer) | [149] | ||
| Phase II, 37 patients, 250 mg/day of gefitinib combined with docetaxel | [150] | ||
| Phase I/II, 31 patients (stage IV HER-2+ metastatic breast cancer), 250 mg/day of gefitinib on days 2–14 combined with trastuzumab and docetaxel | [151] | ||
| Phase II, 148 patients (hormone-positive metastatic breast cancer), 500 mg/day of gefitinib with either anastrozole or fulvestrant | [152] | ||
| Phase II, more than 200 patients (hormone receptor-positive metastatic breast cancer), 250 mg/day of gefitinib with or without tamoxifen | [153] | ||
| Phase II, 174 patients (hormone receptor-positive metastatic breast cancer), anastrozole combined with 250 mg/day of gefinitib or placebo | [154] | ||
| Lapatinib | EGFR, HER-2 | Phase II, 29 patients (CRPC), 1500 mg/day of lapatinib | [155] |
| Phase II, 24 patients (Advanced HER2-positive Breast Cancer), 1250 mg/day of lapatinib and pegylated liposomal doxorubicin | [156] | ||
| Phase II, 23 patients (hormonally untreated advanced prostate cancer), 1500 mg/day of lapatinib | [157] | ||
| Vandetanib | EGFR, VEGFR, RET | Phase II, 39 patients (CPRC), 300 mg/day of vandetanib combined with bicalutamide vs bicalutamide | [158] |
| Phase II, 61 patients (hormone-receptor-positive metastatic breast cancer), fulvestran with either 100 mg/day of vandetanib or placebo | [159] | ||
| Dovotinib | FGFR, VEGFR | Pre-clinical in vitro and in vivo assessment | [160,161] |
(The list of references and clinical trials of this table is not exhaustive). MeCPRC: metastastic castration-resistant prostate cancer including bone metastasis; MeAIPC: androgen-independent prostate cancer with bone metastases.
Although numerous RTK inhibitors initially appeared to be of great interest, based on pre-clinical assessments, most of them have not fulfilled the promise hoped in phase I/II studies. The absence of significant results with their use can be explained by the multiplicity of their targets and the complexity of the mechanisms involved. Indeed, these drugs will affect not only the tumour cells but also its environment. Thus, the Cabozantinib, like dovotinib for instance for which the clinical activity needs to be confirmed, affects the coupling between cancer cells and the bone tumour niche [160,162,163]. The bone tumour microenvironment (in bone sarcoma and bone metastases) is then described as a sanctuary that controls at least in part the tumour growth and contributes to the drug resistance acquisition [164,165]. By modulating the tumour microenvironment, RTK could have a positive and/or a negative impact on the tumour development.
4. Conclusion
In the last 15 years, there have been high expectations in oncology of therapies with RTK inhibitors. Imatinib mesylate was the first to show spectacular clinical success in chronic myeloid leukaemia patients, and has become the first line of treatment. Gastro-intestinal stromal tumour (GIST) is the second success for the use of an RTK inhibitor, and imatinib mesylate is the standard of care in patients who are at high risk for GIST recurrence following resection [166]. Unfortunately, patients develop resistance and relapse due to protein point mutations and/or the introduction of molecular feedback loops. Many other RTK inhibitors have shown disappointing results in clinical applications after encouraging pre-clinical results. In all cases, the efficacy of RTK inhibitors is linked with their ability to disrupt the crosstalk between tumour cells and their environment. A better understanding of both intracellular signal modulating by these RTK inhibitors, and the feedback loops developed during the establishment of resistance, will increase the chances of success for these drugs. In addition, adapted investigational approaches will be needed to define the expression profile of the RTK genuinely activated/mutated/expressed in patients before their inclusion in clinical trials.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
This review was written as a part of a research project which received funding from the Seventh Framework Programme ([FP7/2007-2013]) under Grant Agreement no. 264817-BONE-NET. This study was supported by the Region des Pays de la Loire (CIMATH II research project) and by the Ligue Nationale Contre le Cancer (Equipe LIGUE 2012).
References
- 1.Garbers D.L., Koesling D., Schultz G. Guanylyl cyclase receptors. Mol Biol Cell. 1994;5:1–5. doi: 10.1091/mbc.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya M., Babwah A.V., Ferguson S.S.G. Small GTP-binding protein-coupled receptors. Biochem Soc Trans. 2004;32:1040–1044. doi: 10.1042/BST0321040. [DOI] [PubMed] [Google Scholar]
- 3.Li S., Wong A.H.C., Liu F. Ligand-gated ion channel interacting proteins and their role in neuroprotection. Front Cell Neurosci. 2014;8:125. doi: 10.3389/fncel.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liongue C., Ward A.C. Evolution of class I cytokine receptors. BMC Evol Biol. 2007;7:120. doi: 10.1186/1471-2148-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer J.A., Cutrone E.C., Kotenko S. The class II cytokine receptor (CRF2) family: overview and patterns of receptor–ligand interactions. Cytokine Growth Factor Rev. 2004;15:33–48. doi: 10.1016/j.cytogfr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Liao W., Lin J.X., Leonard W.J. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchard F., Duplomb L., Baud׳huin M., Brounais B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009;20:19–28. doi: 10.1016/j.cytogfr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Heymann D., editor. 2nd ed. Academic Press; San Diego, USA: 2014. Bone cancer, primary bone cancers and bone metastases. [Google Scholar]
- 9.Robins D.R., Wu Y.M., Lin S.F. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 10.Blume-Jensen P., Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 11.Arkin I.T. Structural aspects of oligomerization taking place between the transmembrane α-helices of bitopic membrane proteins. Biochim Biophys Acta. 2002;1565:347–363. doi: 10.1016/s0005-2736(02)00580-1. [DOI] [PubMed] [Google Scholar]
- 12.Moriki T., Maruyama H., Maruyama I.N. Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain. J Mol Biol. 2001;311:1011–1026. doi: 10.1006/jmbi.2001.4923. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard S.R., Till J.H. Protein tyrosine kinase structure and function. Ann Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 14.Lemmon M.A., Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogiso H., Ishitani R., Nureki O., Fukai S., Yamanaka M., Kim J.H. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw R.A., Chalkley R.J., Biarc J., Burlingame A.L. Receptor tyrosine kinase signaling mechanisms: devolving TrkA responses with phosphoproteomics. Adv Biol Regul. 2013;53:87–96. doi: 10.1016/j.jbior.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard S.R. Autoinhibitory mechanisms in receptor tyrosine kinases. Front Biosci. 2002;7:330–340. doi: 10.2741/A778. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard S.R., Miller W.T. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol. 2007;19:117–123. doi: 10.1016/j.ceb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Rohrschneider L.R. The gift of gab. FEBS Lett. 2002;515:1–7. doi: 10.1016/s0014-5793(02)02425-0. [DOI] [PubMed] [Google Scholar]
- 20.Choudhary C., Mann M. Decoding signalling networks by mass spectrometry-based proteomics. Nat Rev Mol Cell Biol. 2010;11:427–439. doi: 10.1038/nrm2900. [DOI] [PubMed] [Google Scholar]
- 21.Cseh B., Doma E., Baccarini M. “RAF” neighborhood: protein–protein interaction in the Raf/Mek/Erk pathway. FEBS Lett. 2014;588:2398–2406. doi: 10.1016/j.febslet.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roskoski R., Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Raman M., Chen W., Cobb M.H. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 24.Cargnello M., et Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song G., Ouyang G., Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ralston R., Bishop J.M. The product of the protooncogene c-Src is modified during the cellular response to platelet-derived growth factor. Proc Natl Acad Sci USA. 1985;82:7845–7849. doi: 10.1073/pnas.82.23.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bromann P.A., Korkaya H., Courtneidge S.A. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 28.Biscardi J.S., Maa M.C., Tice D.A., Cox M.E., Leu T.H., Parsons S.J. C-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 29.Moro L., Dolce L., Cabodi S., Bergatto E., Erba E.B., Smerigli M. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem. 2002;277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- 30.Goi T., Shipitsin M., Lu Z., Foster D.A., Klinz S.G., Feig L.A. An EGF receptor/Ral-GTPase signaling cascade regulates c-Src activity and substrate specificity. EMBO J. 2000;19:623–630. doi: 10.1093/emboj/19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livio T., Berlotti A., Comoglio P.M. MET signaling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 32.Freeman M. Feedback control of intercellular signaling in development. Nature. 2000;408:313–319. doi: 10.1038/35042500. [DOI] [PubMed] [Google Scholar]
- 33.Amit I., Citri A., Shay T., Lu Y., Katz M., Zhang F. A module of negative feedback regulators defines growth factor signaling. Nat Genet. 2007;39:503–512. doi: 10.1038/ng1987. [DOI] [PubMed] [Google Scholar]
- 34.Santos S.D., Verveer P.J., Bastiaens P.I. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat Cell Biol. 2007;9:324–330. doi: 10.1038/ncb1543. [DOI] [PubMed] [Google Scholar]
- 35.Dikic I., Giordano S. Negative receptor signalling. Curr Opin Cell Biol. 2003;15:128–135. doi: 10.1016/s0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 36.Marmor M.D., Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- 37.Jiang X., Huang F., Marusyk A., Sorkin A. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol Biol Cell. 2003;14:858–870. doi: 10.1091/mbc.E02-08-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Z., Jiang G., Jensen P., Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001;21:4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petti L.M., Irusta P.M., DiMaio D. Oncogenic activation of the PDGF beta receptor by the transmembrane domain of p185neu*. Oncogene. 1998;16:843–851. doi: 10.1038/sj.onc.1201590. [DOI] [PubMed] [Google Scholar]
- 40.Meshinchi S., Appelbaum F.R. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin Cancer Res. 2009;15:4263–4269. doi: 10.1158/1078-0432.CCR-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonescu C.R. The GIST paradigm: lessons for other kinase-driven cancers. J Pathol. 2011;223:251–261. doi: 10.1002/path.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 43.Bose R., Kavuri S.M., Searleman A.C., Shen W., Shen D., Koboldt D.C. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakajima H., Ishikawa Y., Furuya M., Sano T., Ohno Y., Horiguchi J. Protein expression, gene amplification, and mutational analysis of EGFR in triple-negative breast cancer. Breast Cancer. 2014;21:66–74. doi: 10.1007/s12282-012-0354-1. [DOI] [PubMed] [Google Scholar]
- 45.Peraldo-Neia C., Migliardi G., Mello-Grand M., Montemurro F., Segir R., Pignochino Y. Epidermal growth factor receptor (EGFR) mutation analysis, gene expression profiling and EGFR protein expression in primary prostate cancer. BMC Cancer. 2011;11:31. doi: 10.1186/1471-2407-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu M., Zhang W., Shan L., Song J., Shang D., Ying J. Mutation status of somatic EGFR and KRAS genes in Chinese patients with prostate cancer (PCa) Virchows Arch. 2014;464:575–581. doi: 10.1007/s00428-014-1566-x. [DOI] [PubMed] [Google Scholar]
- 47.Marie P.J. Signaling pathways affecting skeletal health. Curr Osteoporos Rep. 2012;10:190–198. doi: 10.1007/s11914-012-0109-0. [DOI] [PubMed] [Google Scholar]
- 48.Marie P.J. Fibroblast growth factor signaling controlling bone formation: an update. Gene. 2012;498:1–4. doi: 10.1016/j.gene.2012.01.086. [DOI] [PubMed] [Google Scholar]
- 49.Dai J., Rabie A.B. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- 50.Al-Kharobi H., El-Gendy R., Devine D.A., Beattie J. The role of the insulin‑like growth factor (IGF) axis in osteogenic and odontogenic differentiation. Cell Mol Life Sci. 2014;71:1469–1476. doi: 10.1007/s00018-013-1508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sims N.A., Martin T.J. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy Rep. 2014;3:481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heymann D., Guicheux J., Gouin F., Passuti N., Daculsi G. Cytokines growth factors and osteoclasts. Cytokine. 1998;10:155–168. doi: 10.1006/cyto.1997.0277. [DOI] [PubMed] [Google Scholar]
- 53.Clarkin C.E., Gerstenfeld L.C. VEGF and bone cell signalling: an essential vessel for communication? Cell Biochem Funct. 2013;31:1–11. doi: 10.1002/cbf.2911. [DOI] [PubMed] [Google Scholar]
- 54.Crane J.L., Cao X. Function of matrix IGF-1 in coupling bone resorption and formation. J Mol Med (Berl) 2014;92:107–115. doi: 10.1007/s00109-013-1084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao N.R., Lu M., Bin F.W., Chang Z.Y., Meng J., Zhou L.W. Systematic screen with kinases inhibitors reveals kinases play distinct roles in growth of osteoprogenitor cells. Int J Clin Exp Pathol. 2013;6:2082–2091. [PMC free article] [PubMed] [Google Scholar]
- 56.O’Sullivan S., Lin J.M., Watson M., Callon K., Tong P.C., Naot D. The skeletal effects of the tyrosine kinase inhibitor nilotinib. Bone. 2011;49:281–289. doi: 10.1016/j.bone.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Pinski J., Weeraratna A., Uzgare A.R., Arnold J.T., Denmeade S.R. Isaacs JT.Trk receptor inhibition induces apoptosis of proliferating but not quiescent human osteoblasts. Cancer Res. 2002;62:986–989. [PubMed] [Google Scholar]
- 58.Duan Z., Choy E., Harmon D., Yang C., Ryu K., Schwab J. Insulin-like growth factor-I receptor tyrosine kinase inhibitor cyclolignan picropodophyllin inhibits proliferation and induces apoptosis in multidrug resistant osteosarcoma cell lines. Mol Cancer Ther. 2009;8:2122–2130. doi: 10.1158/1535-7163.MCT-09-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandyke K., Fitter S., Dewar A.L., Hughes T.P., Zannettino A.C. Dysregulation of bone remodeling by imatinib mesylate. Blood. 2010;115(4):766–774. doi: 10.1182/blood-2009-08-237404. [DOI] [PubMed] [Google Scholar]
- 60.Gobin B., Moriceau G., Ory B., Charrier C., Brion R., Blanchard F. Imatinib mesylate exerts anti-proliferative effects on osteosarcoma cells and inhibits the tumour growth in immunocompetent murine models. PLoS One. 2014;9:e90795. doi: 10.1371/journal.pone.0090795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Hajj Dib I., Gallet M., Mentaverri R., Sévenet N., Brazier M., Kamel S. Imatinib mesylate (Gleevec) enhances mature osteoclast apoptosis and suppresses osteoclast bone resorbing activity. Eur J Pharmacol. 2006;551:27–33. doi: 10.1016/j.ejphar.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Rimondi E., Secchiero P., Melloni E., Grill V., Zauli G. Sorafenib inhibits in vitro osteoclastogenesis by down-modulating Mcl-1. Invest New Drugs. 2013;31:780–786. doi: 10.1007/s10637-012-9903-x. [DOI] [PubMed] [Google Scholar]
- 63.Vandyke K., Dewar A.L., Diamond P., Fitter S., Schultz C.G., Sims N.A. The tyrosine kinase inhibitor dasatinib dysregulates bone remodeling through inhibition of osteoclasts in vivo. J Bone Miner Res. 2010;5:1759–1770. doi: 10.1002/jbmr.85. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Gomez A., Ocio E.M., Crusoe E., Santamaria C., Hernández-Campo P., Blanco J.F. Dasatinib as a bone-modifying agent: anabolic and anti-resorptive effects. PLoS One. 2012;7:e34914. doi: 10.1371/journal.pone.0034914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heymann D., Redini F. Bone sarcomas: pathogenesis and new therapeutic approaches. IBMS BoneKEy. 2011;8:402–414. [Google Scholar]
- 66.Heymann D., Redini F. Targeted therapies for bone sarcomas. BoneKEy Rep. 2013;2:378. doi: 10.1038/bonekey.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaspar N., Di Giannatale A., Geoerger B., Redini F., Corradini N., Enz-Werle N. Bone sarcomas: from biology to targeted therapies. Sarcoma. 2012;2012:301975. doi: 10.1155/2012/301975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rettew A.N., Getty P.J., Greenfield E.M. Receptor tyrosine kinases in osteosarcoma: not just the usual suspects. Adv Exp Med Biol. 2014;804:47–66. doi: 10.1007/978-3-319-04843-7_3. [DOI] [PubMed] [Google Scholar]
- 69.McGary E.C., Weber K., Mills L., Doucet M., Lewis V., Lev D.C. Inhibition of platelet-derived growth factor-mediated proliferation of osteosarcoma cells by the novel tyrosine kinase inhibitor STI571. Clin Cancer Res. 2002;8:3584–3591. [PubMed] [Google Scholar]
- 70.Ikeda A.K., Judelson D.R., Federman N., Glaser K.B., Landaw E.M., Denny C.T. ABT-869 inhibits the proliferation of Ewing sarcoma cells and suppresses platelet-derived growth factor receptor beta and c-KIT signaling pathways. Mol Cancer Ther. 2010;9:653–660. doi: 10.1158/1535-7163.MCT-09-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chugh R., Wathen J.K., Maki R.G., Benjamin R.S., Patel S.R., Meyers P.A. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol. 2009;27:3148–3153. doi: 10.1200/JCO.2008.20.5054. [DOI] [PubMed] [Google Scholar]
- 72.Chao J., Budd G.T., Chu P., Frankel P., Garcia D., Junqueira M. Phase II clinical trial of imatinib mesylate in therapy of KIT and/or PDGFRalpha-expressing Ewing sarcoma family of tumours and desmoplastic small round cell tumours. Anticancer Res. 2010;30:547–552. [PubMed] [Google Scholar]
- 73.Bond M., Bernstein M.L., Pappo A., Schultz K.R., Krailo M., Blaney S.M. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumours: a Children’s Oncology Group study. Pediatr Blood Cancer. 2008;50:254–258. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez I., Andreu E.J., Panizo A., Inoges S., Fontalba A., Fernandez-Luna J.L. Imatinib inhibits proliferation of Ewing tumour cells mediated by the stem cell factor/KIT receptor pathway, and sensitizes cells to vincristine and doxorubicin-induced apoptosis. Clin Cancer Res. 2004;10:751–761. doi: 10.1158/1078-0432.ccr-0778-03. [DOI] [PubMed] [Google Scholar]
- 75.Timeus F., Crescenzio N., Fandi A., Doria A., Foglia L., Cordero di Montezemolo L. In vitro antiproliferative and antimigratory activity of dasatinib in neuroblastoma and Ewing sarcoma cell lines. Oncol Rep. 2008;19:353–359. [PubMed] [Google Scholar]
- 76.Hingorani P., Zhang W., Gorlick R., Kolb E.A. Inhibition of Src phosphorylation alters metastatic potential of osteosarcoma in vitro but not in vivo. Clin Cancer Res. 2009;15:3416–3422. doi: 10.1158/1078-0432.CCR-08-1657. [DOI] [PubMed] [Google Scholar]
- 77.Aplenc R., Blaney S.M., Strauss L.C., Balis F.M., Shusterman S., Ingle A.M. Pediatric phase I trial and pharmacokinetic study of dasatinib: a report from the children’s oncology group phase I consortium. J Clin Oncol. 2011;29:839–844. doi: 10.1200/JCO.2010.30.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maris J.M., Courtright J., Houghton P.J., Morton C.L., Kolb E.A., Lock R. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51:42–48. doi: 10.1002/pbc.21535. [DOI] [PubMed] [Google Scholar]
- 79.Dubois S.G., Shusterman S., Ingle A.M., Ahern C.H., Reid J.M., Wu B. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumours: a children’s oncology group study. Clin Cancer Res. 2011;17:5113–5122. doi: 10.1158/1078-0432.CCR-11-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keir S.T., Morton C.L., Wu J., Kurmasheva R.T., Houghton P.J., Smith M.A. Initial testing of the multitargeted kinase inhibitor pazopanib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2012;59:586–588. doi: 10.1002/pbc.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glade Bender J.L., Lee A., Reid J.M., Baruchel S., Roberts T., Voss S.D. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children׳s oncology group phase I consortium report. J Clin Oncol. 2013;31:3034–3043. doi: 10.1200/JCO.2012.47.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar S., Mokhtari R.B., Sheikh R., Wu B., Zhang L., Xu P. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumour. Clin Cancer Res. 2011;17:5656–5667. doi: 10.1158/1078-0432.CCR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pignochino Y., Grignani G., Cavalloni G., Motta M., Tapparo M., Bruno S. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer. 2009;8:118. doi: 10.1186/1476-4598-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Navid F., Baker S.D., McCarville M.B., Stewart C.F., Billups C.A., Wu J. Phase I and clinical pharmacology study of bevacizumab, sorafenib, and low-dose cyclophosphamide in children and young adults with refractory/recurrent solid tumors. Clin Cancer Res. 2013;19:236–246. doi: 10.1158/1078-0432.CCR-12-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grignani G., Palmerini E., Dileo P., Asaftei S.D., D’Ambrosio L., Pignochino Y. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23:508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 86.Han J., Tian R., Yong B., Luo C., Tan P., Shen J. Gas6/Axl mediates tumor cell apoptosis, migration and invasion and predicts the clinical outcome of osteosarcoma patients. Biochem Biophys Res Commun. 2013;435:493–500. doi: 10.1016/j.bbrc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 87.Rettew A.N., Getty P.J., Greenfield E.M. Receptor tyrosine kinases in osteosarcoma: not just the usual suspects. Adv Exp Med Biol. 2014;804:47–66. doi: 10.1007/978-3-319-04843-7_3. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y., Tang Y.J., Man Y., Pan F., Li Z.H., Jia L.S. Knockdown of AXL receptor tyrosine kinase in osteosarcoma cells leads to decreased proliferation and increased apoptosis. Int J Immunopathol Pharmacol. 2013;26:179–188. doi: 10.1177/039463201302600117. [DOI] [PubMed] [Google Scholar]
- 89.Fleuren E.D., Hillebrandt-Roeffen M.H., Flucke U.E., Te Loo D.M., Boerman O.C., van der Graaf W.T. The role of AXL and the in vitro activity of the receptor tyrosine kinase inhibitor BGB324 in Ewing sarcoma. Oncotarget. 2014;5:12753–12768. doi: 10.18632/oncotarget.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fleuren E.D., Roeffen M.H., Leenders W.P., Flucke U.E., Vlenterie M., Schreuder H.W. Expression and clinical relevance of MET and ALK in Ewing sarcomas. Int J Cancer. 2013;133:427–436. doi: 10.1002/ijc.28047. [DOI] [PubMed] [Google Scholar]
- Sampson E.R., Martin B.A., Morris A.E., Xie C., Schwarz E.M., O׳Keefe R.J. The orally bioavailable met inhibitor PF-2341066 inhibits osteosarcoma growth and osteolysis/matrix production in a xenograft model. J Bone Miner Res. 2011;26:1283–1294. doi: 10.1002/jbmr.336. [DOI] [PubMed] [Google Scholar]
- 92.Fritsche-Guenther R., Noske A., Ungethüm U., Kuban R.J., Schlag P.M., Tunn P.U. De novo expression of EphA2 in osteosarcoma modulates activation of the mitogenic signalling pathway. Histopathology. 2010;57:836–850. doi: 10.1111/j.1365-2559.2010.03713.x. [DOI] [PubMed] [Google Scholar]
- 93.Mstuo K., Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adhes Migr. 2012;6:148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Posthumadeboer J., Piersma S.R., Pham T.V., van Egmond P.W., Knol J.C., Cleton-Jansen A.M. Surface proteomic analysis of osteosarcoma identifies EPHA2 as receptor for targeted drug delivery. Br J Cancer. 2013;109:2142–2154. doi: 10.1038/bjc.2013.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuijjer M.L., Peterse E.F., van den Akker B.E., Briaire-de Bruijn I.H., Serra M., Meza-Zepeda L.A. IR/IGF1R signaling as potential target for treatment of high-grade osteosarcoma. BMC Cancer. 2013;13:245. doi: 10.1186/1471-2407-13-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cao Y., Roth M., Piperdi S., Montoya K., Sowers R., Rao P. Insulin-like growth factor 1 receptor and response to anti-IGF1R antibody therapy in osteosarcoma. PLoS One. 2014;9:e106249. doi: 10.1371/journal.pone.0106249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee J.A., Ko Y., Kim D.H., Lim J.S., Kong C.B., Cho W.H. Epidermal growth factor receptor: is it a feasible target for the treatment of osteosarcoma? Cancer Res Treat. 2012;44:202–209. doi: 10.4143/crt.2012.44.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gill J., Geller D., Gorlick R. HER-2 involvement in osteosarcoma. Adv Exp Med Biol. 2014;804:161–177. doi: 10.1007/978-3-319-04843-7_9. [DOI] [PubMed] [Google Scholar]
- 99.Ebb D., Meyers P., Grier H., Bernstein M., Gorlick R., Lipshultz S.E. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children׳s oncology group. J Clin Oncol. 2012;30:2545–2551. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Macheda M.L., Stacker S.A. Importance of Wnt signaling in the tumour stroma microenvironment. Curr Cancer Drug Targets. 2008;8:454–465. doi: 10.2174/156800908785699324. [DOI] [PubMed] [Google Scholar]
- 101.Truitt L., Freywald A. Dancing with the dead: Eph receptors and their kinase-null partners. Biochem Cell Biol. 2011;89:115–129. doi: 10.1139/o10-145. [DOI] [PubMed] [Google Scholar]
- 102.Clézardin P. Pathophysiology of bone metastases and new molecular targets involved in bone remodelling. Bull Cancer. 2013;100:1083–1091. doi: 10.1684/bdc.2013.1836. [DOI] [PubMed] [Google Scholar]
- 103.Uehara H., Kim S.J., Karashima T., Shepherd D.L., Fan D., Tsan R. Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases. J Natl Cancer Inst. 2003;95:458–470. doi: 10.1093/jnci/95.6.458. [DOI] [PubMed] [Google Scholar]
- 104.Kim S.J., Uehara H., Yazici S., Busby J.E., Nakamura T., He J. Targeting platelet-derived growth factor receptor on endothelial cells of multidrug-resistant prostate cancer. J Natl Cancer Inst. 2006;98:783–793. doi: 10.1093/jnci/djj211. [DOI] [PubMed] [Google Scholar]
- 105.Tiffany N.M., Wersinger E.M., Garzotto M., Beer T.M. Imatinib mesylate and zoledronic acid in androgen-independent prostate cancer. Urology. 2004;63:934–939. doi: 10.1016/j.urology.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 106.Mathew P., Thall P.F., Jones D., Perez C., Bucana C., Troncoso P. Platelet-derived growth factor receptor inhibitor imatinib mesylate and docetaxel: a modular phase I trial in androgen-independent prostate cancer. J Clin Oncol. 2004;22:3323–3329. doi: 10.1200/JCO.2004.10.116. [DOI] [PubMed] [Google Scholar]
- 107.Mathew P., Thall P.F., Bucana C.D., Oh W.K., Morris M.J., Jones D.M. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13:5816–5824. doi: 10.1158/1078-0432.CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 108.Liu Y., Karaca M., Zhang Z., Gioeli D., Earp H.S., Whang Y.E. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene. 2010;29:3208–3216. doi: 10.1038/onc.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Araujo J.C., Poblenz A., Corn P., Parikh N.U., Starbuck M.W., Thompson J.T. Dasatinib inhibits both osteoclast activation and prostate cancer PC-3-cell-induced osteoclast formation. Cancer Biol Ther. 2009;8:2153–2159. doi: 10.4161/cbt.8.22.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koreckij T., Nguyen H., Brown L.G., Yu E.Y., Vessella R.L., Corey E. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer. 2009;101:263–268. doi: 10.1038/sj.bjc.6605178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takahashi S., Miyazaki M., Okamoto I., Ito Y., Ueda K., Seriu T. Phase I study of dasatinib (BMS-354825) in Japanese patients with solid tumors. Cancer Sci. 2011;102:2058–2064. doi: 10.1111/j.1349-7006.2011.02041.x. [DOI] [PubMed] [Google Scholar]
- 112.Yu E.Y., Massard C., Gross M.E., Carducci M.A., Culine S., Hudes G. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77:1166–1171. doi: 10.1016/j.urology.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu E.Y., Wilding G., Posadas E., Gross M., Culine S., Massard C. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–7428. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Araujo J.C., Trudel G.C., Saad F., Armstrong A.J., Yu E.Y., Bellmunt J. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14:1307–1316. doi: 10.1016/S1470-2045(13)70479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zwolak P., Jasinski P., Terai K., Gallus N.J., Ericson M.E., Clohisy D.R. Addition of receptor tyrosine kinase inhibitor to radiation increases tumour control in an orthotopic murine model of breast cancer metastasis in bone. Eur J Cancer. 2008;44:2506–2517. doi: 10.1016/j.ejca.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 116.Sonpavde G., Periman P.O., Bernold D., Weckstein D., Fleming M.T., Galsky M.D. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann Oncol. 2010;21:319–324. doi: 10.1093/annonc/mdp323. [DOI] [PubMed] [Google Scholar]
- 117.Michaelson M.D., Oudard S., Ou Y.C., Sengeløv L., Saad F., Houede N. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J Clin Oncol. 2014;32:76–82. doi: 10.1200/JCO.2012.48.5268. [DOI] [PubMed] [Google Scholar]
- 118.Bachelot T., Garcia-Saenz J.A., Verma S., Gutierrez M., Pivot X., Kozloff M.F. Sunitinib in combination with trastuzumab for the treatment of advanced breast cancer: activity and safety results from a phase II study. BMC Cancer. 2014;14:166. doi: 10.1186/1471-2407-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beuselinck B., Oudard S., Rixe O., Wolter P., Blesius A., Ayllon J. Negative impact of bone metastasis on outcome in clear-cell renal cell carcinoma treated with sunitinib. Ann Oncol. 2011;22:794–800. doi: 10.1093/annonc/mdq554. [DOI] [PubMed] [Google Scholar]
- 120.Keizman D., Ish-Shalom M., Pili R., Hammers H., Eisenberger M.A., Sinibaldi V. Bisphosphonates combined with sunitinib may improve the response rate, progression free survival and overall survival of patients with bone metastases from renal cell carcinoma. Eur J Cancer. 2012;48:1031–1037. doi: 10.1016/j.ejca.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 121.Merz M., Komljenovic D., Zwick S., Semmler W., Bäuerle T. Sorafenib tosylate and paclitaxel induce anti-angiogenic, anti-tumour and anti-resorptive effects in experimental breast cancer bone metastases. Eur J Cancer. 2011;47:277–286. doi: 10.1016/j.ejca.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 122.Dahut W.L., Scripture C., Posadas E., Jain L., Gulley J.L., Arlen P.M. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 123.Aragon-Ching J.B., Jain L., Gulley J.L., Arlen P.M., Wright J.J., Steinberg S.M. Final analysis of a phase II trial using sorafenib for metastatic castration-resistant prostate cancer. BJU Int. 2009;103:1636–1640. doi: 10.1111/j.1464-410X.2008.08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sciarra A., Autran Gomez A.M., Gentilucci A., Parente U., Salciccia S., Gentile V. Adjuvant therapy with sorafenib in bone metastases bilateral renal carcinoma: a case report. Eur Urol. 2007;52:597–599. doi: 10.1016/j.eururo.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 125.Humphrey P.A., Zhu X., Zarnegar R., Swanson P.E., Ratliff T.L., Vollmer R.T. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol. 1995;147:386–396. [PMC free article] [PubMed] [Google Scholar]
- 126.Pisters L.L., Troncoso P., Zhau H.E., Li W., von Eschenbach A.C., Chung L.W. c-met proto-oncogene expression in benign and malignant human prostate tissues. J Urol. 1995;154:293–298. [PubMed] [Google Scholar]
- 127.Nagy J., Curry G.W., Hillan K.J., McKay I.C., Mallon E., Purushotham A.D. Hepatocyte growth factor/scatter factor expression and c-met in primary breast cancer. Surg Oncol. 1996;5:15–21. doi: 10.1016/s0960-7404(96)80017-x. [DOI] [PubMed] [Google Scholar]
- 128.Tuck A.B., Park M., Sterns E.E., Boag A., Elliott B.E. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol. 1996;148:225–232. [PMC free article] [PubMed] [Google Scholar]
- 129.Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G.F. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 130.Ponzo M.G., Lesurf R., Petkiewicz S., O׳Malley F.P., Pinnaduwage D., Andrulis I.L. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc Natl Acad Sci USA. 2009;106:12903–12908. doi: 10.1073/pnas.0810402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Previdi S., Scolari F., Chilà R., Ricci F., Abbadessa G., Broggini M. Combination of the c-Met inhibitor tivantinib and zoledronic acid prevents tumor bone engraftment and inhibits progression of established bone metastases in a breast xenograft model. PLoS One. 2013;8:e79101. doi: 10.1371/journal.pone.0079101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.T.J. Graham, G. Box, N. Tunariu, M. Crespo, T.J. Spinks, S. Miranda, et al., Preclinical evaluation of imaging biomarkers for prostate cancer bone metastasis and response to cabozantinib, J Natl Cancer Inst, in press [DOI] [PubMed]
- 133.Lee R.J., Saylor P.J., Michaelson M.D., Rothenberg S.M., Smas M.E., Miyamoto D.T. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res. 2013;19:3088–3094. doi: 10.1158/1078-0432.CCR-13-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith M.R., Sweeney C.J., Corn Smith M.R., Sweeney C.J., Corn P.G., Rathkopf D.E. Cabozantinib in chemotherapy-pretreated metastatic castration-resistant prostate cancer: results of a phase II nonrandomized expansion study. J Clin Oncol. 2014;32:3391–3399. doi: 10.1200/JCO.2013.54.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith D.C., Smith M.R., Sweeney C., Elfiky A.A., Logothetis C., Corn P.G. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.E. Basch, K.A. Autio, M.R. Smith, A.V. Bennett, A.L. Weitzman, C. Scheffold, et al., Effects of cabozantinib on pain and narcotic use in patients with castration-resistant prostate cancer: results from a phase 2 nonrandomized expansion cohort, Eur Urol, in press [DOI] [PubMed]
- 137.Previdi S., Scolari F., Chilà R., Ricci F., Abbadessa G., Broggini M. Combination of the c-Met inhibitor tivantinib and zoledronic acid prevents tumor bone engraftment and inhibits progression of established bone metastases in a breast xenograft model. PLoS One. 2013;8:e79101. doi: 10.1371/journal.pone.0079101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Previdi S., Abbadessa G., Dalò F., France D.S., Broggini M. Breast cancer-derived bone metastasis can be effectively reduced through specific c-MET inhibitor tivantinib (ARQ 197) and shRNA c-MET knockdown. Mol Cancer Ther. 2012;11:214–223. doi: 10.1158/1535-7163.MCT-11-0277. [DOI] [PubMed] [Google Scholar]
- 139.Yin J.J., Zhang L., Munasinghe J., Linnoila R.I., Kelly K. Ceranib/AZD2171 inhibits bone and brain metastasis in a preclinical model of advanced prostate cancer. Cancer Res. 2010;70:8662–8673. doi: 10.1158/0008-5472.CAN-10-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ryan C.J., Stadler W.M., Roth B., Hutcheon D., Conry S., Puchalski T. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest New Drugs. 2007;25(5):445–451. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 141.Bachelier R., Confavreux C.B., Peyruchaud O., Croset M., Goehrig D., van der Pluijm G. Combination of anti-angiogenic therapies reduces osteolysis and tumor burden in experimental breast cancer bone metastasis. Int J Cancer. 2014;135:1319–1329. doi: 10.1002/ijc.28787. [DOI] [PubMed] [Google Scholar]
- 142.Furugaki K., Moriya Y., Iwai T., Yorozu K., Yanagisawa M., Kondoh K. Erlotinib inhibits osteolytic bone invasion of human non-small-cell lung cancer cell line NCI-H292. Clin Exp Metastasis. 2011;28:649–659. doi: 10.1007/s10585-011-9398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nabhan C., Lestingi T.M., Galvez A., Tolzien K., Kelby S.K., Tsarwhas D. Erlotinib has moderate single-agent activity in chemotherapy-naïve castration-resistant prostate cancer: final results of a phase II trial. Urology. 2009;74:665–671. doi: 10.1016/j.urology.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 144.Gross M., Higano C., Pantuck A., Castellanos O., Green E., Nguyen K. A phase II trial of docetaxel and erlotinib as first-line therapy for elderly patients with androgen-independent prostate cancer. BMC Cancer. 2007;7:142. doi: 10.1186/1471-2407-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.D׳Alessio A., De Luca A., Maiello M.R., Lamura L., Rachiglio A.M., Napolitano M. Effects of the combined blockade of EGFR and ErbB-2 on signal transduction and regulation of cell cycle regulatory proteins in breast cancer cells. Breast Cancer Res Treat. 2010;123:387–396. doi: 10.1007/s10549-009-0649-x. [DOI] [PubMed] [Google Scholar]
- 146.Borghese C., Cattaruzza L., Pivetta E., Normanno N., De Luca A., Mazzucato M. Gefitinib inhibits the cross-talk between mesenchymal stem cells and prostate cancer cells leading to tumor cell proliferation and inhibition of docetaxel activity. J Cell Biochem. 2013;114:1135–1144. doi: 10.1002/jcb.24456. [DOI] [PubMed] [Google Scholar]
- 147.Sgambato A., Camerini A., Faraglia B., Ardito R., Bianchino G., Spada D. Targeted inhibition of the epidermal growth factor receptor-tyrosine kinase by ZD1839 (׳Iressa׳) induces cell-cycle arrest and inhibits proliferation in prostate cancer cells. J Cell Physiol. 2004;201:97–105. doi: 10.1002/jcp.20045. [DOI] [PubMed] [Google Scholar]
- 148.Pezaro C., Rosenthal M.A., Gurney H., Davis I.D., Underhill C., Boyer M.J. An open-label, single-arm phase two trial of gefitinib in patients with advanced or metastatic castration-resistant prostate cancer. Am J Clin Oncol. 2009;32:338–341. doi: 10.1097/COC.0b013e31818b946b. [DOI] [PubMed] [Google Scholar]
- 149.Boccardo F., Rubagotti A., Conti G., Battaglia M., Cruciani G., Manganelli A. Prednisone plus gefitinib versus prednisone plus placebo in the treatment of hormone-refractory prostate cancer: a randomized phase II trial. Oncology. 2008;74:223–228. doi: 10.1159/000151391. [DOI] [PubMed] [Google Scholar]
- 150.Salzberg M., Rochlitz C., Morant R., Thalmann G., Pedrazzini A., Roggero E. An open-label, noncomparative phase II trial to evaluate the efficacy and safety of docetaxel in combination with gefitinib in patients with hormone-refractory metastatic prostate cancer. Onkologie. 2007;30:355–360. doi: 10.1159/000102452. [DOI] [PubMed] [Google Scholar]
- 151.Somlo G., Martel C.L., Lau S.K., Frankel P., Ruel C., Gu L. A phase I/II prospective, single arm trial of gefitinib, trastuzumab, and docetaxel in patients with stage IV HER-2 positive metastatic breast cancer. Breast Cancer Res Treat. 2012;131:899–906. doi: 10.1007/s10549-011-1850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carlson R.W., O׳Neill A., Vidaurre T., Gomez H.L., Badve S.S., Sledge G.W. A randomized trial of combination anastrozole plus gefitinib and of combination fulvestrant plus gefitinib in the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat. 2012;133:1049–1056. doi: 10.1007/s10549-012-1997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Osborne C.K., Neven P., Dirix L.Y., Mackey J.R., Robert J., Underhill C. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011;17:1147–1159. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cristofanilli M., Valero V., Mangalik A., Royce M., Rabinowitz I., Arena F.P. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16:1904–1914. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 155.Whang Y.E., Armstrong A.J., Rathmell W.K., Godley P.A., Kim W.Y., Pruthi R.S. A phase II study of lapatinib, a dual EGFR and HER-2 tyrosine kinase inhibitor, in patients with castration-resistant prostate cancer. Urol Oncol. 2013;31:82–86. doi: 10.1016/j.urolonc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 156.Pircher M., Mlineritsch B., Fridrik M.A., Dittrich C., Lang A., Petru E. Lapatinib-plus-pegylated liposomal doxorubicin in advanced HER2-positive breast cancer following trastuzumab: a phase II trial. Anticancer Res. 2015;35:517–521. [PubMed] [Google Scholar]
- 157.Sridhar S.S., Hotte S.J., Chin J.L., Hudes G.R., Gregg R., Trachtenberg J. A multicenter phase II clinical trial of lapatinib (GW572016) in hormonally untreated advanced prostate cancer. Am J Clin Oncol. 2010;33:609–613. doi: 10.1097/COC.0b013e3181beac33. [DOI] [PubMed] [Google Scholar]
- 158.Azad A.A., Beardsley E.K., Hotte S.J., Ellard S.L., Klotz L., Chin J. A randomized phase II efficacy and safety study of vandetanib (ZD6474) in combination with bicalutamide versus bicalutamide alone in patients with chemotherapy naïve castration-resistant prostate cancer. Invest New Drugs. 2014;32:746–752. doi: 10.1007/s10637-014-0091-8. [DOI] [PubMed] [Google Scholar]
- 159.Clemons M.J., Cochrane B., Pond G.R., Califaretti N., Chia S.K., Dent R.A. Randomised, phase II, placebo-controlled, trial of fulvestrant plus vandetanib in postmenopausal women with bone only or bone predominant, hormone-receptor-positive metastatic breast cancer (MBC): the OCOG ZAMBONEY study. Breast Cancer Res Treat. 2014;146:153–162. doi: 10.1007/s10549-014-3015-6. [DOI] [PubMed] [Google Scholar]
- 160.Wan X., Corn P.G., Yang J., Palanisamy N., Starbuck M.W., Efstathiou E. Prostate cancer cell-stromal cell crosstalk via FGFR1 mediates antitumor activity of dovitinib in bone metastases. Sci Transl Med. 2014;6:122–252. doi: 10.1126/scitranslmed.3009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vallo S., Mani J., Stastny M., Makarević J., Juengel E., Tsaur I. The prostate cancer blocking potential of the histone deacetylase inhibitor LBH589 is not enhanced by the multi receptor tyrosine kinase inhibitor TKI258. Invest New Drugs. 2013;31:265–272. doi: 10.1007/s10637-012-9851-5. [DOI] [PubMed] [Google Scholar]
- 162.Nguyen H.M., Ruppender N., Zhang X., Brown L.G., Gross T.S., Morrissey C. Cabozantinib inhibits growth of androgen-sensitive and castration-resistant prostate cancer and affects bone remodeling. PLoS One. 2013;8:e78881. doi: 10.1371/journal.pone.0078881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee R.J., Smith M.R. Cabozantinib and prostate cancer: inhibiting seed and disrupting soil? Clin Cancer Res. 2014;20:525–527. doi: 10.1158/1078-0432.CCR-13-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Meads M.B., Hazlehurst L.A., Dalton W.S. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- 165.David E., Blanchard F., Heymann M.F., De Pinieux G., Gouin F., Rédini F. The bone niche of chondrosarcoma: a sanctuary for drug resistance, tumour growth and also a source of new therapeutic targets. Sarcoma. 2011;2011:932451. doi: 10.1155/2011/932451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Trent J.C., Subramanian M.P. Managing GIST in the imatinib era: optimization of adjuvant therapy. Expert Rev Anticancer Ther. 2014;14:1445–1459. doi: 10.1586/14737140.2014.952284. [DOI] [PubMed] [Google Scholar]