Abstract
Asthma is a pulmonary disorder, with an estimated 300 million people affected worldwide. While it is thought that endogenous reactive oxygen species (ROS) and reactive nitrogen species (RNS) such as hydrogen peroxide and nitric oxide, are important mediators of natural physiological processes, inflammatory cells recruited to the asthmatic airways have an exceptional capacity for producing a variety of highly reactive ROS and RNS believed to contribute to tissue damage and chronic airways inflammation. Antioxidant defense systems form a tightly regulated network that maintains the redox environment of the intra- as well as extracellular environment. Evidence for an oxidant-antioxidant imbalance in asthmatic airways is demonstrated in a number of studies, revealing decreased total antioxidant capacity as well as lower levels of individual antioxidants. Thiols in the form of GSH and sulfhydryl groups of proteins are among the most susceptible oxidant-sensitive targets, and hence, studies investigating protein thiol redox modifications in biology and disease have emerged. This perspective offers an overview of the combined efforts aimed at the elucidation of mechanisms whereby cysteine oxidations contribute to chronic inflammation and asthma, as well as insights into potential cysteine thiol-based therapeutic strategies.
I. Asthma
Asthma is a disorder characterized by chronic airway inflammation, mucus secreting cell hyperplasia and metaplasia, airway hyperreactivity (AHR) and airway remodeling [Hansbro et al., 2011]. The prevalence of asthma has increased dramatically over the past two decades, affecting 300 million people globally. As this number grows, the demand for therapeutic intervention also increases, and hundreds of molecules are currently in development [Short et al., 1996]. Current therapeutics, however, are of limited efficacy as will be discussed further in this review.
The etiology of asthma is complex and multifactorial. Clinical manifestations include allergic asthma, severe and steroid-resistant asthma, as well as exacerbations induced by air pollution, cigarette smoke, obesity and exercise-associated, or even aspirin-induced asthma [Kim et al., 2010; Narayanankutty et al., 2013]. The cause(s) of asthma remain incompletely understood, but it is believed that the complex interplay between genetic and environmental factors becomes unbalanced and predisposes individuals to hypersensitive reactions. In the mid-1980’s, type 2 CD4+ lymphocytes (TH2 cells) were thought to be integral to the pathogenesis of allergic airway disease based on the observation that specific TH2 subtypes existed in asthmatic patients [Hansbro et al., 2011]. These T cells were able to secrete cytokines (IL-3, -4, -5, -9, -13 and GM-CSF) responsible for the recruitment, priming and survival of the primary effector cells (e.g., mast cells and eosinophils) [Holgate, 2010]. TH2-type cytokines are now known to contribute to tissue damage, mucus metaplasia, airway obstruction and chronic inflammation of the airways; all hallmarks of persistent asthma [Whitehead et al., 2003]. Antigen-presenting dendritic cells (DCs) were also identified in individuals susceptible to indoor and outdoor environmental allergens [Steinman, 1991]. In the last decade, studies have been aimed at addressing how specific DC subsets recognize allergens and communicate with naive T cells through Class II MHC T cell receptor (CD3) interaction and engagement of co-stimulatory molecules [Holgate, 2010]. Based upon these collective studies, among the potential new targets being evaluated for treatment of asthma are the immune-stimulating cytokines IL-4 and IL-13. These cytokines are critical to asthma pathophysiology, and may hold promise in the treatment of specific patient populations [May, 2011].
Patients suffering from allergic airway disease often exhibit increased serum IgE levels and predominant eosinophilic infiltration [Hamelmann et al., 1999] and eosinophilic driven chemokines CCL-11 and -24 within the airways. The discovery that administration of a monoclonal antibody targeting FcepsilonR1, the high affinity IgE binding site on mast cells, blocks mast cell activation and degranulation [McKeage, 2013], led to clinical trials using the monoclonal antibody, Omalizumab. Omalizumab led to an almost total inhibition of the early and late asthmatic responses to inhaled allergen. However, only one third to one half of patients with severe allergic asthma appeared to respond to Omalizumab, potentially corresponding to the extent that blockade of IgE binding to mast cells and dendritic cells produce down-regulation of FcεR1 [Holgate, 2010]. More recently, multiple studies have investigated the ability of Mepolizumab, a humanized monoclonal antibody against interleukin-5 (IL-5) to selectively inhibit eosinophilic inflammation, reduce the number of eosinophils in both sputum and blood, and reduce the frequency of exacerbations and the need for treatment with systemic glucocorticoids in patients with severe asthma and persistent eosinophilic inflammation. Following a trial evaluating dose ranging efficacy and safety in response to intravenous Mepolizumab [Pavord et al., 2012], a newly reported study utilizing the same defining characteristics (blood eosinophil count, number of previous exacerbations and dose of inhaled corticosteroids) evaluated subcutaneous versus intravenous administration of Mepolizumab to determine whether the use of anti-IL-5 therapy would mitigate the requirement for frequent glucocorticoid use in patients with severe asthma. The occurrence of exacerbations was significantly lessened in those patients receiving both intravenous and subcutaneous Mepolizumab and was associated with improvements in markers of asthma control [Ortega et al., 2014].
Recent clinical and experimental observations have suggested however, that asthma is much more heterogeneous than proposed by the TH2 paradigm, and roles for TH1 cells, regulatory T (TReg) cells, TH17, and innate lymphoid cells (ILC) have been identified. While it is known that multiple T cell lineages contribute to the pathogenesis of allergic airway disease, the extent and degree to which these cells orchestrate the respective pheno(endo)types of human asthma remains unknown. Non- TH2 responses, such as IL-17A, E, or F production are frequently found in the lungs of asthma patients, particularly those with severe or corticosteroid-resistant asthma [Kim et al., 2010]. IL-17E, released by alveolar macrophages, is associated with TH1 and TH17 immunity and may be involved in the mixed T-cell response [Hansbro et al., 2011]. IL-17 and IL-22 have been shown to sustain inflammation in allergic diseases and affect a variety of cell types, particularly epithelial cells in the inflamed tissues [Souwer et al., 2010]. Abundant neutrophilic infiltration may also play a role in asthma pathophysiology possibly through release of ROS, RNS, cytokines, lipid mediators and enzymes including elastase, myeloperoxidases and cathepsin G [Hamid and Tulic, 2009]. Notably, the presence of neutrophils in lungs of severe asthmatics has been linked to IL-17 production, and is associated with steroid resistance [Chang et al., 2013; Ryu et al., 2013; Sturrock et al., 2006; Sutcliffe et al., 2012].
Originally described as TH2 cytokine producing non-B/non-T cells, Innate Lymphoid Cells (ILCs) were identified by multiple groups in 2010. One study identified c-Kit, Sca-1, IL-17R and IL-33-expressing ‘natural helper’ cells, distinct from lymphoid progenitors in adipose tissue of the peritoneal cavity in response to IL-2, important in the protection against helminth infection [Waghray et al., 2005]. Similar observations were reported characterizing a distinct population of ‘nuocytes’ in the mesenteric lymph nodes expanded in vivo in response to IL-25 and IL-33, and were an early source of IL-13 during helminth infection with N. brasiliensis [Kim et al., 2012], and additional studies also identified these cells in the spleen and liver, and labeled them ‘innate helper type 2 cells’ [Muijsers et al., 2001]. Despite subtle differences reported among natural helper cells, nuocytes and innate helper type 2 cells [Bacsi et al., 2005], these cells are now categorized as group 2 innate lymphoid cells (ILC2s). Specific functions for ILC subsets have been identified, as ILC2 cells expressing GATA3 and IL-33R are implicated in allergen-induced asthma [Dobashi et al., 2001; Murata et al., 2003], while ILC3 cells are speculated to contribute to obesity-induced asthma [Koike et al., 2007]. ILC2s produce TH2 cytokines and are stimulated by IL-25 and IL-33 release [Kim et al., 2010] and recent studies in wild-type (WT) mice showed expansion of the ILC2 population in the lung and bronchoalveolar lavage fluid in a model of house dust mite (HDM)-induced allergic asthma, and were a major source of IL-5 and IL-13 [Boldogh et al., 2005]. Additionally, in vivo adoptive transfer experiments demonstrated that IL-13 produced by ILC2s was sufficient to mediate IL-33-induced airway inflammation [Muijsers et al., 2001; Pasqualini et al., 2011; Traidl-Hoffmann et al., 2002]. ILC2s may therefore be critical mediators of allergic airway inflammation in the lung.
In addition to deregulation of the immune response, there is evolving awareness of the active participation of structural elements, such as the airway epithelium, airway smooth muscle, and endothelium in asthma, especially as the disease progresses to a more severe and chronic phenotype [Holgate, 2010]. Hallmark pathological features of asthma, such as remodeling of the epithelium, are thought to induce the clinical symptoms of the disease: airway obstruction, coughing, dyspnea, and wheezing [Holgate, 2010]. Inhaled corticosteroids and combination therapies with long-acting β-agonists are the mainstay pharmacotherapies for controlling the symptoms of asthma. Although several more immunotherapies are currently in development as described above (IgE mAb, IL-5 mAb, and IL-4 mAb) [Umetsu et al., 2002], none of these treatments can cure established asthma. Therefore, further elucidation of the biochemical processes that culminate in the diverse phenotypes of human asthma, and the contribution of immune and structural cells herein are paramount toward the identification of new therapeutic targets.
II. Protein thiol redox homeostasis
Oxidants, such as hydrogen peroxide, are largely thought to be harmful, as excessive amounts can induce cellular damage and have been implicated in a variety of diseases. Contrasting this notion, other studies have demonstrated that oxidants play a much more complex and not always harmful role in the regulation of cellular processes. A key manifestation of this is the oxidation and modification of cysteines (Figure 1), which holds significant biological importance as many of these modifications can control protein function [Adachi et al., 2004; Barrett et al., 1999; Janssen-Heininger et al., 2008; Michalek et al., 2007; Nolin et al., 2014; Sakai et al., 2012]. While all cysteines contain a sulfhydryl moiety, not all are susceptible to oxidation. Reactive cysteines generally have a pKa value at or lower than neutral pH, compared to the standard cysteine pKa around 8–8.5. This decrease in pKa is due in large part to the microenvironment surrounding the cysteine, which allows the formation of a thiolate anion (-S−) that can be readily oxidized. Upon exposure to oxidants such as hydrogen peroxide, nitric oxide, peroxynitrite or other organic peroxides, the thiolate form of reactive cysteines can become oxidized to sulfenic acid (S-OH, sulfenylation), which is considered the “gateway oxidation” that can give rise to further oxidations [Finkel, 2011]. Nitric oxide gives rise to nitrosylated protein cysteines (S-NO, S-nitrosylation), a post-translational modification thought to significantly contribute to biological function of nitric oxide [Evangelista et al., 2013]. Further reversible modifications of sulfenic acid include thiolation, cysteinylation, and S-glutathionylation. S-glutathionylation (P-SSG) represents the conjugation of the tri-peptide, glutathione (GSH), with a reactive cysteine in a protein. Both S-NO and S-SG are believed to be important in protection against irreversible protein oxidation, including the formation of cysteine sulfinic acid (S-O2H) and sulfonic acid (S-O3H), the hyper-oxidized forms of cysteine which typically lead to degradation. The exact mechanisms that govern inter-conversations between the various oxidized states in intact cells and tissues remain unknown. In addition to protecting the target protein from further oxidation, S-glutathionylation can have profound effects on protein activity, and protein activation and inactivation and other functional changes have been reported, depending on the target protein. Conversely, prolonged exposure of sulfenic acid intermediates to oxidants can generate irreversiblly oxidized sulfinic and sulfonic acid forms of cysteine (Figure 1).
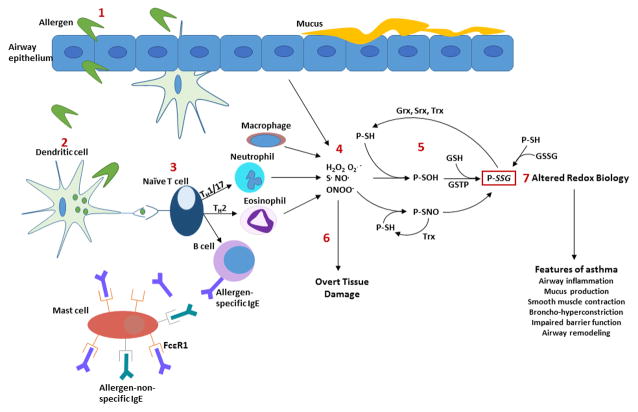
Figure 1.
Overview of allergen-initiated innate and adaptive immune responses and associated changes in protein thiol redox status. (1) Inhalation of allergens leads to disruption of epithelial cell homeostasis and impaired barrier function, allowing the allergen to interact with dendritic cells and other immune cells, including T cells. (2) Allergens are engulfed and processed by dendritic cells, which present antigenic epitopes to naïve T cells. (3) Naïve T cells polarize leading to recruitment of neutrophils and eosinophils (innate immune response) and activation of B cells, driving the adaptive immune response. (4) Reactive oxygen and nitrogen species are produced by inflammatory cells and structural cells including epithelial cells, resulting in oxidative modifications of reactive cysteines within proteins (P) (5). The downstream effects of oxidant overproduction have traditionally been associated with tissue damage (6). However, altered protein thiol redox biology (7) has emerged as a putative mechanism where oxidants contribute to the chronic features of asthma. This (patho) biological role of oxidants has the potential to be exploited towards the development of new and targeted therapeutics for patients with asthma. H2O2, hydrogen peroxide; O2·−, superoxide; S·, thiyl; NO· nitric oxide; ONOO− peroxynitrite; P-SH, protein thiol; P-SOH, protein sulfenic acid; P-SNO, S-nitrosylation; P-SSG, S-glutathionylation; Grx, glutaredoxin; Srx, sulfiredoxin; Trx, thioredoxin; GSTP, glutathione S-transferase pi; GSSG, glutathione disulfide.
The existence of enzymes involved in maintaining protein cysteine redox homeostasis provides further evidence for the biological importance of these modifications. Glutaredoxins (Grx), under normal physiological conditions, can specifically reverse S-glutathionylation of target proteins [Reynaert et al., 2006]. Thioredoxins (Trx), can reduce disulfide bonds as well as reverse S-nitrosylation of proteins [Benhar et al., 2008], and sulfiredoxins (Srx) can reverse S-glutathionylation of certain targets [Findlay et al., 2006; Park et al., 2009]. Other enzymes involved in cysteine chemistry include protein disulfide isomerases (PDI), which are largely responsible for disulfide bond formation, as well as glutathione S-transferases (GST). Of these, GSTP was recently described as a putative catalyst of S-glutathionylation [Townsend et al., 2009], although the specific targets of GSTP-catalyzed S-glutathionylation remain unknown.
III. Redox perturbations in chronic inflammation and allergic asthma: ROS sources
Despite the aforementioned importance of cysteine redox modifications, the source(s) of oxidants that regulate these events is difficult to determine. Numerous potential exogenous and endogenous sources of oxidants exist. Activation of NADPH oxidase in phagocytic cells, a quintessential factor in the host defense system, promotes intra-phagosomal ROS production to kill ingested pathogens. NADPH oxidase also contributes to activation of secreted proteolytic enzymes and receptor-mediated activation of pro-inflammatory signaling pathways [Terada, 2006]. While conflicting results exist, the role of active NADPH oxidase in allergic airway disease has been demonstrated by studies utilizing Gp91phox (subunit of flavocytochrome b558, the catalytic core of NADPH oxidase) deficient mice (Gp91phox−/−) in the Ovalbumin (OVA) model of allergic airway disease. It has been reported that Gp91 deficiency decreased ROS production, airway inflammation, and airway mucus production in Gp91phox−/− mice [Sevin et al., 2013]. In contrast, it was also demonstrated that mice lacking Gp91phox exhibited enhanced influx of inflammatory cells and TH2 cytokine levels [Banerjee and Henderson, 2012; Banerjee and Henderson, 2013]. Mechanistically, bone marrow-derived DCs from Gp91phox−/− animals were found to produce more IL-12 in response to LPS, suggesting enhanced TH1 differentiation. Moreover, splenocytes isolated from sensitized Gp91phox−/− mice produced less IL-13 in response to OVA challenge in vitro, indicating decreased TH2 differentiation. T cell differentiation appeared unaffected, however, as cytokine production by T cells was unaltered by the absence of Gp91phox under TH0, TH1, TH2 as well as TH17 polarizing conditions [Sevin et al., 2013].
In DCs, Gp91phox is recruited to early phagosomes, mediating sustained production of low levels of ROS which cause alkalinization of the phagosomal lumen. Although, under certain conditions, such as prolonged contact between DCs and pro-inflammatory ligands, substantial production of ROS may occur and affect DC responses. Interaction of DCs with TLR ligands can for instance enhance the level of ROS production by DCs [Vulcano et al., 2004]. Phagosomal alkalinization normally observed in DCs however, was lost in bone marrow derived DCs of Gp91phox−/− mice. This lower phagosomal pH resulted in enhanced degradation of proteins and decreased efficiency of antigen cross-presentation [Jancic et al., 2007; Savina et al., 2006]. These studies implicate Gp91phox in the processing of antigens rather than in killing pathogens in DCs.
Gp91phox is also present in non-hematopoietic cells and its role in these cells in allergic airway disease has been investigated using chimeric mice. These experiments showed that the absence Gp91phox in non-hematopoietic cells resulted in reduced AHR in response to OVA, and inhibition of eosinophil migration across the endothelium. The effects on AHR could be bypassed by intratracheal administration of eosinophils, suggesting that the induction of NADPH oxidase in endothelium by VCAM1 is necessary for eosinophil recruitment [Abdala-Valencia et al., 2007]. Virtually all cell types express a non-phagocytic isoform of NADPH oxidase (NOX). Superoxide or hydrogen peroxide production by these NOX enzymes is tightly controlled and localized, and is involved in many physiological processes, relaying signals via posttranslational modifications in target proteins, notably via cysteine oxidations, described above. Their role in allergic airway inflammation and asthma has recently been elegantly reviewed [Schroer et al., 2011; van der Vliet, 2011], and recent studies have also demonstrated their importance in the development of allergic airway disease. Duox1 is already known to be induced by the typical TH2 cytokines IL-4 and IL-13, and is associated with features of asthma such as mucin production. Interestingly, histamine, via the H1 receptor, has been shown to induce ROS production through Duox1 in primary human bronchial epithelial cells. Moreover, a synergistic effect with IL-4 inducing Duox1 gene and protein expression, and histamine further enhancing Duox activity has been observed [Rada et al., 2014]. Duox2 has also been shown to associate with TLR4 in bronchial and TLR2 in nasal epithelium upon HDM extract stimulation and shown to be necessary for the development of allergic airway disease and allergic rhinitis respectively [Ryu et al., 2013]. The relevance of Duox-mediated ROS production in the development of allergic airway disease in vivo was furthermore shown using DUOXA deficient mice in the OVA model. Mice deficient in both Duox1 and 2, displayed reduced mucus cell metaplasia, lower levels of TH2 cytokines and somewhat surprisingly lower numbers of neutrophils, but not eosinophils in bronchoalveolar lavage fluid (BALF) in conjunction with attenuated AHR to methacholine [Chang et al., 2013]. To date, no reports on Duox levels in patients with asthma have been published, but these enzymes likely contribute to asthma pathology as they can affect the aforementioned processes.
Another non-phagocytic NAPDH oxidase of interest in asthma is NOX4 [Sutcliffe et al., 2012], as intrinsic NOX4 mRNA and protein expression in primary airway smooth muscle cells isolated from asthma patients was increased relative to healthy controls. Importantly, the enhanced contractility displayed by asthmatic smooth muscle cells was attenuated following NOX4 knockdown and pharmacological inhibition [Sutcliffe et al., 2012]. Additionally, NOX4 is required for expression of differentiation markers in smooth muscle cells in vitro and mediates proliferation and hypertrophy induced by TGFβ [Sturrock et al., 2006]. NOX4 also has been implicated in other disorders of the lung, such as IPF [Hecker et al., 2009], and initiation of NOX4-induced ROS production at the surface of fibroblasts can enhance epithelial cell apoptosis [Waghray et al., 2005]. Together, these NOX-mediated cellular responses are thought to be important in the pathophysiology of asthma, and application of the general NOX inhibitor apocynin in the OVA model decreased AHR and inflammation and affected activation of redox sensitive transcription factors including activator protein-1 (AP1) and Nuclear Factor kappa B (NF-κB) in vitro [Kim et al., 2012]. Moreover, a prior study observed attenuation of AHR by apocynin treatment, but did not demonstrate effects on inflammation [Muijsers et al., 2001]. Importantly, a number of allergens have been shown to contain NADPH oxidase activity as well [Bacsi et al., 2005]. A variety of pollen grain extracts not only contained intrinsic NADPH oxidase activity, but this activity was crucial to the induction of ROS production in mouse lung epithelial cells, as well as markers of oxidative stress prior to inflammatory cell influx [Boldogh et al., 2005]. Furthermore, this initial induction of local oxidative stress by these extracts was important in augmenting antigen-induced allergic airway inflammation. It has also been shown that challenging mice with ragweed extract lacking NADPH oxidase activity resulted in diminished recruitment of eosinophils and mucus metaplasia, an effect that is not accompanied by altered production of TH2 cytokines [Bacsi et al., 2005].
Lastly, exacerbations of allergic airway disease are often associated with enhanced exposure to oxidants and oxidizing particulates and gases such as pollution and cigarette smoke. Air pollution is thought to contribute to the increase in incidence of allergy and allergic asthma in industrial countries. One reason for this increase could be the interaction of pollutants with known allergens thereby increasing their allergenicity, or with non-allergic substances causing them to become allergens. These possibilities were investigated in vitro by examining the effect of ozone fumigation of ragweed pollen. Ozone fumigation was found to cause damage to the pollen membrane system, which can lead to enhanced release of pollen-associated lipid immune-modulators [Traidl-Hoffmann et al., 2002], but it did not affect the content of allergens per se. Ozone fumigation did significantly increase NAD(P)H oxidase enzyme activity [Pasqualini et al., 2011] however, which could increase the pollen’s allergenicity [Boldogh et al., 2005].
IV. Cysteine redox perturbations in chronic inflammation and allergic asthma: Glutathione and its regulatory enzymes
Within the lung tissue, thiols in the form of glutathione (GSH) and sulfhydryl groups of proteins are among the most susceptible oxidant-sensitive targets and can be either reversibly oxidized to sulfenic acids (-SOH) or disulfides (S-S), or irreversibly oxidized to sulfinic (-SO2H) and sulfonic (SO3H) acids, as discussed earlier. Although reversible oxidations are believed to protect proteins from irreversible oxidation, the sulfenic acid moiety is very unstable and readily reacts with other thiols to form intra- or intermolecular disulfides, potentially altering protein function. Protein S-glutathionylation (PSSG), the reversible conjugation of a GSH molecule to a reactive cysteine via a disulfide bond, is a critical regulator of cellular function by controlling diverse signaling pathways and has been associated with inflammation. Accumulation of PSSG occurs in different cell types under a variety of oxidative conditions [Shelton and Mieyal, 2008]. Although total levels of glutathione in BALF, sputum and EBC have been reported to be increased in asthma, few studies examined levels of GSSG and these show conflicting data as reviewed by Reynaert, N.L. [Reynaert, 2011]. A recent study on a relatively large cohort of children predominantly with severe asthma found increased levels of GSSG in both macrophages and BALF, which were associated with decreased HDAC activity, increased apoptosis and impaired phagocytosis [Fitzpatrick et al., 2011a]. An associated study also found lower levels of reduced GSH and protein cysteines in the airways and plasma of children with severe asthma. These results were associated with dysfunctional Nrf2 activity, despite elevated Nrf2 mRNA and protein expression [Fitzpatrick et al., 2011b]. Regardless, evidence suggests that the state of GSH redox equilibrium in immune cells is crucial in determining the balance of TH1/TH2 immune responses and antigen presentation. For instance, lowering intracellular GSH levels was shown to negatively impact antigen processing in DCs, especially of antigens containing disulfide bonds [Short et al., 1996]. Nrf2 has also been shown to influence antigen presentation activity by controlling the redox equilibrium of DCs [Chan et al., 2006]. These data correlate with the reports described above on the role of Gp91phox in antigen processing in DCs. Further, higher intracellular levels of reduced GSH favored TH1 development through IL-12 production in antigen presenting cells [Murata et al., 2003]. Conversely, exposure of macrophages to the TH1 cytokine IFNγ increased the GSH/GSSG ratio, whereas exposure to the TH2 cytokine IL-4 decreased this ratio [Dobashi et al., 2001]. Administration of γ-glutamylcysteinylethyl ester, a GSH precursor, to monocytes in vitro as well as in the OVA model in vivo also increased IL-12 and suppressed production of TH2 cytokines IL-4 and IL-5 and eosinophil chemotaxis [Koike et al., 2007].
Glutathione peroxidase
At present, it is not clear if the expression of the enzymes involved in GSH synthesis is altered in asthmatics. However, enzymes that regulate GSH redox status and that use GSH have been studied in the context of asthma. Glutathione peroxidase 3 (GPx3) or extracellular GPx, the most abundant GPx isoform in the lung, was found to be increased in the BALF and bronchial epithelial cells of asthma patients compared to healthy controls [Comhair et al., 2001]. GPx1, the most abundant intracellular GPx isoform, was increased in the OVA model of allergic asthma, and furthermore, GPx1-deficient mice exhibited attenuated eosinophilia, goblet cell hyperplasia, collagen deposition and AHR. Moreover, in vitro studies demonstrated that CD4+ T helper cells derived from GPx1-deficient mice exhibited enhanced proliferation and produced more ROS and IL-2 after stimulation compared to WT cells. Gpx1−/− cells were also predominantly TH1 and TH17-biased. Together, these data indicate that the effects of GPx1 on immune cell proliferation and differentiation may predominate over its effects on protection against oxidative damage [Won et al., 2010]. Expression of GPx2 was shown to be elevated in the airway epithelium in the OVA model of allergic airway disease, but unlike GPx1-deficient mice, GPx2-deficient mice showed enhanced airway inflammation and reactivity in this model [Dittrich et al., 2010]. Discrete localization and functions of different GPx isoforms could explain both protective versus enhancing roles in allergic airway disease. With respect to the latter, indications exist that GPx2 might function less as a GSH peroxidase and could be more involved in inhibiting prostaglandin synthesis and enhance other anti-inflammatory mechanisms [Dittrich et al., 2010].
Glutathione-S-transferase
Members of the glutathione-S-transferase (GST) superfamily have been under investigation in asthma since they are critical in the protection of cells against toxic products of ROS-mediated reactions. GSTP1 is the predominant cytosolic GST in the human lung epithelium [Fryer et al., 1986], and is located on chromosome 11q13, a locus linked to asthma susceptibility [Doull et al., 1996; Kano et al., 1987]. Furthermore, specific GSTP1 polymorphisms are strongly associated with asthma [Spiteri et al., 2000]. Interestingly, challenge of ragweed-sensitive patients with allergen alone or in combination with diesel exhaust particles induced IgE and histamine production in patients expressing Ile105, a variant of GSTP1 [Gilliland et al., 2004]. GSTP expression was found to be decreased in nasal epithelial cells derived from children with asthma compared to non-asthmatic children [Schroer et al., 2011]. Further, GSTP1 is functionally involved in specific pathophysiological processes that underlie clinical features of asthma. Eosinophilia, goblet cell hyperplasia, IL-5 levels, airway remodeling and lung resistance were enhanced in GSTP-deficient mice compared to WT controls in the OVA model. As in humans, strain-dependent effects were observed in mice that correlated with endogenous levels of GSTP expression [Zhou et al., 2008]. GSTP-null mice also displayed increased cysteine disulfide levels in response to HDM relative to WT animals [Schroer et al., 2011].
Glutaredoxin
Glutaredoxin (Grx) is a specific and well-characterized catalyst of protein deglutathionylation [Shelton and Mieyal, 2008]. A member of the thiol-disulfide oxidoreductase family, Grx promotes reversible reduction of protein-SSG to free sulfhydryl groups (Figure 1). In addition, Grx is unique in that it regenerates itself through the oxidation of GSH, notably at high concentrations of GSH [Reynaert et al., 2007]. In this reaction, Grx itself is S-glutathionylated at Cys-22, and the reduced state of Grx is restored by the coupling GSH to a second GSH molecule, forming a glutathione disulfide (GSSG) [Reynaert et al., 2006]. So far, Grx and PSSG have only been reported in one study in patients with asthma. Our group has recently shown that Grx1 levels are increased, and PSSG levels are decreased, in the sputum from asthmatics. These alterations in Grx1 seemed largely independent of the inflammatory cells present in the sputum, but expression of Grx1 was increased in bronchial epithelial cells isolated from asthma patients [Kuipers et al., 2013]. PSSG levels on the other hand were only decreased in asthmatics with a dominant eosinophilic or neutrophilic sputum cellular profile, and positively correlated with the percentage of lymphocytes in the sputum. Sputum PSSG levels in asthma are therefore likely influenced not only by Grx1 levels, but also by other factors such as oxidative changes related to inflammation. Whether decreases in sputum PSSG are associated with enhanced protein deglutathionylation and whether this increases the vulnerability of these proteins to oxidative damage has yet to be determined. Importantly, decreased lung function was associated with higher sputum Grx1 levels and lower PSSG levels. Additionally, PSSG levels correlated with asthmatic controls in neutrophilic patients [Kuipers et al., 2013]. These data correspond with the increases in Grx1 expression and activity predominantly in bronchial epithelium in the OVA model of allergic airway disease [Reynaert et al., 2006]. A follow-up study also demonstrated that additional OVA challenges lead to further increases in Grx1 expression within the whole lung. No clear effects on lung PSSG were observed in WT mice in response to OVA, but marked increases in PSSG content was detected 48h after repeated antigen challenge in the lungs of Grx1-deficient mice. To investigate the impact of Grx1/PSSG alterations on the development of allergic airway disease, Grx1-deficient mice (Glrx1−/−) were sensitized and challenged with OVA. No effect of Grx1 ablation on OVA-specific IgE levels were observed, but inflammatory cell influx and expression of proinflammatory mediators were decreased in Glrx1−/− mice. WT and Glrx1−/− mice demonstrated comparable increases in AHR, however, Glrx1−/− mice showed enhanced resolution of AHR 7 days post challenge cessation. This was accompanied by marked decreases in mucus metaplasia and IL-13 expression, in the absence of effects on inflammation. These results demonstrate that the Grx1/S-glutathionylation redox status in mice is a critical regulator of AHR and its resolution, and suggest that avenues to increase S-glutathionylation of specific targets may attenuate AHR [Hoffman et al., 2012].
Thioredoxin
Thioredoxin (Trx), represents another class of thiol-oxidoreductases important in cellular redox homeostasis, for which numerous target proteins have been identified, and has emerged as a critical regulator of biological processes [Lee et al., 2013]. Serum levels of Trx were significantly increased in patients during asthmatic exacerbation, and serum Trx levels correlated with lung function and eosinophil cationic protein levels during these times. Levels of serum Trx may therefore correspond to the extent of airway obstruction and eosinophil activation in the lung, and could represent a possible protective response [Ito et al., 2011].
This latter possibility alludes to several animal studies that show a beneficial effect of Trx in animal models of asthma. Reduced eosinophilia, mucus metaplasia and AHR were observed in Trx-overexpressing mice, for example, while effects on TH2 responses were not [Torii et al., 2010]. Furthermore, studies have shown that exogenously administered recombinant Trx suppressed allergic inflammation in animal models of asthma. Intraperitoneal administration of recombinant human (rh) Trx1 during antigen challenge significantly inhibited airway remodeling, lung eosinophilia and AHR, and resulted in decreased eotaxin, macrophage inflammatory protein-1alpha and IL-13 expression in the lung. Importantly, administration of rhTrx1 significantly ameliorated existing goblet cell hyperplasia in OVA-sensitized and -challenged WT mice [Imaoka et al., 2009]. Administration of WT Trx also suppressed AHR and increased mRNA expression of several TH1 cytokines including including IL-1β and IL-18 in the lungs of OVA-sensitized mice. However, the 32S/35S mutant Trx, the catalytically-inactive form of this enzyme, did not affect these endpoints, confirming that the effects of Trx are dependent on its enzymatic activity. Trx may also regulate the TH1 response by increasing expression of IFNγ and IL-12 in monocytes and T cells in vitro, but these effects were not observed in the Trx transgenic mice described above [Torii et al., 2010]. The effects of Trx on AHR and airway inflammation are subject to some controversy however [Ichiki et al., 2005], and differences in genetic background, immunization protocols, as well as the potential divergent functions of intracellular vs extracellular and endogenous vs exogenous Trx could be responsible for these discrepancies. Repression of eosinophilia could be attributed to multiple mechanisms of action of Trx. Interestingly, preincubation with Trx was found to directly suppress eotaxin and RANTES-induced chemotaxis of eosinophils. Trx did not affect expression of the receptor of these two chemokines, CCR3, but attenuated the downstream activation of ERK 1/2 and p38 MAPK [Kobayashi et al., 2009].
The anti-inflammatory effects of Trx are generally thought to be dependent on its catalytic function. Importantly however, Trx also directly attenuates eosinophil and neutrophil chemotaxis, and may therefore have clinical benefits. Along these lines, it is important to note that the half-life of Trx in lung tissue is 6 times longer than in sera (51.3 hrs vs 8.5 hrs) [Hoshino et al., 2003].
V. Potential for therapeutic intervention and targeting of oxidative events and cysteine oxidations in asthma
Asthma represents a major health concern worldwide and while current mainstay therapies, namely β-agonists and glucocorticoids reduce airway inflammation, reverse bronchoconstriction and improve quality of life, there remains a significant patient population for whom these treatments are ineffective [Umetsu et al., 2002]. Additionally, these treatments have little or no effect on structural alterations of the disease, and do not reverse or prevent disease progression. Current popular belief holds that oxidants are “bad,” contributing to cellular damage and tissue pathology. The possibility that oxidants can exert regulatory biological functions, and even constitute anti-inflammatory mechanisms, however, is not implausible and has great clinical relevance. Given that our understanding of means to inhibit inflammation in allergic asthma is incomplete to date, ongoing and future studies are significant in that they attempt to identify the contribution of specific ROS-producing enzymes and resultant modification of protein cysteine residues. We envision the use of compounds to specifically target S-glutathionylation in the context of allergic asthma. Of direct translational relevance NOV-002, a dimeric version of oxidized glutathione (GSSG), which may enhance PSSG by altering the GSH:GSSG ratio [Fitzpatrick et al., 2011a; Fitzpatrick et al., 2011b]. Such studies have the potential to unravel beneficial redox reactions catalyzed by oxidants that are targeted with the goal to dampen the extent of and enhance resolution of allergic inflammation.
Acknowledgments
This work was supported by the National Heart, Lung and Blood institute (R01 HL085464 and HL060014, to Y J-H)
References
- Abdala-Valencia H, Earwood J, Bansal S, Jansen M, Babcock G, Garvy B, Wills-Karp M, Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1111–25. doi: 10.1152/ajplung.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–62. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- Bacsi A, Dharajiya N, Choudhury BK, Sur S, Boldogh I. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. J Allergy Clin Immunol. 2005;116:836–43. doi: 10.1016/j.jaci.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee ER, Henderson WR., Jr Defining the molecular role of gp91phox in the immune manifestation of acute allergic asthma using a preclinical murine model. Clin Mol Allergy. 2012;10:2. doi: 10.1186/1476-7961-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee ER, Henderson WR., Jr Role of T cells in a gp91phox knockout murine model of acute allergic asthma. Allergy Asthma Clin Immunol. 2013;9:6. doi: 10.1186/1710-1492-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–4. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–79. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Wang M, Li N, Yanagawa Y, Onoe K, Lee JJ, Nel AE. Pro-oxidative diesel exhaust particle chemicals inhibit LPS-induced dendritic cell responses involved in T-helper differentiation. J Allergy Clin Immunol. 2006;118:455–65. doi: 10.1016/j.jaci.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Chang S, Linderholm A, Franzi L, Kenyon N, Grasberger H, Harper R. Dual oxidase regulates neutrophil recruitment in allergic airways. Free Radic Biol Med. 2013;65:38–46. doi: 10.1016/j.freeradbiomed.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comhair SA, Bhathena PR, Farver C, Thunnissen FB, Erzurum SC. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. FASEB J. 2001;15:70–78. doi: 10.1096/fj.00-0085com. [DOI] [PubMed] [Google Scholar]
- Dittrich AM, Meyer HA, Krokowski M, Quarcoo D, Ahrens B, Kube SM, Witzenrath M, Esworthy RS, Chu FF, Hamelmann E. Glutathione peroxidase-2 protects from allergen-induced airway inflammation in mice. Eur Respir J. 2010;35:1148–54. doi: 10.1183/09031936.00026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobashi K, Aihara M, Araki T, Shimizu Y, Utsugi M, Iizuka K, Murata Y, Hamuro J, Nakazawa T, Mori M. Regulation of LPS induced IL-12 production by IFN-gamma and IL-4 through intracellular glutathione status in human alveolar macrophages. Clin Exp Immunol. 2001;124:290–6. doi: 10.1046/j.1365-2249.2001.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doull IJ, Lawrence S, Watson M, Begishvili T, Beasley RW, Lampe F, Holgate T, Morton NE. Allelic association of gene markers on chromosomes 5q and 11q with atopy and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 1996;153:1280–4. doi: 10.1164/ajrccm.153.4.8616554. [DOI] [PubMed] [Google Scholar]
- Evangelista AM, Kohr MJ, Murphy E. S-nitrosylation: specificity, occupancy, and interaction with other post-translational modifications. Antioxid Redox Signal. 2013;19:1209–19. doi: 10.1089/ars.2012.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay VJ, Townsend DM, Morris TE, Fraser JP, He L, Tew KD. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res. 2006;66:6800–6. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AM, Teague WG, Burwell L, Brown MS, Brown LA Program NNSAR. Glutathione oxidation is associated with airway macrophage functional impairment in children with severe asthma. Pediatr Res. 2011a;69:154–9. doi: 10.1203/PDR.0b013e3182026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Aujla S, Castro M, Bacharier LB, Gaston BM, Bleecker ER, Moore WC National Institutes of Health/National Heart, L, Blood Institute Severe Asthma Research P. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011b;127:382–389. e1–13. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer AA, Hume R, Strange RC. The development of glutathione S-transferase and glutathione peroxidase activities in human lung. Biochim Biophys Acta. 1986;883:448–53. doi: 10.1016/0304-4165(86)90283-7. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet. 2004;363:119–25. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model. Allergy. 1999;54:297–305. doi: 10.1034/j.1398-9995.1999.00085.x. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- Hansbro PM, Kaiko GE, Foster PS. Cytokine/anti-cytokine therapy - novel treatments for asthma? Br J Pharmacol. 2011;163:81–95. doi: 10.1111/j.1476-5381.2011.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–81. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman SM, Tully JE, Lahue KG, Anathy V, Nolin JD, Guala AS, van der Velden JL, Ho YS, Aliyeva M, Daphtary N, Lundblad LK, Irvin CG, Janssen-Heininger YM. Genetic ablation of glutaredoxin-1 causes enhanced resolution of airways hyperresponsiveness and mucus metaplasia in mice with allergic airways disease. Am J Physiol Lung Cell Mol Physiol. 2012;303:L528–38. doi: 10.1152/ajplung.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate ST. A brief history of asthma and its mechanisms to modern concepts of disease pathogenesis. Allergy Asthma Immunol Res. 2010;2:165–71. doi: 10.4168/aair.2010.2.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Nakamura H, Okamoto M, Kato S, Araya S, Nomiyama K, Oizumi K, Young HA, Aizawa H, Yodoi J. Redox-active protein thioredoxin prevents proinflammatory cytokine- or bleomycin-induced lung injury. Am J Respir Crit Care Med. 2003;168:1075–83. doi: 10.1164/rccm.200209-982OC. [DOI] [PubMed] [Google Scholar]
- Ichiki H, Hoshino T, Kinoshita T, Imaoka H, Kato S, Inoue H, Nakamura H, Yodoi J, Young HA, Aizawa H. Thioredoxin suppresses airway hyperresponsiveness and airway inflammation in asthma. Biochem Biophys Res Commun. 2005;334:1141–8. doi: 10.1016/j.bbrc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Imaoka H, Hoshino T, Okamoto M, Sakazaki Y, Sawada M, Takei S, Kinoshita T, Kawayama T, Kato S, Aizawa H. Endogenous and exogenous thioredoxin 1 prevents goblet cell hyperplasia in a chronic antigen exposure asthma model. Allergol Int. 2009;58:403–10. doi: 10.2332/allergolint.09-OA-0086. [DOI] [PubMed] [Google Scholar]
- Ito W, Kobayashi N, Takeda M, Ueki S, Kayaba H, Nakamura H, Yodoi J, Chihara J. Thioredoxin in allergic inflammation. Int Arch Allergy Immunol. 2011;155(Suppl 1):142–6. doi: 10.1159/000327501. [DOI] [PubMed] [Google Scholar]
- Jancic C, Savina A, Wasmeier C, Tolmachova T, El-Benna J, Dang PM, Pascolo S, Gougerot-Pocidalo MA, Raposo G, Seabra MC, Amigorena S. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9:367–78. doi: 10.1038/ncb1552. [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano T, Sakai M, Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987;47:5626–30. [PubMed] [Google Scholar]
- Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11:577–84. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, Cho YS, Chun E, Lee KY. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol. 2012;90:441–8. doi: 10.1038/icb.2011.60. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Yamada Y, Ito W, Ueki S, Kayaba H, Nakamura H, Yodoi J, Chihara J. Thioredoxin reduces C-C chemokine-induced chemotaxis of human eosinophils. Allergy. 2009;64:1130–5. doi: 10.1111/j.1398-9995.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- Koike Y, Hisada T, Utsugi M, Ishizuka T, Shimizu Y, Ono A, Murata Y, Hamuro J, Mori M, Dobashi K. Glutathione redox regulates airway hyperresponsiveness and airway inflammation in mice. Am J Respir Cell Mol Biol. 2007;37:322–9. doi: 10.1165/rcmb.2006-0423OC. [DOI] [PubMed] [Google Scholar]
- Kuipers I, Louis R, Manise M, Dentener MA, Irvin CG, Janssen-Heininger YM, Brightling CE, Wouters EF, Reynaert NL. Increased glutaredoxin-1 and decreased protein S-glutathionylation in sputum of asthmatics. Eur Respir J. 2013;41:469–72. doi: 10.1183/09031936.00115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim SM, Lee RT. Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxid Redox Signal. 2013;18:1165–207. doi: 10.1089/ars.2011.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. Drug companies hope to breathe life into asthma pipeline. Nat Med. 2011;17:642–3. doi: 10.1038/nm0611-642b. [DOI] [PubMed] [Google Scholar]
- McKeage K. Omalizumab: a review of its use in patients with severe persistent allergic asthma. Drugs. 2013;73:1197–212. doi: 10.1007/s40265-013-0085-4. [DOI] [PubMed] [Google Scholar]
- Michalek RD, Nelson KJ, Holbrook BC, Yi JS, Stridiron D, Daniel LW, Fetrow JS, King SB, Poole LB, Grayson JM. The requirement of reversible cysteine sulfenic acid formation for T cell activation and function. J Immunol. 2007;179:6456–67. doi: 10.4049/jimmunol.179.10.6456. [DOI] [PubMed] [Google Scholar]
- Muijsers RB, van Ark I, Folkerts G, Koster AS, van Oosterhout AJ, Postma DS, Nijkamp FP. Apocynin and 1400 W prevents airway hyperresponsiveness during allergic reactions in mice. Br J Pharmacol. 2001;134:434–40. doi: 10.1038/sj.bjp.0704235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Amao M, Hamuro J. Sequential conversion of the redox status of macrophages dictates the pathological progression of autoimmune diabetes. Eur J Immunol. 2003;33:1001–11. doi: 10.1002/eji.200323575. [DOI] [PubMed] [Google Scholar]
- Narayanankutty A, Resendiz-Hernandez JM, Falfan-Valencia R, Teran LM. Biochemical pathogenesis of aspirin exacerbated respiratory disease (AERD) Clin Biochem. 2013;46:566–78. doi: 10.1016/j.clinbiochem.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Nolin JD, Tully JE, Hoffman SM, Guala AS, van der Velden JL, Poynter ME, van der Vliet A, Anathy V, Janssen-Heininger YM. The glutaredoxin/S-glutathionylation axis regulates interleukin-17A-induced proinflammatory responses in lung epithelial cells in association with S-glutathionylation of nuclear factor kappaB family proteins. Free Radic Biol Med. 2014;73:143–53. doi: 10.1016/j.freeradbiomed.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, Chanez P the MI. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- Park JW, Mieyal JJ, Rhee SG, Chock PB. Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin. J Biol Chem. 2009;284:23364–74. doi: 10.1074/jbc.M109.021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini S, Tedeschini E, Frenguelli G, Wopfner N, Ferreira F, D’Amato G, Ederli L. Ozone affects pollen viability and NAD(P)H oxidase release from Ambrosia artemisiifolia pollen. Environ Pollut. 2011;159:2823–30. doi: 10.1016/j.envpol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- Rada B, Boudreau HE, Park JJ, Leto TL. Histamine stimulates hydrogen peroxide production by bronchial epithelial cells via histamine H1 receptor and dual oxidase. Am J Respir Cell Mol Biol. 2014;50:125–34. doi: 10.1165/rcmb.2013-0254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaert NL. Glutathione biochemistry in asthma. Biochim Biophys Acta. 2011;1810:1045–51. doi: 10.1016/j.bbagen.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A. 2006;103:13086–91. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaert NL, Wouters EF, Janssen-Heininger YM. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am J Respir Cell Mol Biol. 2007;36:147–51. doi: 10.1165/rcmb.2006-0259RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, Ryu JC, Choi MK, Joo JH, Kim CH, Lee SN, Lee WJ, Kim J, Shin DM, Kweon MN, Bae YS, Yoon JH. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol. 2013;131:549–61. doi: 10.1016/j.jaci.2012.07.050. [DOI] [PubMed] [Google Scholar]
- Sakai J, Li J, Subramanian KK, Mondal S, Bajrami B, Hattori H, Jia Y, Dickinson BC, Zhong J, Ye K, Chang CJ, Ho YS, Zhou J, Luo HR. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity. 2012;37:1037–49. doi: 10.1016/j.immuni.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–18. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Schroer KT, Gibson AM, Sivaprasad U, Bass SA, Ericksen MB, Wills-Karp M, Lecras T, Fitzpatrick AM, Brown LA, Stringer KF, Hershey GK. Downregulation of glutathione S-transferase pi in asthma contributes to enhanced oxidative stress. J Allergy Clin Immunol. 2011;128:539–48. doi: 10.1016/j.jaci.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevin CM, Newcomb DC, Toki S, Han W, Sherrill TP, Boswell MG, Zhu Z, Collins RD, Boyd KL, Goleniewska K, Huckabee MM, Blackwell TS, Peebles RS., Jr Deficiency of gp91phox inhibits allergic airway inflammation. Am J Respir Cell Mol Biol. 2013;49:396–402. doi: 10.1165/rcmb.2012-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25:332–46. [PMC free article] [PubMed] [Google Scholar]
- Short S, Merkel BJ, Caffrey R, McCoy KL. Defective antigen processing correlates with a low level of intracellular glutathione. Eur J Immunol. 1996;26:3015–20. doi: 10.1002/eji.1830261229. [DOI] [PubMed] [Google Scholar]
- Souwer Y, Szegedi K, Kapsenberg ML, de Jong EC. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol. 2010;22:821–6. doi: 10.1016/j.coi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Spiteri MA, Bianco A, Strange RC, Fryer AA. Polymorphisms at the glutathione S-transferase, GSTP1 locus: a novel mechanism for susceptibility and development of atopic airway inflammation. Allergy. 2000;55(Suppl 61):15–20. doi: 10.1034/j.1398-9995.2000.00502.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- Sutcliffe A, Hollins F, Gomez E, Saunders R, Doe C, Cooke M, Challiss RA, Brightling CE. Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am J Respir Crit Care Med. 2012;185:267–74. doi: 10.1164/rccm.201107-1281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol. 2006;174:615–23. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Wang L, Ma N, Saito K, Hori T, Sato-Ueshima M, Koyama Y, Nishikawa H, Katayama N, Mizoguchi A, Shiku H, Yodoi J, Kuribayashi K, Kato T. Thioredoxin suppresses airway inflammation independently of systemic Th1/Th2 immune modulation. Eur J Immunol. 2010;40:787–96. doi: 10.1002/eji.200939724. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284:436–45. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traidl-Hoffmann C, Kasche A, Jakob T, Huger M, Plotz S, Feussner I, Ring J, Behrendt H. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J Allergy Clin Immunol. 2002;109:831–8. doi: 10.1067/mai.2002.124655. [DOI] [PubMed] [Google Scholar]
- Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–20. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- van der Vliet A. Nox enzymes in allergic airway inflammation. Biochim Biophys Acta. 2011;1810:1035–44. doi: 10.1016/j.bbagen.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulcano M, Dusi S, Lissandrini D, Badolato R, Mazzi P, Riboldi E, Borroni E, Calleri A, Donini M, Plebani A, Notarangelo L, Musso T, Sozzani S. Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J Immunol. 2004;173:5749–56. doi: 10.4049/jimmunol.173.9.5749. [DOI] [PubMed] [Google Scholar]
- Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–6. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. Am J Physiol Lung Cell Mol Physiol. 2003;285:L32–42. doi: 10.1152/ajplung.00390.2002. [DOI] [PubMed] [Google Scholar]
- Won HY, Sohn JH, Min HJ, Lee K, Woo HA, Ho YS, Park JW, Rhee SG, Hwang ES. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid Redox Signal. 2010;13:575–87. doi: 10.1089/ars.2009.2989. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wolf CR, Henderson CJ, Cai Y, Board PG, Foster PS, Webb DC. Glutathione transferase P1: an endogenous inhibitor of allergic responses in a mouse model of asthma. Am J Respir Crit Care Med. 2008;178:1202–10. doi: 10.1164/rccm.200801-178OC. [DOI] [PubMed] [Google Scholar]