Abstract
Background
Mucous hypersecretion increases asthma morbidity and mortality. Tumor necrosis factor α (TNF-α) levels are elevated in bronchoalveolar fluid, sputum, and monocyte membranes in some patients with asthma. Anti–TNF-α decreased asthma exacerbations and improved forced expiratory volume in 1 second in these patients. Whether anti–TNF-α reduces mucous cell metaplasia or hyperplasia has not been evaluated.
Objective
To investigate the role of anti–TNF-α in mucous hypersecretion.
Methods
BALB/c mice sensitized intraperitoneally and challenged intratracheally with ovalbumin were treated with 250 μg of anti–TNF-α before ovalbumin sensitization and challenge or before only ovalbumin challenge. Control groups were sham treated. The tumor necrosis factor receptor (TNFR) mice (TNFR−/−and TNFR+/+) were identically sensitized and challenged. Seventy-two hours after the final challenge, the airway pressure time index (APTI), which measures airway hyperresponsiveness, was recorded. Mucous cell metaplasia was accessed by quantitative polymerase chain reaction for MUC-5AC (the epithelial cell mucous–inducing gene) and the percentage of periodic acid–Schiff (PAS) staining of bronchial epithelial cells. A human airway cell line (constitutively expressing MUC-5AC) was pretreated with a NF-κB inhibitor before TNF-α culture.
Results
The mean (SE) fold change of MUC-5AC expression (compared with naive controls), the percentage of PAS-positive bronchiole epithelial cells, and the APTI decreased in BALB/c mice treated with anti–TNF-α before sensitization and challenge (4.9 [1.14], P = .007; 28.9% [6.8%], P < .001; and 545.8 [104.5] cm H2O/s, P < .001, respectively) and before challenge alone (9.3 [1.8], P = .03; 43.6% [10.7%], P = .009; and 896.8 [81.23] cm H2O/s, P = .06, respectively) compared with sham-treated mice (20.9 [3.9], 82.4% [1.8%], and 1,055 [30.6] cm H2O/s, respectively). MUC-5AC expression decreased in ovalbumin sensitized or challenged TNFR−/− (2.41 [0.4]) compared with ovalbumin sensitized or challenged TNFR+/+ mice (18.4 [2.5], P < .001). TNF-α–induced MUC-5AC expression in human airway culture significantly decreased with pretreatment of a NF-κB inhibitor.
Conclusions
Anti–TNF-α treatment reduces airway mucous cell metaplasia in a mouse model of asthma, which may in part underlie its beneficial effect as asthma therapy.
INTRODUCTION
Mucous hypersecretion is linked with asthma fatality.1 Furthermore, a decreasing forced expiratory volume in 1 second (FEV1) is independently associated with a history of sputum production, suggesting that increased mucous production increases asthma severity.2 Mucin glycoproteins, the primary constituents of mucus, are produced by goblet cells and submucosal glands. MUC-5AC is the predominant airway mucin gene. Lung tissue from asthma animal models and asthmatic patients have increased MUC-5AC expression.3,4 TH2 cytokines interleukin (IL) 4, IL-5, IL-9, IL-13, and IL-17 induce mucous gene expression and secretion in vitro and in vivo.5–8 Tumor necrosis factor α (TNF-α) and IL-1β induce mucin gene expression in vitro.9–11 We demonstrated that TNF-α significantly increased mucous cell metaplasia in naive mice.12
TNF-α is important in severe asthma.13,14 Besides inducing mucous cell metaplasia, TNF-α increases airway contraction15 and induces airway hyperresponsiveness,16,17 which may occur secondary to recruiting and activating eosinophils and neutrophils to the airways18,19 and increasing cytokine release by mast and T cells.20,21 Anti–TNF-α appears to have the greatest effect in patients with severe asthma13,14 and those with specific alleles of TNF receptor (TNFR) genes.22 However, patients with moderate asthma given infliximab experienced decreased exacerbations, asthma symptom scores, use of rescue short-acting β2-agonist, and mean diurnal variation of peak expiratory flow compared with placebo treatment but not a change in FEV1.23 This study evaluated whether anti–TNF-α antibody reduces mucous cell metaplasia in a murine model of allergic asthma.
METHODS
Mice and Reagents
Six-week-old female BALB/c, TNFR knockout mice (TNFR−/−) (p55 and p75 deficient, derived from a mixed 129S and B57BL/6 background, backcrossed onto C57BL/6) and B6129/J (TNFR+/+, control) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Neutralizing hamster antimouse monoclonal anti–TNF-α antibody (endotoxin level, <0.001 ng/μg; azide free) was purchased from eBio-science (San Diego, California) and hamster IgG isotype antibody from Jackson ImmunoResearch Laboratories Inc (West Grove, Pennsylvania).
Antigen Sensitization and Challenge
BALB/c, TNFR−/−, and TNFR+/+ mice were sensitized intraperitoneally with 200 μg of alum-absorbed ovalbumin (grade V; Sigma Aldrich, St. Louis, Missouri) (days 0 and 8). Mice were challenged intratracheally 3 times (days 18, 25, and 32) with 100 μg of ovalbumin (Fig 1). Strain-matched naive mice were controls (n = 5 per group). We did not administer phosphate-buffered saline intratracheally to control mice because bronchoalveolar lavage fluid (BALF) cell numbers and differential and airway hyperresponsiveness (AHR) are not significantly different when compared with naive mice. Furthermore, allergen sensitized and phosphate-buffered saline challenged mice do not develop airway inflammation or AHR significantly different from naive mice (P.J.B. and X.-M.L., unpublished data, April 2005). Mice were killed 72 hours after final ovalbumin challenge.
Figure 1.

Experimental protocol. BALB/c mice were sensitized intraperitoneally (i.p.) with ovalbumin (200 μg per mouse) twice at weekly intervals and then challenged intratracheally (i.t.) on days 18, 25, and 32 with ovalbumin (100 μg per mouse) (ovalbumin mice). One group of mice received i.p. anti–tumor necrosis factor α (TNF-α) (250 μg per mouse) 4 hours before each ovalbumin sensitization and challenge (n = 5) (protocol 1), and another group received anti–TNF-α (250 μg per mouse) only before ovalbumin challenge (n = 5) (protocol 2). Sham-treated ovalbumin mice received isotype control IgG i.p. (250 μg per mouse) 4 hours before ovalbumin sensitization and challenge (n = 7) (protocol 1). Naive BALB/c mice were controls (n = 5). Analysis was performed 72 hours after the final ovalbumin challenge (day 35) to determine airway hyperresponsiveness (AHR), bronchoalveolar lavage fluid (BALF) cell number and differential, messenger RNA cytokine, and MUC-5AC expression from lung tissue and histologic analysis.
Anti–TNF-α Antibody Treatment
One group of BALB/c mice was intraperitoneally injected with anti–TNF-α (250 μg per mouse) 4 hours before each ovalbumin sensitization and ovalbumin challenge (n = 5). Another group of ovalbumin sensitized BALB/c mice only received intraperitoneal anti–TNF-α (250 μg per mouse) 4 hours before ovalbumin challenge (n = 5). Sham-treated BALB/c ovalbumin mice (n = 7) received isotype control IgG intraperitoneally (250 μg per mouse) 4 hours before each ovalbumin sensitization and challenge (Fig 1). Dose and timing of anti–TNF-α were based on murine (BALB/c) colitis models using commercially available anticytokine antibody.24,25 (Doses of anti–TNF-α in BALB/c models of allergic asthma range from 10 μg to 2 mg.26,27)
Airway Pressure Recording
Seventy-two hours after the final ovalbumin challenge, AHR to acetylcholine was measured in BALB/c mice.28 Mice were given ventilatory assistance and decamethonium bromide (25 mg/kg) was administered into the inferior vena cava to eliminate muscle movements. Airway pressure was measured with a transducer via a port in the tracheal cannula. A 2-minute baseline was recorded, acetylcholine (50 μg/kg) injected into the inferior vena cava, and the recording continued for 4 minutes. AHR was defined as the airway pressure time index (APTI). Because C57BL/6 mice develop airway inflammation but not AHR after antigen sensitization and challenge,29 we did not measure APTI in this strain.
BALF Preparation and Cell Differential Counts
After APTI, BALF was collected and processed.30 Total cell numbers were determined using a hemocytometer and cell differential counts by counting at least 500 cells per slide by light microscopy.
Lung Histologic Findings
Seventy-two hours after the final ovalbumin challenge, the right lower lobe was fixed in neutral buffered formaldehyde and 5-μm paraffin sections stained with PAS for evaluation of goblet cells. Mucous expression was calculated by determining the percentage of PAS-positive epithelial cells (number of PAS-positive cells divided by the total epithelial cell number) in at least 6 randomly selected bronchioles per animal.31 Results are expressed as the mean percentage of PAS-positive cells per bronchiole and were conducted by a blinded reviewer.
Cell Culture
The human mucoepidermoid cell line, NCI-H292 (American Tissue Culture Collection, Manassas, Virginia), which constituently expresses low-level MUC-5AC messenger RNA (mRNA), was plated at 5 to 6 × 105 cells in RPMI-1640 supplemented with 10% fetal bovine serum, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 25-mmol/L HEPES at 37°C in a humidified chamber with 5% carbon dioxide. Two days after cells reached confluence, they were serum deprived for 24 hours. Cells were cultured with 50 ng of recombinant human TNF-α (rhTNF-α) for 12, 24, 48, and 72 hours in serum-supplemented media to determine the optimal time for increased MUC-5AC mRNA expression. Additional samples were pretreated with BAY-11–7082 (Sigma), a IκB-a phosphorylation inhibitor, at 100 and 10μM for 2 hours before culture with rhTNF-α for 24 hours (determined to be the optimal time). Control samples were cultured in media alone or with 50 ng of rhTNF-α and did not receive pretreatment with BAY-11–7082. Cell culture experiments included at least 5 samples per group and were repeated.
RNA Extraction
Total RNA was isolated from NCI-292 cells and the right upper and middle lung tissue using TRIZOL (Invitrogen, Carlsbad, California) according to the manufacturer’s directions.
Semiquantitation of Polymerase Chain Reaction Products
Reverse transcription–polymerase chain reaction (RT-PCR) was performed on cell culture samples. Complementary DNA was synthesized with random hexamers (Superscript First-strand synthesis RT-PCR kit; Invitrogen). Primers for MUC-5AC and β2-microglobulin are previously described.11 PCR products were examined by electrophoresis on a 1.5% agarose gel.
Quantitive Real-Time PCR
Real-time quantitative RT-PCR (qPCR) was performed using a previously published SYBR green protocol using ABI7900 HT (Foster City, California).32 Each transcript in each sample was assayed 3 times and the median threshold cycle was used to calculate the fold change values and control changes for each gene. Three housekeeping genes were used for global normalization in each experiment (β-actin, RPS11, α-tubulin). Primer sequences for murine IL-4, IL-5, IL-13, interferon γ (IFN-γ), and human MUC-5AC have been previously described.9,30
Statistical Analysis
Differences between groups were analyzed by 1-way analysis of variance followed by Bonferroni t test for all pairwise comparisons if the data passed the normality test. P < .05 was considered statistically significant. Analyses were performed using SigmaPlot/Stat (Systat Software Inc, Point Richmond, California).
RESULTS
Suppression of Mucous Cell Metaplasia by Anti–TNF-α Treatment of Ovalbumin Mice
We first determined whether anti–TNF-α treatment decreased MUC-5AC expression in lung tissues from BALB/c ovalbumin mice by qPCR. Results are expressed as fold increase in MUC-5AC mRNA copy number in tissues from ovalbumin mice compared with the average mRNA copy number in age-matched tissues from naive mice. Figure 2A demonstrates that anti–TNF-α treatment administered during both antigen sensitization and challenge significantly (P = .007) decreased MUC-5AC expression to 4.97 (1.14) from 20.88 (3.8) (sham-treated group). Similar to initiating anti–TNF-α treatment before sensitization, initiating treatment before antigen challenge also significantly decreased MUC-5AC expression (9.3 [1.85], P = .03) (Fig 2A). Although beginning anti–TNF-α before antigen sensitization tended to decrease MUC-5AC to a greater extent, the difference between both treatment groups was not significant.
Figure 2.

Real-time quantitative polymerase chain reaction (qPCR). qPCR was performed using primers specific for MUC-5AC and normalized to control genes (β-actin, α-tubulin, and RPS11). Specific messenger RNA levels are expressed as a fold increase in copy number compared with strain and aged-matched naive mice for (A) sham-treated and anti–tumor necrosis factor α (TNF-α)–treated (sensitization and challenge or challenge alone) BALB/c ovalbumin (OVA) mice and (B) tumor necrosis factor receptor (TNFR−/− and TNFR+/+) OVA mice. Results are expressed as means (SE). *P < .05 and **P < .01 compared with sham-treated OVA mice; ***P < .001 compared with OVA TNFR−/− mice.
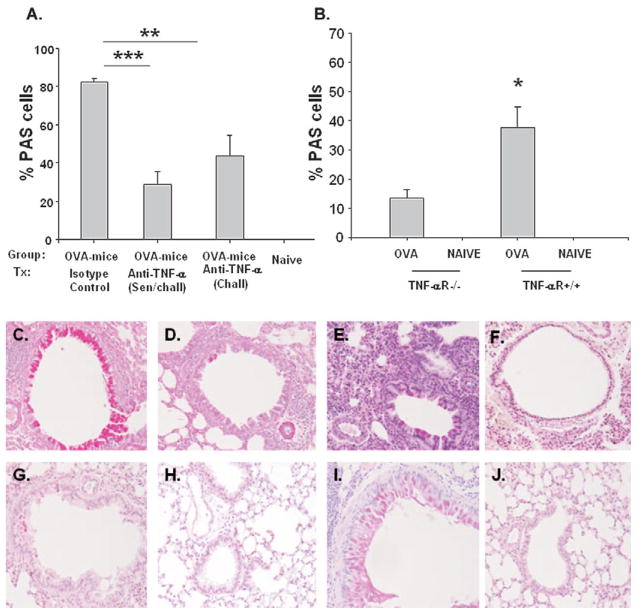
To correlate mRNA MUC-5AC expression with mucous cell metaplasia, sections of lung tissue were PAS stained. The percentage of PAS-positive cells in bronchioles was significantly lower (P < .001) in mice treated with anti–TNF-α antibody before antigen sensitization and challenge (28.9% [6.7%]) compared with sham-treated mice (82.29% [1.8%]; Fig 3A). Likewise, PAS staining was significantly decreased (P = .009) in BALB/c ovalbumin mice treated with anti–TNF-α only before challenge (43.57% [10.72%]) when compared with sham-treated ovalbumin mice. However, like MUC-5AC expression data, the difference in PAS staining was not significantly different between the 2 treatment groups but trended toward decreased mucous staining in mice treated with anti–TNF-α during both sensitization and challenge periods. Figure 3C–E shows representative PAS-stained (deep purple) lung tissue samples in BALB/c ovalbumin mice treated with anti–TNF-α before challenge only (D), before sensitization and challenge (E), and with sham treatment (C), demonstrating decreased staining in the 2 active treatment groups. There were no PAS-positive airway epithelial cells in naive mice (Fig 3F).
Figure 3.
Goblet cell metaplasia. Percentage of period acid–Schiff (PAS) staining in the right upper lung was determined for (A) BALB/c ovalbumin (OVA) mice receiving sham or anti–tumor necrosis factor α (TNF-α) treatment during (sensitization and challenge or challenge alone) and naive BALB/c mice and (B) effect of tumor necrosis factor receptor (TNFR) presence on goblet cell metaplasia in OVA mice. PAS staining was performed to access mucous metaplasia (deep purple) in (C) BALB/c OVA mice (isotype control treated), (D) BALB/c OVA mice (anti–TNF-α treatment, challenge), (E) BALB/c OVA mice (anti–TNF-α treatment, sensitization and challenge), (F) naive BALB/c mice, (G) TNFR−/− OVA mice, (H) TNFR−/− naive mice, (I) TNFR+/+ OVA mice, and (J) TNFR+/+ naive mice. One representative histologic sample per group shown (all original magnifications ×20). *P < .05; **P < .01; ***P < .001 compared with 6-week OVA mice. **P < .01 and ***P < .001 compared with sham-treated OVA mice or TNFR−/− OVA mice.
Decrease of Mucous Cell Metaplasia in TNFR−/− Ovalbumin Mice
To confirm that treatment with anti–TNF-α decreased airway MUC-5AC expression and PAS staining of lung tissue, we sensitized and challenged TNFR−/− and TNFR+/+ mice with ovalbumin. Results are expressed as fold increase in MUC-5AC mRNA copy number from ovalbumin mice compared with the average mRNA copy number in age-matched naive mice for both the TNFR−/− and TNFR+/+ mice. The fold increase in MUC-5AC expression was significantly greater (P < .001) in TNFR+/+ ovalbumin mice (18.37 [2.54]) compared with the fold increase in TNFR−/− ovalbumin mice (2.41 [0.41]; Fig 2B). Similar to airways of antigen sensitized and challenged BALB/c anti–TNF-α< treated ovalbumin mice, TNFR−/− ovalbumin mice contained fewer PAS-positive airway epithelial cells (13.57% [3.0%], P = .02) than similarly treated (control) TNFR+/+ ovalbumin mice (37.58% [7.19%]; Fig 3B). Figure 3G–J demonstrates decreased PAS staining from representative lung tissue samples from TNFR−/− ovalbumin mice (Fig 3G) compared with TNFR+/+ ovalbumin mice (Fig 3I). There were no PAS-positive airway epithelial cells in naive mouse lungs (Fig 3H and J).
Reduction of AHR by Anti–TNF-α Treatment in Ovalbumin Mice
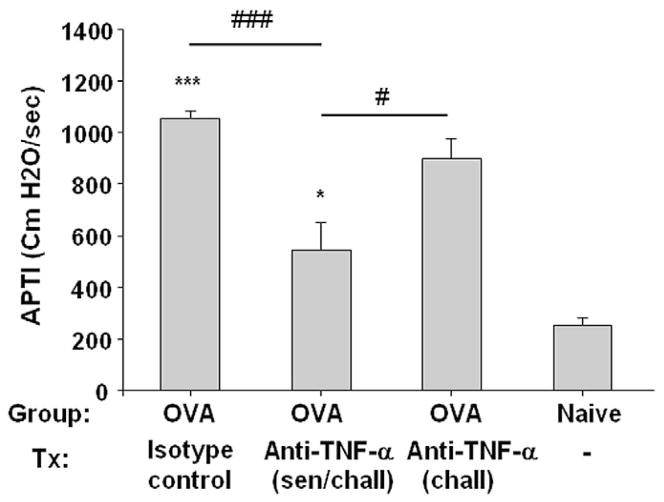
We previously demonstrated that intratracheal administration of rhTNF-α to naive mice induces mucous metaplasia and AHR.12 To determine whether administration of anti–TNF-α decreases AHR and mucous cell metaplasia, we measured APTI after intravenous acetylcholine in BALB/c mice. APTI was significantly greater (P < .001) in sham-treated BALB/c ovalbumin mice (1,055 [30.6] cm H2O/s) compared with naive mice (249.6 [34.1] cm H2O/s), confirming AHR in our positive controls (Fig 4). APTI in mice treated with anti–TNF-α antibody during both antigen sensitization and challenge (545.8 [104.5] cm H2O/s) was significantly decreased (P < .001) compared with sham-treated ovalbumin mice. Treatment in the challenge period only, however, reduced APTI compared with sham-treated mice but did not reach statistical significance (P = .06). These results suggest that treatment with anti–TNF-α antibody during both sensitization and challenge can significantly reduce AHR.
Figure 4.
Effect of anti–tumor necrosis factor α (TNF-α) on airway hyperresponsiveness. Peak airway pressure time index (APTI) after acetylcholine significantly decreased in BALB/c anti–TNF-α–treated ovalbumin (OVA) mice compared with BALB/c sham-treated OVA mice and naive age-matched controls. Data are mean (SE). *P < .05 and ***P < .001 compared with age-matched naive controls; #P < .05 and ###P < .001 compared with BALB/c anti–TNF-α–treated (sensitization and challenge) OVA mice.
Reduction of BALF Eosinophils by Anti–TNF-α Treatment of Ovalbumin Mice
To investigate a potential mechanism for reduction of mucous cell metaplasia by anti–TNF-α, we determined the total number of BALF cells and eosinophils from BALB/c mice. The total BALF cell number in BALB/c ovalbumin mice treated with anti–TNF-α antibody during antigen sensitization and challenge was significantly reduced (24.17 [3.96] × 104, P = .01) when compared with the BALF cell number in the sham-treated ovalbumin mice (44.17 [5.07] × 104; Fig 5A). The BALF cell number in the former treatment group did not differ significantly from naive samples (18.0 [1.22] × 104). The BALF cell number in ovalbumin mice treated with anti–TNF-α antibody only during challenge (32.0 [4.36] × 104) was fewer compared with sham-treated ovalbumin mice; however, this number did not reach statistical significance. No significant difference was found in total BALF cell counts between the ovalbumin mice treated with anti–TNF-α antibody during challenge vs treatment during both challenge and sensitization.
Figure 5.
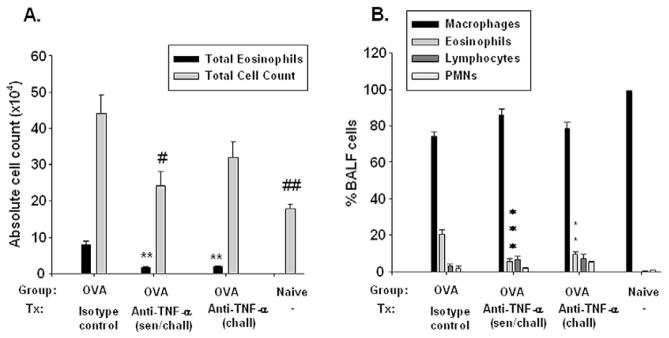
Effect of anti–tumor necrosis factor α (TNF-α) treatment on pulmonary inflammation. After the mice were killed, their lungs underwent lavage, the total leukocytes were counted, and bronchoalveolar lavage fluid (BALF) was cytocentrifuged on slides, fixed, and stained for leukocyte differential. A, Total BALF cell numbers for BALB/c mice. B, Leukocyte BALF differential from BALB/c mice. Data are presented as means (SE). **P < .01 and ***P < .001 compared with BALB/c sham-treated ovalbumin (OVA) mice. #P < .05 and ##P < .01 compared with sham-treated OVA mice. PMNs indicates polymorphonuclear cells; sen, sensitization; chall, challenge.
The eosinophil cell number (Fig 5A) and eosinophil percentage (Fig 5B) were significantly decreased in ovalbumin mice treated with anti–TNF-α antibody during both sensitization and challenge (1.66 [0.27], P = .001; 5.56% [1.48%], P = .006) when compared with the sham-treated ovalbumin mice (7.88 [1.01] and 20.49% [2.77%]). In addition, the total number and percentage of BALF eosinophils from BALB/c ovalbumin mice treated with anti–TNF-α antibody during challenge only was significantly reduced (1.77 [0.44], P < .01; and 9.55% [1.66%], P < .01; respectively) when compared with sham-treated mice (Fig 5A and B).
Expression of Other Proinflammatory Cytokines
To investigate whether the decrease in mucous cell metaplasia, AHR, and BALF eosinophilia resulting from administration of anti–TNF-α could be attributed to modulation of other proinflammatory cytokines, we performed qPCR on lung tissue for IL-4, IL-5, IL-13, and IFN-γ in the BALB/c mice. The fold increase of mRNA for IL-4, IL-5, IL-13, and IFN-γ was not significantly different between the sham-treated and the anti–TNF-α treated groups (data not shown).
Cell Culture Results
Our group previously demonstrated that rhTNF-α induces MUC-5AC expression in vivo.12 To investigate whether rhTNF-α increases MUC-5AC expression in a human lung epithelial cell line, NCI-H292 cells were first cultured with 50 ng of rhTNF-α for 12, 24, 48, or 72 hours. RT-PCR demonstrated that TNF-α increased MUC-5AC RNA expression at 12 hours, with maximal increase at 24 hours (data not shown). MUC-5AC RNA expression returned to baseline by 48 hours. To determine if the increase in MUC-5AC RNA expression induced by TNF-α occurred through a NF-κB pathway, samples were pretreated with 10 or 100μM of BAY-11–7082. Treatment with BAY-11–7082 of NCI-H292 cells significantly decreased the TNF-α–induced increase in MUC-5AC expression: TNF-α alone, 3.10-fold (0.17-fold) increase compared with untreated control; and 10 and 100μM BAY-11–7082, 0.77 (0.17) and 0.71 (0.12), respectively, P < .001 (Fig 6). Microscopically, no morphologic differences were seen in cells from different conditions (media alone, TNF-α supplemented, BAY-11–7082 treated).
Figure 6.
Effect of NF-κB inhibition of tumor necrosis factor α (TNF-α)–induced MUC-5AC expression. Human lung epithelial cells, NCI-H292, were cultured with 50 ng of recombinant human TNF-α for 24 hours. Some samples were pretreated with a NF-κB inhibitor (BAY-11–7082) at either 10 or 100μM. Quantitative real-time polymerase chain reaction was performed for human MUC-5AC and normalized to control genes (β-actin, α-tubulin, and RPS11). Specific messenger RNA levels are expressed as a fold increase in copy number compared with samples cultured in media alone. Data are mean (SE). ***P < .001 compared with samples cultured in media alone.
DISCUSSION
Mucous hypersecretion is associated with increased asthma morbidity and mortality. The mechanisms of mucous hyper-secretion in asthma are not completely understood. However, several cytokines, including IL-4, IL-5, IL-9, IL-13, and IL-17, have been shown to increase the major mucin-producing gene in the airway, MUC-5AC.5–8 TNF-α increases MUC-5AC expression in vitro9,11 and in vivo.12 This study examined whether administration of anti–TNF-α decreased mucous cell metaplasia in antigen sensitized and challenged mice, supporting its potential use as an adjunct therapy for asthma. We demonstrated for the first time that anti–TNF-α antibody treatment of ovalbumin mice before either antigen sensitization or antigen challenge significantly reduced mucous metaplasia as determined by PAS staining and MUC-5AC RNA expression.
MUC-5AC expression was significantly decreased by anti–TNF-α to a greater extent when given before antigen sensitization. Three studies have shown that anti–TNF-α administered to BALB/c26,27 and CBA/J mice18 before antigen challenge significantly decreased BALF total cell numbers, including eosinophils and neutrophils and lung inflammation, but did not address mucous hypersecretion. Our anti–TNF-α protocol challenged mice during a longer period to represent chronic asthma, whereas previous studies had shorter challenge periods. Furthermore, we challenged our mice intratracheally, which directly introduces the antigen into the airways, resulting in less gastric deposition of antigen.33 Another study in CBA/J mice examined the effects of daily administration of rhTNFR p80 fusion protein (etanercept; Wyeth Pharmaceuticals, Berks, England), starting in the antigen sensitization period. This protocol resulted in a significantly decreased BALF eosinophil count and lung tissue inflammatory cell infiltrate but also did not access for mucous metaplasia.34
To confirm our findings in anti–TNF-α–treated ovalbumin mice, we sensitized and challenged TNFR−/− mice. Airway MUC-5AC was significantly decreased in TNFR-deficient mice compared with wild-type mice. Lung tissue PAS staining paralleled our qPCR data. The differences in the percentages of PAS staining between sham-treated BALB/c ovalbumin mice (82.29% [1.8%]) and ovalbumin TNFR+/+ mice (37.58% [7.19%]) can be explained by strain differences. BALB/c mice typically develop increased mucous cell metaplasia with antigen sensitization compared with C57BL/6 mice.35 However, both anti–TNF-α–treated BALB/c ovalbumin mice and TNFR−/− ovalbumin mice showed approximately a 65% reduction in PAS staining. Therefore, the data from the TNFR−/− mice were consistent with our findings on the reduction of mucous cell metaplasia in our anticytokine BALB/c–treated mice. Studies have demonstrated that antigen sensitization and challenge of TNFR−/− mice decrease lung inflammation (hematoxylin-eosin staining and fewer total BALF cells, BALF eosinophils, lymphocytes, and neutrophils26,36,37) but have not investigated mucous metaplasia. Rudmann et al,38 in contrast, did not find decreased BALF total leukocytes or eosinophils in antigen-treated TNFR−/− mice. The protocol for this study differed from ours in that mice received aerosolized antigen challenge during a shorter interval.
To investigate possible mechanisms that anti–TNF-α therapy attenuates mucous metaplasia, we examined the BALF eosinophil number. The total number and percentage of BALF eosinophils from BALB/c ovalbumin mice treated with anti–TNF-α antibody during challenge had significantly fewer eosinophils when compared with sham-treated mice. The effect was more pronounced in mice treated with anti–TNF-α antibody during both the sensitization and challenge periods. This finding is consistent with other studies administering anti–TNF-α to antigen-treated mice.18,26,27,34 The eosinophil has been implicated in mucous metaplasia, although controversy exists.39–41
To indentify whether reduction of the BALF eosinophils was accompanied by a reduction in TH cytokines, we performed qPCR for IL-4, IL-5, IL-13, and IFN-γ. We did not detect any significant difference of these cytokines between mice receiving anti–TNF-α treatment and sham-treated mice. We previously demonstrated no increase in IL-4, IL-5, or IL-13 with rhTNF-α administration to naive mice.12 Furthermore, Kim et al27 demonstrated that anti–TNF-α administration to dust mite sensitized and challenge mice increased BALF IL-4, IL-5, and IL-13 but decreased eotaxin. TNF-α increases eotaxin42,43; therefore, an explanation for decreased BALF eosinophils (despite no reduction in IL-5) with anti–TNF-α treatment in our model may be via reduced eotaxin.
To study other mechanisms that TNF-α regulates mucous cell metaplasia, we investigated the NF-κB pathway. TNF-α induces NF-κB,44 and the MUC-5AC promoter has several NF-κB binding sites.45 Transgenic mice with repressed NF-κB activity had decreased mucous metaplasia after antigen exposure.46 We found that TNF-α enhances MUC-5AC expression.12 Therefore, we hypothesized that NF-κB is involved in TNF-α–induced activation of MUC-5AC and that blocking the NF-κB pathway could decrease mucous hyper-secretion. We pretreated H292 cells with the NF-κB inhibitor, BAY-11–7082,47 before TNF-α culture. BAY-11–7082–treated cells had significantly decreased MUC-5AC expression compared with nontreated cells, after TNF-α culture. Lora et al48 noted that adenoviral transfection of the H292 cell line using dominant-negative IKKβ decreased MUC-5AC expression measured by immunofluorescence staining after TNF-α culture.
Although increased mucous metaplasia most likely produces increased AHR,49 not all reports have established a clear relationship.46,50,51 We reported that rhTNF-α induced mucous metaplasia and AHR in naive mice.12 The current study demonstrated that anti–TNF-α given during both sensitization and challenge or only in challenge decreased AHR (significantly only in the former). Although the effects of anti–TNF-α on mucous metaplasia and APTI paralleled each other, it is unclear if it was a direct relationship or secondary to decreased BALF eosinophilia or relaxation of airway smooth muscle. Two studies measured effects of anti–TNF-α on AHR in murine models of allergic asthma.26,27 These studies administered anticytokine treatment during challenge periods only and, unlike our data, when administered in the challenge-only protocol suggested that anti–TNF-α significantly (P < .05 in both studies) suppressed AHR.26,27 These studies differed from ours in that AHR was determined by whole-body plethysmography, which may not be as accurate as invasive APTI.52 Furthermore, one study used 2 mg of anti–TNF-α,26 larger than our 250-μg dose. The other study sensitized mice to dust mite.27
In conclusion, we have demonstrated that inhibition of TNF-α decreases MUC-5AC and mucous cell histologic features in a murine model of allergic asthma. Although mechanisms of TNF-α regulation of mucous metaplasia are not entirely clear and further studies are necessary, it appears to involve a decrease in the airway eosinophils and NF-κB regulation.
Acknowledgments
The authors acknowledge Mount Sinai Real Time PCR Core Facility. We appreciate technical support from Dr. Steven Bezdecny.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Carroll N, Carello S, Cooke C, James A. Airway structure and inflammatory cells in fatal attacks of asthma. Eur Respir J. 1996;9:709–715. doi: 10.1183/09031936.96.09040709. [DOI] [PubMed] [Google Scholar]
- 2.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 3.Zuhdi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol. 2000;22:253–260. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 4.Ordonez CL, Khashayar R, Wong HH, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 5.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999;162:6233–6237. [PubMed] [Google Scholar]
- 6.Reader JR, Hyde DM, Schelegle ES, et al. Interleukin-9 induces mucous cell metaplasia independent of inflammation. Am J Respir Cell Mol Biol. 2003;28:664–672. doi: 10.1165/rcmb.2002-0207OC. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 9.Hauber HP, Daigneault P, Frenkiel S, et al. Niflumic acid and MSI-2216 reduce TNF-alpha-induced mucin expression in human airway mucosa. J Allergy Clin Immunol. 2005;115:266–271. doi: 10.1016/j.jaci.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH, Song KS, Koo JS, et al. IL-13 suppresses MUC5AC gene expression and mucin secretion in nasal epithelial cells. Acta Otolaryngol. 2002;122:638–643. doi: 10.1080/000164802320396321. [DOI] [PubMed] [Google Scholar]
- 11.Song KS, Lee WJ, Chung KC, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem. 2003;278:23243–23250. doi: 10.1074/jbc.M300096200. [DOI] [PubMed] [Google Scholar]
- 12.Busse PJ, Zhang TF, Srivastava K, et al. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116:1256–1263. doi: 10.1016/j.jaci.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 13.Howarth PH, Babu KS, Arshad HS, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60:1012–1018. doi: 10.1136/thx.2005.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry MA, Hargadon B, Shelley M, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 15.Amrani Y, Chen H, Panettieri RA., Jr Activation of tumor necrosis factor receptor 1 in airway smooth muscle: a potential pathway that modulates bronchial hyper-responsiveness in asthma? Respir Res. 2000;1:49–53. doi: 10.1186/rr12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med. 1995;152:76–80. doi: 10.1164/ajrccm.152.1.7599866. [DOI] [PubMed] [Google Scholar]
- 17.Thomas PS, Heywood G. Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax. 2002;57:774–778. doi: 10.1136/thorax.57.9.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukacs NW, Strieter RM, Chensue SW, Widmer M, Kunkel SL. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol. 1995;154:5411–5417. [PubMed] [Google Scholar]
- 19.Slungaard A, Vercellotti GM, Walker G, Nelson RD, Jacob HS. Tumor necrosis factor alpha/cachectin stimulates eosinophil oxidant production and toxicity towards human endothelium. J Exp Med. 1990;171:2025–2041. doi: 10.1084/jem.171.6.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheurich P, Thoma B, Ucer U, Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987;138:1786–1790. [PubMed] [Google Scholar]
- 21.Coward WR, Okayama Y, Sagara H, Wilson SJ, Holgate ST, Church MK. NF-kappa B and TNF-alpha: a positive autocrine loop in human lung mast cells? J Immunol. 2002;169:5287–5293. doi: 10.4049/jimmunol.169.9.5287. [DOI] [PubMed] [Google Scholar]
- 22.Meyers DAHGA, Wenzel SA, Lo K, Watt R, Bleecker ER. Pharmacogenetic identification of increased responsiveness in severe asthma with anti-TNF (Golimumab) therapy. J Allergy Clin Immunol. 2008;121:LB29. [Google Scholar]
- 23.Erin EM, Leaker BR, Nicholson GC, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med. 2006;174:753–762. doi: 10.1164/rccm.200601-072OC. [DOI] [PubMed] [Google Scholar]
- 24.Totsuka T, Kanai T, Uraushihara K, et al. Therapeutic effect of anti-OX40L and anti-TNF-alpha MAbs in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G595–603. doi: 10.1152/ajpgi.00450.2002. [DOI] [PubMed] [Google Scholar]
- 25.Uno JK, Kolek OI, Hines ER, et al. The role of tumor necrosis factor alpha in down-regulation of osteoblast Phex gene expression in experimental murine colitis. Gastroenterology. 2006;131:497–509. doi: 10.1053/j.gastro.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Choi IW, Sun K, Kim YS, et al. TNF-alpha induces the late-phase airway hyperresponsiveness and airway inflammation through cytosolic phospholipase A(2) activation. J Allergy Clin Immunol. 2005;116:537–543. doi: 10.1016/j.jaci.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, McKinley L, Natarajan S, et al. Anti-tumor necrosis factor-alpha antibody treatment reduces pulmonary inflammation and methacholine hyper-responsiveness in a murine asthma model induced by house dust. Clin Exp Allergy. 2006;36:122–132. doi: 10.1111/j.1365-2222.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 28.Li XM, Chopra RK, Chou TY, Schofield BH, Wills-Karp M, Huang SK. Mucosal IFN-gamma gene transfer inhibits pulmonary allergic responses in mice. J Immunol. 1996;157:3216–3219. [PubMed] [Google Scholar]
- 29.Levitt RC, Mitzner W. Expression of airway hyperreactivity to acetylcholine as a simple autosomal recessive trait in mice. FASEB J. 1988;2:2605–2608. doi: 10.1096/fasebj.2.10.3384240. [DOI] [PubMed] [Google Scholar]
- 30.Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007;37:1392–1403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Martin LD, Minnicozzi M, et al. Enhanced expression of mucin genes in a guinea pig model of allergic asthma. Am J Respir Cell Mol Biol. 2001;25:644–651. doi: 10.1165/ajrcmb.25.5.4485. [DOI] [PubMed] [Google Scholar]
- 32.Yuen T, Wurmbach E, Pfeffer RL, Ebersole BJ, Sealfon SC. Accuracy and calibration of commercial oligonucleotide and custom cDNA microarrays. Nucleic Acids Res. 2002;30:e48. doi: 10.1093/nar/30.10.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyles JE, Spiers ID, Williamson ED, Alpar HO. Tissue distribution of radioactivity following intranasal administration of radioactive micro-spheres. J Pharm Pharmacol. 2001;53:601–607. doi: 10.1211/0022357011775929. [DOI] [PubMed] [Google Scholar]
- 34.Hutchison S, Choo-Kang BS, Bundick RV, et al. Tumour necrosis factor-alpha blockade suppresses murine allergic airways inflammation. Clin Exp Immunol. 2008;151:114–122. doi: 10.1111/j.1365-2249.2007.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med. 2003;168:959–967. doi: 10.1164/rccm.200210-1188OC. [DOI] [PubMed] [Google Scholar]
- 36.Nakae S, Ho LH, Yu M, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 37.Broide DH, Stachnick G, Castaneda D, Nayar J, Sriramarao P. Inhibition of eosinophilic inflammation in allergen-challenged TNF receptor p55/p75–and TNF receptor p55-deficient mice. Am J Respir Cell Mol Biol. 2001;24:304–311. doi: 10.1165/ajrcmb.24.3.4071. [DOI] [PubMed] [Google Scholar]
- 38.Rudmann DG, Moore MW, Tepper JS, et al. Modulation of allergic inflammation in mice deficient in TNF receptors. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1047–57. doi: 10.1152/ajplung.2000.279.6.L1047. [DOI] [PubMed] [Google Scholar]
- 39.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 40.Cohn L, Homer RJ, MacLeod H, Mohrs M, Brombacher F, Bottomly K. Th2-induced airway mucus production is dependent on IL-4Ralpha, but not on eosinophils. J Immunol. 1999;162:6178–6183. [PubMed] [Google Scholar]
- 41.Shen HH, Ochkur SI, McGarry MP, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170:3296–3305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 43.Wong CK, Zhang JP, Ip WK, Lam CW. Activation of p38 mitogen-activated protein kinase and nuclear factor-kappaB in tumour necrosis factor-induced eotaxin release of human eosinophils. Clin Exp Immunol. 2002;128:483–489. doi: 10.1046/j.1365-2249.2002.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Nickola TJ, DiFronzo NL, Colberg-Poley AM, Rose MC. Dexamethasone-mediated repression of MUC5AC gene expression in human lung epithelial cells. Am J Respir Cell Mol Biol. 2006;34:338–347. doi: 10.1165/rcmb.2005-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poynter ME, Cloots R, van Woerkom T, et al. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol. 2004;173:7003–7009. doi: 10.4049/jimmunol.173.11.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pierce JW, Schoenleber R, Jesmok G, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 48.Lora JM, Zhang DM, Liao SM, et al. TNF-alpha triggers mucus production in airway epithelium through an IKKbeta dependent mechanism. J Biol Chem. 2005 doi: 10.1074/jbc.M507977200. [DOI] [PubMed] [Google Scholar]
- 49.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4:241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Bai TR, Knight DA. Structural changes in the airways in asthma: observations and consequences. Clin Sci (Lond) 2005;108:463–477. doi: 10.1042/CS20040342. [DOI] [PubMed] [Google Scholar]
- 51.Wu CA, Puddington L, Whiteley HE, et al. Murine cytomegalovirus infection alters Th1/Th2 cytokine expression, decreases airway eosinophilia, and enhances mucus production in allergic airway disease. J Immunol. 2001;167:2798–2807. doi: 10.4049/jimmunol.167.5.2798. [DOI] [PubMed] [Google Scholar]
- 52.Finkelman FD. Use of unrestrained, single-chamber barometric plethysmography to evaluate sensitivity to cholinergic stimulation in mouse models of allergic airway disease. J Allergy Clin Immunol. 2008;121:334–335. doi: 10.1016/j.jaci.2007.11.028. [DOI] [PubMed] [Google Scholar]