Abstract
Delivery of antigen in particulate form using either synthetic or natural particles induces stronger immunity than soluble forms of the antigen. Among naturally occurring particles, virus-like particles (VLPs) have been genetically engineered to express tumor-associated antigens (TAAs) and have shown to induce strong TAA-specific immune responses due to their nano-particulate size and ability to bind and activate antigen-presenting cells. In this report, we demonstrate that influenza VLPs can be modified by a protein transfer technology to express TAAs for induction of effective antitumor immune responses. We converted the breast cancer HER-2 antigen to a glycosylphosphatidylinositol (GPI)-anchored form and incorporated GPI-HER-2 onto VLPs by a rapid protein transfer process. Expression levels on VLPs depended on the GPI-HER-2 concentration added during protein transfer. Vaccination of mice with protein transferred GPI-HER-2-VLPs induced a strong Th1 and Th2-type anti-HER-2 antibody response and protected mice against a HER-2-expressing tumor challenge. Soluble form of GPI-HER-2 induced only a weak Th2 response under similar conditions. These results suggest that influenza VLPs can be enriched with TAAs by protein transfer to develop effective VLP-based subunit vaccines against cancer without chemical or genetic modifications and thus preserve the immune stimulating properties of VLPs for easier production of antigen-specific therapeutic cancer vaccines.
Keywords: protein transfer, virus-like particles, breast cancer, HER-2, vaccine
Introduction
Overexpression of the tumor associated antigen (TAA) human epidermal growth factor receptor 2 (HER-2) found on a subset of breast cancers is correlated with poor prognosis and clinical outcome (Press et al. 1997; Slamon et al. 1987). Therefore, HER-2 is an ideal candidate for targeted immunotherapies against cancer. Currently, multiple antibody-based treatment regimens exist to target HER-2 in HER-2-positive breast cancer (Thery et al. 2014). However, these passive antibody-mediated immunotherapies do not develop cellular immune responses and thus eventually fail. To fully combat cancer, active immunotherapies which develop humoral and cellular adaptive immune responses are needed.
Active immunotherapies include HER-2-expressing tumor cells that also secrete the cytokine GM-CSF (Kim et al. 2008; Srivatsan et al. 2014), patient derived peripheral blood mononuclear cells (PBMCs) activated with fusion proteins consisting of HER-2 and GM-CSF (Park et al. 2007; Peethambaram et al. 2009), ex vivo loaded dendritic cells transfected with adenoviruses encoding HER-2 along with immunostimulatory molecules (Chen et al. 2001; Chen et al. 2002; Sakai et al. 2004) or pulsed with HER-2 peptides (Brossart et al. 2000; Czerniecki et al. 2007), as well as HER-2 encoding DNA vaccines (Gallo et al. 2005; Piechocki et al. 2001b; Rovero et al. 2000; Wei et al. 1999), HER-2 peptide-based vaccines (Disis et al. 2002; Holmes et al. 2008; Mittendorf et al. 2008), and protein-based vaccines (Disis et al. 2004). Although these immunotherapies induce HER-2-specific immunity, complete remission is not seen. Further, obtaining and maintaining patient-derived DCs and PBMCs for ex vivo manipulation is expensive. Peptide-based and protein-based vaccination strategies are also poorly immunogenic due to stability issues and DNA-based vaccines do not allow for targeted expression. Therefore, there is a need for a more efficient approach to deliver the HER-2 antigen to the immune system.
Delivering antigens to the immune system as a particulate form has been shown to induce a stronger antigen-specific immune response than soluble antigens (Peacey et al. 2007; Zhang et al. 2014). In this regard, many biocompatible micro/nano-particles have been explored as delivery platforms. Among these, virus-like particles (VLPs) have shown to be effective. VLPs are nanoparticles that resemble viral counterparts in size and structure however they lack viral genome. They are derived from the expression of viral envelope and/or capsid proteins and express repetitive molecules in an ordered structure. The nanosize and particulate nature of VLPs makes them ideal for uptake by antigen presenting cells (APCs) (Cubas et al. 2009; Manolova et al. 2008) and the presence of highly ordered repetitive structures on their surface provides a danger signal to initiate immune activation (Jennings and Bachmann 2008). These features make VLPs strong immunogens that induce robust cellular and humoral anti-viral immune responses upon vaccination (Lua et al. 2014).
The intrinsic immunogenicity of VLPs has been exploited to deliver tumor antigens to elicit antigen-specific immunity. Non-enveloped VLPs derived from murine polyomavirus VP1 and VP2 protein have been modified through genetic means to express HER-2 by fusing the extracellular domain of HER-2 to the VP2 protein (Tegerstedt et al. 2005). Vaccination with these chimeric HER-2-expressing VLPs led to protection against HER-2-expressing tumors. Further, vaccination with VLPs produced using rBVs expressing Gag and mTrop2, an antigen associated with pancreatic cancer, enhanced tumor infiltrating lymphocyte populations and generated mTrop2-specific antibodies and cytotoxic T lymphocytes that led to enhanced survival of pancreatic tumor-bearing mice (Cubas et al. 2011). Both of these strategies involve genetic alterations to produce chimeric VLPs, however genetic engineering to produce chimeric VLPs may affect VLP proteins leading to lower VLP production and altered immunogenicity. Further, although genetic modification may produce a homogeneous product, the amount of expression of TAAs per VLP cannot be easily manipulated due to the limitations of gene transfer technologies.
In this report we investigated whether enveloped VLPs, such as influenza VLPs, can be used as a tumor antigen delivery vehicle without the use of genetic manipulation but using a protein transfer approach. Previously, our lab has shown that cells or membrane vesicles that contain lipid bilayers can be modified by protein transfer to homogenously express glycophosphatidylinositol (GPI)-anchored immunostimulatory molecules, B7-1 or IL-12 (McHugh et al. 1995; McHugh et al. 1999; Nagarajan and Selvaraj 2006; Poloso et al. 2001). Protein transfer involves a simple incubation of purified GPI-proteins with cells or membrane vesicles for 2-4 hours and leads to stable expression of the incorporated protein which in turn remains functional (McHugh et al. 1995; McHugh et al. 1999; Nagarajan and Selvaraj 2006; Poloso et al. 2001). Influenza VLPs are enveloped VLPs that can be derived from the expression of hemagglutinin (HA) and matrix (M1) proteins and contain lipid bilayer envelopes derived from host cells. Vaccination with influenza VLPs has led to robust cellular and humoral immunity against HA expressed on the VLPs (Galarza et al. 2005; Kang et al. 2009; Pushko et al. 2005; Song et al. 2010).
Based on these observations, we hypothesized that enveloped VLPs such as influenza VLPs can be modified to express TAAs by protein transfer similar to cellular membranes and used to induce tumor immunity. Herein, we show for the first time that influenza VLPs can be modified by protein transfer to express a TAA to induce TAA-specific immunity. We found that vaccination with GPI-HER-2 anchored to VLPs (GPI-HER-2-VLPs) by protein transfer led to enhanced protection against HER-2-expressing tumor growth in a murine breast cancer model. Further, GPI-HER-2-VLPs led to HER-2-specific IgG production and enhanced HER-2-specific Th1-type immunity compared to vaccination with GPI-HER-2 alone.
Methods
Animals, antibodies, and cell lines
6-8 week old female BALB/c mice were purchased from Jackson Laboratory. All experiments were conducted in accordance with Emory University IACUC guidelines. Hybridoma secreting anti-HER-2 (TA1) monoclonal antibody (mAb) was obtained from ATCC. CHO-K1 cell transfectants expressing either full-length transmembrane HER-2 or GPI-HER-2 were maintained in RPMI 1640 and 10% Cosmic Calf Serum (Invitrogen) containing 10 μg/ml Blasticidin (Invitrogen). Panning was used to select for cells that expressed high levels of HER-2 and expression was analyzed by flow cytometry. Sheep red blood cells (RBCs) were purchased from HemoStat Laboratories. D2F2 and D2F2/E2 cells were a kind gift from Wei-Zen Wei from Wayne State University (Piechocki et al. 2001a; Pilon et al. 2001). In brief, the D2F2 cell line is a murine breast cancer cell line that was transfected with full length transmembrane HER-2 DNA to create the HER-2-expressing cell line, D2F2/E2.
GPI-HER-2 DNA Construct
GPI-HER-2 was constructed by attaching the nucleotides corresponding to the GPI-signal sequence from CD59 to the C-terminal end of the extracellular domain sequence of HER-2 (GenBank X03363.1; amino acids 1-652) as previously described (Nagarajan and Selvaraj 2002; Poloso et al. 2001; Poloso et al. 2002). An EcoRI restriction enzyme site was placed separating the two sequences. Nucleotide 1305 (1479 of Genbank sequence) was changed from a T to a C to remove an internal EcoRI restriction enzyme site in HER-2 without changing the amino acid sequence. HindIII and KpnI restriction enzyme sites were introduced into the N-terminal end before the HER-2 sequence. The resulting GPI-HER-2 construct was then placed in a pUB6blast vector (Invitrogen) and transfected into CHO-K1 cells to establish transfectants expressing GPI-HER-2.
Phosphatidylinositol-specific phospholipase C (PI-PLC) treatment of transfected CHO-K1 cells
Transfected CHO-K1 cells were washed and resuspended in PBS/EDTA/0.1% ovalbumin to a final concentration of 10×106 cells/ml. 1U PI-PLC from Bacillus cereus (Invitrogen) that specifically cleaves the GPI-anchor was added to 1ml of cell suspension and incubated at 37°C for 45 min. Cells were washed and protein expression was analyzed by flow cytometry.
Purification of GPI-HER-2
Cell pellets of CHO-K1 cell transfectants expressing GPI-HER-2 were lysed with 50 mM Tris-HCl pH 8, 2% n-octyl-β-D-glucopyranoside (A.G. Scientific), 1:100 dilution of Protease Inhibitor Cocktail (Sigma), 5 mM EDTA, 20 mM sodium iodoacetate, and 2 mM PMSF overnight at 4°C. The lysate was centrifuged at 14,000 rpm using a JA-20 rotor (Beckman) for 1 h at 4°C. The supernatant was passed through a Sepharose 4B bead (Sigma-Aldrich) pre-column. The effluent was then passed through a TA1 mAb-affinity chromatography column followed by a wash with 50 mM Tris-HCl, pH 8.0, 1% Triton X-100, 200 mM NaCl and then with 20 mM Tris-HCl, pH 7.5, 0.1% n-octyl-β-D-glucopyranoside. GPI-HER-2 was eluted from the column with 100mM Triethylamine, 1% n-octyl-β-D-glucopyranoside, pH 11.6. Eluted fractions were analyzed by SDS PAGE followed by western blot using the anti-HER-2 mAb TA1 or silver stain. Fractions that contained purified GPI-HER-2 were concentrated in a 10-14 kDa MWCO dialysis bag (Fisherbrand) using polyvinylpyrrolidone (Sigma-Aldrich) and then dialyzed with PBS containing 0.05% n-octyl-β-D-glucopyranoside. Quantification of GPI-HER-2 was performed using a micro BCA kit (Thermo Scientific).
Protein transfer of GPI-HER-2 onto sheep RBCs
Sheep RBCs were washed with PBS two times by centrifugation followed by a wash with PBS containing 0.1% ovalbumin. 14 μg of GPI-HER-2 was then incubated with 2 × 106 sheep RBCs in 200 μL PBS containing 0.1% ovalbumin for 4 h at 37°C in an end-over-end rotation. Unincorporated GPI-HER-2 was washed out by centrifugation at 2000 rpm for 5 min using PBS, 5 mM EDTA, and 1% cosmic calf serum.
VLP preparation
VLPs were produced using the recombinant baculovirus (rBV) expression system as described (Kang et al. 2009; Quan et al. 2007). Briefly, rBVs individually expressing influenza M1 protein and HA protein from H1N1 influenza A/PR/8/1934 virus were co-infected into Sf9 insect cells to produce VLPs. The cell culture supernatant containing the VLPs was collected after 2-3 days and centrifuged (6000 rpm, 30 min at 4°C). VLPs were concentrated by the QuixStand hollow fiber ultrafiltration system (GE Healthcare, Piscataway, NJ). The VLPs were then purified by 20%-60% (wt/vol) sucrose gradient ultracentrifugation at 30,000 rpm using a SW32Ti Rotor (Beckman) for 60 min at 4°C. The VLPs were collected at the interphase between 20% and 60% sucrose layers, diluted in PBS and pelleted at 30,000 rpm for 30 min at 4°C. The VLP pellet was resuspended with PBS and stored at -80°C until use. Quantification of VLPs was performed by the Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA).
Protein transfer of GPI-HER-2 onto VLPs
Purified GPI-HER-2 was first centrifuged at 13,200 rpm for 1 h at 4°C to exclude any precipitate formed during storage and then used for protein transfer. Approximately 250 μg of purified GPI-HER-2 was incubated with 1 mg of VLP in 4 ml PBS for 4 h at 37°C in an end-over-end rotation. To remove any unbound GPI-HER-2, the VLPs were pelleted by centrifugation at 35,000 rpm for 1 h at 4°C using a SW-41Ti rotor (Beckman) and the supernatant containing unincorporated GPI-HER-2 was removed. Then the protein transferred VLPs were resuspended in PBS and stored at 4°C until use. GPI-HER-2 incorporation onto VLPs was analyzed by western blot using TA1 mAb and quantified by Image J. Known concentrations of purified GPI-HER-2 were used to make a standard curve.
Quantification of GPI-HER-2-VLPs by ELISA
GPI-HER-2-VLPs and known concentrations of unmodified VLPs were coated onto 96-well ELISA plates overnight at 4°C. Polyclonal anti-VLP mouse antibodies were used as a primary antibody against the VLPs followed by secondary antibody detection using goat-anti-mouse-IgG-HRP (Southern Biotech). The TMB-1 substrate (BioLegend) was used to develop color followed by addition of 2N H2SO4 to stop the reaction. Absorbance was read at 450 nm. Values from known concentrations of unmodified VLPs were used as standards to quantify GPI-HER-2 VLPs.
Vaccination and tumor challenge studies
BALB/c mice were vaccinated at day 0 and boosted at day 14 with 25 μg of GPI-HER-2-VLPs each time in 100 μl PBS subcutaneously on the left hind flank. For tumor challenge, mice were challenged with 2 × 105 D2F2/E2 (HER-2 positive) cells or 2 × 105 wild-type D2F2 (HER-2 negative) cells, in 100 μl PBS on the right hind flank 7 days after boost. Tumor area was measured by multiplying the length and width of each tumor.
Detection of anti-HER-2 serum IgG responses
Flow cytometry (FACSCalibur, Becton Dickinson) analysis of serum anti-HER-2 IgG responses was carried out using a 1:200 diluted serum (obtained 7 days post boost) from vaccinated mice as primary antibody and 2.5 × 105 D2F2/E2 cells in 100 μl FACS Buffer (PBS, 5 mM EDTA, 1% cosmic calf serum). Goat-anti-mouse-FITC (Jackson Immunoresearch) was used as the secondary antibody. To determine HER-2 specific IgG subtypes, a cell ELISA (Bumgarner et al. 2005; Cobern and Selvaraj 1995) using 5 × 104 D2F2/E2 cells per well with 50 μl of 1:100 diluted serum obtained from mice 7 days after boost was performed. The secondary antibody used was rat-anti-mouse-IgG1-HRP, rat-anti-mouse-IgG2a-HRP, or goat-anti-mouse-IgG2b-HRP (Southern Biotech) and the absorbance was measured at 450 nm. D2F2 cells were used as a negative control.
Detection of anti-VLP serum IgG responses
ELISA of serum from vaccinated mice collected 7 days after boost was performed by plating 100 μl of 1 μg/ml VLP in triplicate in 96-well flat bottom ELISA plates overnight at 4°C. The unbound VLPs were removed and then the wells were blocked with PBS containing 0.05% Tween-20 and 3% bovine serum albumin. A non-saturating (1:10,000) dilution of serum was added to wells as the primary antibody. Goat-anti-mouse IgG-HRP was used as the secondary antibody followed by detection with TMB-1 substrate (BioLegend). The color development was stopped with 2N H2SO4 and absorbance was read at 450 nm.
Statistical Analysis
All data are expressed as mean ± SEM. GraphPad Prism 6 was used to perform statistical analysis. Student's t-test, One-way ANOVA or Two-way ANOVA using Tukey's multiple comparisons post-test was used for analysis as described in the figure legends. A p < 0.05 value was considered significant.
Results
Construction and expression of GPI-anchored-HER-2
HER-2 is a transmembrane protein that contains both an extracellular and intracellular domain. Since protein transfer requires a GPI-anchor, HER-2 was converted into a GPI-anchored form by attaching DNA encoding the GPI-signal sequence obtained from the naturally GPI-anchored protein, CD59, to the C-terminal end of DNA encoding the extracellular domain of HER-2. The resulting GPI-HER-2 DNA construct was placed in a pUB6blast vector that was transfected into CHO-K1 cells. To determine the presence of the GPI-anchor, transfected CHO-K1 cells were subjected to PI-PLC treatment. PI-PLC is a bacterial enzyme that specifically cleaves the GPI-anchor and releases GPI-anchored proteins from the cell surface (Low and Saltiel 1988). CHO-K1 cells expressing full-length transmembrane HER-2 showed similar HER-2 surface expression whether subjected to PI-PLC treatment or not (Figure 1A; left panel). However, CHO-K1 cells transfected with the GPI-HER-2 DNA construct showed 97.3% decrease in HER-2 surface expression after PI-PLC treatment (Figure 1A; right panel) suggesting that HER-2 expressed on these cells contains a GPI-anchor.
Figure 1. Construction and purification of GPI-HER-2.
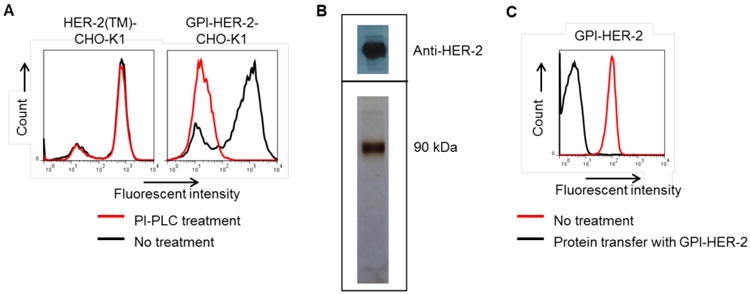
(A) HER-2 expressed on the surface of GPI-HER-2-CHO-K1 cell transfectants is GPI-anchored. PI-PLC treatment (red) was performed on CHO-K1 cells transfected with full-length transmembrane (TM) HER-2 (left panel) and CHO-K1 cells transfected with the GPI-HER-2 DNA construct. Surface expression was analyzed by flow cytometry. (B) Purification of GPI-HER-2. GPI-HER-2 was purified from the lysate of transfected CHO-K1 cells by mAb-affinity chromatography. HER-2 containing fractions were pooled, concentrated and dialyzed with PBS containing 0.05% octyl-β-glucopyranoside. The resulting concentrated fraction was subjected to SDS-PAGE under non-reducing conditions in a 10% gel followed by western blot (top panel) or silver stain (bottom panel) analysis. (C) Purified GPI-HER-2 retains the GPI-anchor domain and the ability to incorporate onto cell surfaces. Protein transfer of purified GPI-HER-2 was performed on sheep RBCs (2 × 106) in PBS containing 0.1% ovalbumin for 4 h at 37°C. The sheep RBCs were then washed with PBS by centrifugation and incorporation was analyzed by flow cytometry.
Purification of GPI-HER-2
GPI-HER-2 was purified from transfected CHO-K1 cell lysates using mAb-affinity chromatography. Analysis of purified GPI-HER-2 by SDS-PAGE followed by western blot (Figure 1B, top panel) and silver staining (Figure 1B, bottom panel) showed that the molecular weight of GPI-HER-2 is approximately 90 kDa.
To determine if purified GPI-HER-2 contained an intact GPI-anchor and was capable of incorporating onto cell membranes, protein transfer of GPI-HER-2 was performed on sheep RBCs for 4 h at 37°C as an assay system. As shown in Figure 1C, purified GPI-HER-2 was incorporated onto sheep RBCs after protein transfer as detected by flow cytometry analysis suggesting that purified GPI-HER-2 contained an intact GPI-anchor.
Protein transfer-mediated incorporation of GPI-HER-2 onto VLPs
Immunization of antigen in a particulate form enhances antigen-specific immune responses compared to immunization of antigen in a soluble form (Peacey et al. 2007; Zhang et al. 2014). Therefore, we hypothesized that VLPs modified to express HER-2 by protein transfer would induce enhanced HER-2-specific immunity compared to administration of HER-2 alone. Since protein transfer led to incorporation of GPI-HER-2 onto sheep RBCs that contain lipid bilayers, we also hypothesized that purified GPI-HER-2 could incorporate onto lipid envelopes of influenza VLPs. As shown in Figure 2, GPI-HER-2 was able to incorporate onto influenza VLPs by protein transfer. As the concentration of purified GPI-HER-2 incubated with VLPs was increased, HER-2 incorporation on the VLPs increased as well showing that protein transfer of GPI-HER-2 onto VLPs is concentration dependent (Figure 2A, upper panel). Further, the overall structure (i.e. epitopes recognized by the anti-VLP antibodies) of the VLPs was not significantly modified after protein transfer as detected by western blot (Figure 2A, bottom panel). Incubation of 200 μg of GPI-HER-2 with 800 μg VLPs during protein transfer resulted in incorporation of 0.235 μg of GPI-HER-2 per μg of VLP as detected by ELISA and ImageJ analysis. This was further confirmed by western blot analysis of GPI-HER-2 present in the solution before and after protein transfer, which showed that 80.6% of the 200 μg of GPI-HER-2 was incorporated onto VLPs by protein transfer (Figure 2B).
Figure 2. Protein transfer of GPI-HER-2 onto influenza VLPs.
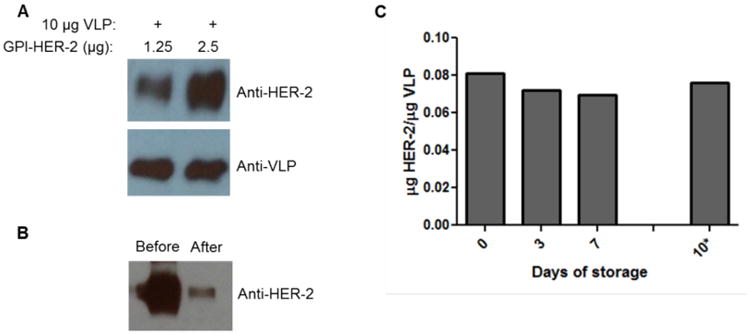
(A) Concentration dependent-incorporation of GPI-HER-2 onto influenza VLPs by protein transfer. Purified GPI-HER-2 was incubated with 10 μg VLPs at different concentrations for 4 h at 37°C. Incorporation of HER-2 was detected by subjecting VLPs to SDS-PAGE and western blot analysis (top panel). The presence of VLP proteins was analyzed (bottom panel) by staining with a 1:1000 dilution of VLP-specific serum from vaccinated mice. (B) High level of added GPI-HER-2 incorporates into VLPs by protein transfer. Supernatant before (lane 1) and after (lane 2) protein transfer of 200 μg GPI-HER-2 with 800 μg VLPs was analyzed by SDS-PAGE and western blot by blotting against HER-2. 80.6% GPI-HER-2 incorporated onto VLPs as analyzed by Image J. (C) GPI-HER-2 incorporated into influenza VLPs remains stably associated with VLPs after storage. From day 0 to day 7, GPI-HER-2-VLPs were stored at 4°C. The GPI-HER-2-VLPs were then transferred to room temperature for another 3 days (from day 7 to day 10*). On each day, an aliquot of GPI-HER-2-VLPs was washed with PBS by centrifugation at 13,200 rpm for 30 min. The VLP pellet was then resuspended with Laemmli sample buffer and then subjected to SDS PAGE and western blot analysis to detect VLP and GPI-HER-2 concentrations using anti-VLP serum and TA1 mAb, respectively.
Protein transfer results in stable expression of incorporated GPI-HER-2
Next, we determined the stability of GPI-HER-2 incorporated onto VLPs by protein transfer. GPI-HER-2-modified VLPs were stored at 4°C for 7 days. As shown in Figure 2C, HER-2 expression on the VLPs remained stable during this time period. From day 7 to day 10, the GPI-HER-2-modified-VLPs were then transferred to storage at room temperature. Storage at room temperature for an additional 3 days also did not affect the expression levels of HER-2 on the protein transfer-modified-VLPs (Figure 2C).
Vaccination with GPI-HER-2-VLPs induces HER-2-specific antibodies
To determine if vaccination with VLPs protein transferred with GPI-HER-2 (GPI-HER-2-VLPs) induced HER-2-specific immunity, antibody responses were analyzed after vaccination of mice with 25 μg GPI-HER-2-VLPs on day 0 and day 14. Serum was collected from vaccinated mice 7 days post boost and analyzed by flow cytometry using D2F2/E2 cells. A strong anti-D2F2/E2 serum IgG antibody response was detected in GPI-HER-2-VLP-vaccinated mice whereas unmodified VLP-vaccinated mice did not show any detectable levels of anti-D2F2/E2 IgG antibodies (Figure 3A). Interestingly, vaccination with purified GPI-HER-2 also induced anti-D2F2/E2 antibody levels similar to GPI-HER-2-VLPs. Further, minimum or no serum IgG antibody was detected against the parental HER-2-negative D2F2 (Figure 3B) cells showing that reactivity against D2F2/E2 was from HER-2 specific antibodies. Mice vaccinated with unmodified VLPs or GPI-HER-2-modified-VLPs displayed a similar serum antibody response against viral proteins present on VLPs (Figure 3C), suggesting that protein transfer does not affect the antigenicity of intrinsic VLP proteins.
Figure 3. Vaccination with GPI-HER-2-protein transferred-VLPs induces HER-2-specific antibody responses.
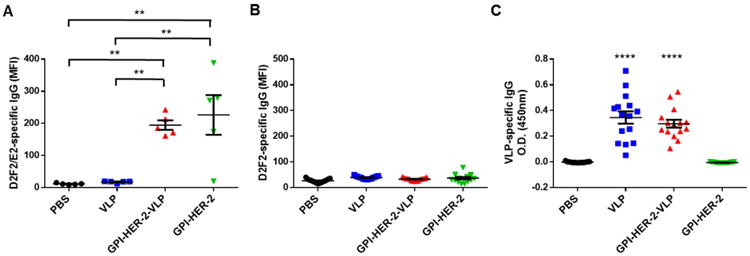
(A) Protein transferred GPI-HER-2-VLP vaccination induces HER-2-specific serum IgG. Mice were vaccinated with GPI-HER-2-VLPs (25 μg) on day 0 and day 14, and serum was collected from vaccinated mice on day 21. Flow cytometry analysis was performed to detect anti-D2F2/E2 (HER-2-positive cell line) specific (n = 5) or anti-D2F2 (HER-2-negative cell line) specific (n = 15) IgG antibodies by using 1:200 diluted serum and 250,000 cells per well. (B) Minimum or no serum IgG was detected against D2F2 cells showing HER-2-specific antibody reactivity against D2F2/E2. (C) Influenza VLPs protein transferred with or without GPI-HER-2 induce similar levels of VLP-specific serum IgG antibodies. ELISA was performed using 1:10,000 diluted serum collected 1 week post boost from groups of vaccinated mice (n = 15).
Vaccination with GPI-HER-2-VLPs induces HER-2-specific Th1 and Th2-type immunity
Th1-type immune responses play an important role in antitumor immunity (Nishimura et al. 2000) and many studies have shown that both Th1 and Th2-type cellular immunity play synergistic roles in eradicating tumors (Nishimura et al. 1999). To elucidate the type of immunity induced by vaccination with GPI-HER-2-VLPs, a cell ELISA was conducted on sera from vaccinated mice using D2F2/E2 cells and HER-2-specific IgG subtypes were analyzed. IgG2a and IgG2b subtypes are indicative of a Th1-type immune response whereas IgG1 production represents a Th2-type immune response. As shown in Figure 4, GPI-HER-2-VLP vaccination significantly raised levels of anti-HER-2 IgG2a (Figure 4B) and IgG2b (Figure 4C) antibody subtypes compared to those induced by soluble GPI-HER-2 vaccination. Whereas IgG1 levels, which is the predominant subtype in soluble GPI-HER-2 vaccinated mice, were similarly detected in both groups (Figure 4A). These results suggest that delivery of an antigen physically linked to nanoparticulate VLPs induces both Th1 and Th2-type IgG isotype responses as opposed to the soluble form of antigen which induces mostly Th2-type antibody responses.
Figure 4. Vaccination with GPI-HER-2-VLPs enhances Th1-type antibody responses.
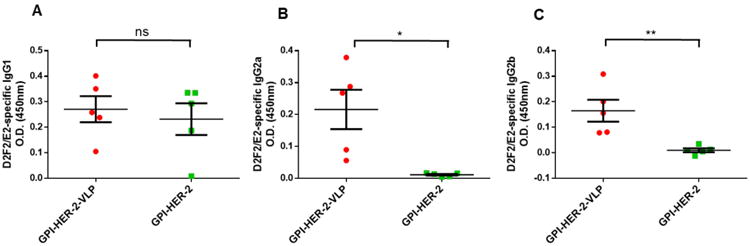
Mice were vaccinated with GPI-HER-2-VLPs (25 μg) on day 0 and day 14, and serum was collected on day 21. Anti-D2F2/E2-specific serum (A) IgG1, (B) IgG2a, and (C) IgG2b subtype antibodies were analyzed by cell ELISA using 1:200 diluted serum from vaccinated mice (n = 5) with 50,000 D2F2/E2 cells per well.
Immunization with GPI-HER-2-VLPs protects mice against HER-2-expressing tumor challenge
To test the protective efficacy of GPI-HER-2-VLPs, mice were vaccinated and boosted 14 days later with 25 μg of VLPs, GPI-HER-2-VLPs, or similar concentrations (0.757 μg) of soluble GPI-HER-2. At 7 days after boost, vaccinated mice were challenged with 2 × 105 D2F2/E2 cells, a HER-2 expressing murine breast cancer cell line. Mice vaccinated with GPI-HER-2-modified-VLPs had on average significantly smaller tumors compared to mice vaccinated with similar concentrations of GPI-HER-2 alone (Figure 5A, left panel). Further, a higher percentage of mice remained tumor free in the GPI-HER-2-VLP vaccinated group (66.67%) by the end of the study (day 58 post challenge) compared to either PBS (10%), unmodified VLPs (0%) or GPI-HER-2 alone (20%) (Figure 5A, right panel) vaccinated groups. The HER-2-specific antibody response in the vaccinated mice that succumbed to tumor growth was comparable to the antibody response in the vaccinated mice that cleared the tumor (Figure 3A). At present, it is not clear as to why some of these vaccinated mice succumbed to tumor growth despite the production of HER-2-specific antibodies. Intriguingly, the average tumor size in unmodified VLP vaccinated groups was significantly lower compared to control mice suggesting that VLP vaccination alone affected tumor growth, perhaps through activation of a more robust polyclonal immune response that could have primed mice against the HER-2 antigen expressed on the tumor cells used for challenge (Figure 5A, left panel). Mice vaccinated with GPI-HER-2 alone also portrayed some protection against tumor growth compared to control mice although not as enhanced as GPI-HER-2-VLP vaccinated mice (Figure 5A). On the other hand, when GPI-HER-2-VLP vaccinated mice were challenged with HER-2-negative D2F2 cells, tumors grew steadily similar to unvaccinated mice (Figure 5B, left panel) with 100% tumor incidence (Figure 5B, right panel). These results show that vaccination with VLPs protein transferred with GPI-HER-2 induced anti-tumor immune responses that are specific to the HER-2 antigen delivered by the VLPs.
Figure 5. Vaccination with GPI-HER-2-VLPs induces protection against challenge with a HER-2-expressing breast cancer cell line.
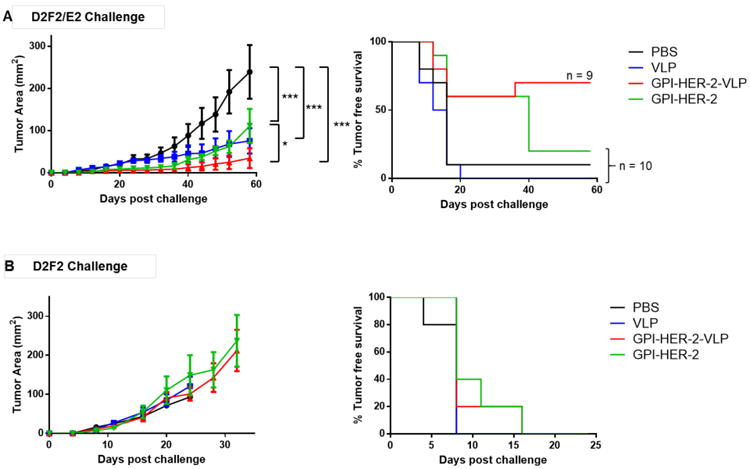
(A) Tumor growth (left panel; n = 5) and incidence (right panel; n = 9 or 10) after vaccination with protein transferred GPI-HER-2-VLPs followed by D2F2/E2 cell challenge. Mice were vaccinated with GPI-HER-2-VLPs (25 μg) on day 0 and day 14, and then challenged with 200,000 D2F2/E2 cells on day 21. (B) GPI-HER-2-VLPs did not protect against challenge with HER-2-negative D2F2 breast cancer cells. Vaccinated mice were challenged with 200,000 D2F2 (HER-2 negative) cells 7 days post boost. Tumor growth (left panel) and incidence (right panel) was measured.
Discussion
VLPs are nanoparticles that express antigens with a pattern of repetitive structures. The ordered and repetitive nature of antigen presentation on VLPs are suggested to enhance antibody production (Bachmann et al. 1993) by acting like pathogen associated molecular patterns (PAMPs), which produce danger signals activating both innate and adaptive immune responses (Jennings and Bachmann 2008). The particulate form and size of VLPs further allow for optimal uptake by APCs, in particular dendritic cells (Zhang et al. 2004). In addition, the nanosize of VLPs is likely to permit for direct transport of VLPs to the lymph nodes for further activation of immune cells (Cubas et al. 2009; Manolova et al. 2008). Notably, cross-presentation of VLP-expressing proteins is considered to activate both CD4+ helper T-cells and cytotoxic T lymphocytes (CTLs), and incubation of VLPs with splenocytes was shown to induce IgG2a producing plasma cell differentiation (Zhang et al. 2009). These characteristics might have contributed to making VLPs strong immunogens. Thus, the potent inherent immunogenicity of VLPs make them ideal candidates for antigen delivery.
Our approach described here made use of this inherent immunogenicity of influenza VLPs to induce immunity against the new antigen HER-2 protein transferred onto the nanoparticles. Herein, we show as a proof of concept that protein transfer is a simple and rapid modification system to introduce TAAs onto lipid enveloped VLPs for induction of antitumor immunity. Protein transfer mediated modification of VLPs by GPI-HER-2 is concentration dependent and occurred within 4 h. The level of incorporation of GPI-HER-2 on VLPs can be varied by simply adjusting the concentration of purified GPI-HER-2 added during protein transfer. After incorporation, HER-2 was expressed on the VLPs stably for at least one week at 4°C and even for 3 days at room temperature. We demonstrated that mice vaccinated with GPI-HER-2-VLPs induced strong anti-HER-2 specific IgG responses and protected mice against challenge with HER-2-expressing D2F2/E2 cells. Interestingly, GPI-HER-2 attached to the VLPs by protein transfer induced both Th1 and Th2-type anti-HER-2 antibody responses whereas GPI-HER-2 alone induced mostly Th2-type antibody responses. The Th1-type antibody induction by GPI-HER-2-VLPs is likely due to the intrinsic nature of the nanoparticulate VLPs that have been shown to enhance Th1 and Th2-type immune responses against viral antigens (Ko et al. 2014; Song et al. 2010; Zhang et al. 2009). These results suggest that delivering antigen attached to these nanoparticles by protein transfer has the ability to modulate immune responses toward a more dual Th1/Th2 phenotype that appears correlative with enhanced therapeutic efficacy. A dual Th1/Th2 phenotype response is highly desirable in a tumor immunotherapy setting, as a combination of Th1 and Th2-tupe responses has shown to be more effective in combating tumor growth (Hung et al. 1998; Kim and Cantor 2014; Nishimura et al. 1999).
The VLP-based delivery system described here has many advantages over other approaches to express TAAs on VLPs. When engineering chimeric VLPs to express tumor antigens by genetic means, the expression of tumor antigens on the VLPs cannot be easily controlled. Further, inclusion of tumor antigens may alter proteins intrinsic to the VLPs which may in turn influence the structural integrity of the VLPs. For generation of VLPs using the recombinant baculovirus (rBV) expression system, the multiplicity of infection (MOI) of rBVs for expression of viral capsid and envelop proteins have to be optimized to produce sufficient quantities of VLPs with high viral protein expression. Introducing foreign antigens such as TAAs onto VLPs using this rBV expression system, alters the optimized MOIs needed for viral protein expression and thus may lower VLP production. More importantly, post-translational modification of proteins expressed in insect cells is different from that of mammalian cells, which may limit proper conformation of mammalian cell-derived tumor antigens. Protein transfer would overcome these drawbacks and allow for easy modification of VLP surfaces after production. Further, protein transfer-mediated modification of enveloped VLPs does not involve any chemical changes (Peacey et al. 2007) thus preserving the immune stimulating properties of VLPs as well as antigenicity of the incorporated TAA. For use in protein transfer, the protein antigens are precisely modified at the C-terminus, which does not affect the functionality or antigenicity of the proteins. We found that protein transfer of HER-2 did not alter the antigenicity of viral proteins expressed on the VLPs, as GPI-HER-2-VLPs still induced VLP-specific serum IgG responses similar to unmodified VLPs (Figure 3C).
For protein transfer to incorporate antigens onto the surface of lipid bilayers, only a GPI-anchor attached to the antigen is needed (Selvaraj et al. 1987; Shashidharamurthy et al. 2012; Srivatsan et al. 2014; Zhang et al. 1992). Since more than one GPI-anchored protein can be incorporated onto VLPs by protein transfer simultaneously, this approach can be used to expand the repertoire of immunity induced to target multiple TAAs. Thus, protein transfer-mediated incorporation of TAAs onto influenza VLPs provides a quick and simple method for developing antigen-specific therapeutic vaccines to induce antitumor immune responses to treat cancer.
Acknowledgments
We thank W.Z. Wei (Wayne State University) for the D2F2 and D2F2/E2 tumor cell line and we thank Archana Boopathy for critical reading of the manuscript. This work was supported in part by NIH/NIAID grants 1 R01 CA138993 (P.S.), 1 F31 CA165632 (J.M.P.) and R01 AI093772 (S.M.K.) and R01 AI105170 (S.M.K.).
Dr. Selvaraj is a co-founder & equity holder of Metaclipse Therapeutics Corporation - a startup company formed to develop therapeutic cancer vaccines for humans using the protein transfer technology described here for which he is a co-inventor.
References
- Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262(5138):1448–51. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96(9):3102–8. [PubMed] [Google Scholar]
- Bumgarner GW, Zampell JC, Nagarajan S, Poloso NJ, Dorn AS, D'Souza MJ, Selvaraj P. Modified cell ELISA to determine the solubilization of cell surface proteins: Applications in GPI-anchored protein purification. J Biochem Biophys Methods. 2005;64(2):99–109. doi: 10.1016/j.jbbm.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Emtage P, Zhu Q, Foley R, Muller W, Hitt M, Gauldie J, Wan Y. Induction of ErbB-2/neu-specific protective and therapeutic antitumor immunity using genetically modified dendritic cells: enhanced efficacy by cotransduction of gene encoding IL-12. Gene Ther. 2001;8(4):316–23. doi: 10.1038/sj.gt.3301396. [DOI] [PubMed] [Google Scholar]
- Chen Z, Huang H, Chang T, Carlsen S, Saxena A, Marr R, Xing Z, Xiang J. Enhanced HER-2/neu-specific antitumor immunity by cotransduction of mouse dendritic cells with two genes encoding HER-2/neu and alpha tumor necrosis factor. Cancer Gene Ther. 2002;9(9):778–86. doi: 10.1038/sj.cgt.7700498. [DOI] [PubMed] [Google Scholar]
- Cobern L, Selvaraj P. An enzymatic method to determine receptor-mediated endocytosis. J Biochem Biophys Methods. 1995;30(4):249–55. doi: 10.1016/0165-022x(95)00013-3. [DOI] [PubMed] [Google Scholar]
- Cubas R, Zhang S, Kwon S, Sevick-Muraca EM, Li M, Chen C, Yao Q. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J Immunother. 2009;32(2):118–28. doi: 10.1097/CJI.0b013e31818f13c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas R, Zhang S, Li M, Chen C, Yao Q. Chimeric Trop2 virus-like particles: a potential immunotherapeutic approach against pancreatic cancer. J Immunother. 2011;34(3):251–63. doi: 10.1097/CJI.0b013e318209ee72. [DOI] [PubMed] [Google Scholar]
- Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R, Nisenbaum H, Pasha T, Xu M, Fox KR, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–52. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20(11):2624–32. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- Disis ML, Schiffman K, Guthrie K, Salazar LG, Knutson KL, Goodell V, dela Rosa C, Cheever MA. Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein--based vaccine. J Clin Oncol. 2004;22(10):1916–25. doi: 10.1200/JCO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18(1):244–51. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- Gallo P, Dharmapuri S, Nuzzo M, Maldini D, Iezzi M, Cavallo F, Musiani P, Forni G, Monaci P. Xenogeneic immunization in mice using HER2 DNA delivered by an adenoviral vector. Int J Cancer. 2005;113(1):67–77. doi: 10.1002/ijc.20536. [DOI] [PubMed] [Google Scholar]
- Holmes JP, Benavides LC, Gates JD, Carmichael MG, Hueman MT, Mittendorf EA, Murray JL, Amin A, Craig D, von Hofe E, et al. Results of the first phase I clinical trial of the novel II-key hybrid preventive HER-2/neu peptide (AE37) vaccine. J Clin Oncol. 2008;26(20):3426–33. doi: 10.1200/JCO.2007.15.7842. [DOI] [PubMed] [Google Scholar]
- Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188(12):2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389(5):521–36. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- Kang SM, Yoo DG, Lipatov AS, Song JM, Davis CT, Quan FS, Chen LM, Donis RO, Compans RW. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS One. 2009;4(3):e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Cantor H. CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer Immunol Res. 2014;2(2):91–8. doi: 10.1158/2326-6066.CIR-13-0216. [DOI] [PubMed] [Google Scholar]
- Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, Huang LQ, Murata S, Sgouros G, Emens LA, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;118(5):1700–11. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EJ, Kwon YM, Lee JS, Hwang HS, Yoo SE, Lee YN, Lee YT, Kim MC, Cho MK, Lee YR, et al. Virus-like nanoparticle and DNA vaccination confers protection against respiratory syncytial virus by modulating innate and adaptive immune cells. Nanomedicine. 2014 doi: 10.1016/j.nano.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low MG, Saltiel AR. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988;239(4837):268–75. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Lua LH, Connors NK, Sainsbury F, Chuan YP, Wibowo N, Middelberg AP. Bioengineering virus-like particles as vaccines. Biotechnol Bioeng. 2014;111(3):425–40. doi: 10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–13. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- McHugh RS, Ahmed SN, Wang YC, Sell KW, Selvaraj P. Construction, purification, and functional incorporation on tumor cells of glycolipid-anchored human B7-1 (CD80) Proc Natl Acad Sci U S A. 1995;92(17):8059–63. doi: 10.1073/pnas.92.17.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RS, Nagarajan S, Wang YC, Sell KW, Selvaraj P. Protein transfer of glycosylphosphatidylinositol-B7-1 into tumor cell membranes: a novel approach to tumor immunotherapy. Cancer Res. 1999;59(10):2433–7. [PubMed] [Google Scholar]
- Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother. 2008;57(10):1511–21. doi: 10.1007/s00262-008-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan S, Selvaraj P. Glycolipid-anchored IL-12 expressed on tumor cell surface induces antitumor immune response. Cancer Res. 2002;62(10):2869–74. [PubMed] [Google Scholar]
- Nagarajan S, Selvaraj P. Human tumor membrane vesicles modified to express glycolipid-anchored IL-12 by protein transfer induce T cell proliferation in vitro: a potential approach for local delivery of cytokines during vaccination. Vaccine. 2006;24(13):2264–74. doi: 10.1016/j.vaccine.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190(5):617–27. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, Ohta A, Koda T, Nishimura S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(Suppl):S52–61. doi: 10.1007/pl00014051. [DOI] [PubMed] [Google Scholar]
- Park JW, Melisko ME, Esserman LJ, Jones LA, Wollan JB, Sims R. Treatment with autologous antigen-presenting cells activated with the HER-2 based antigen Lapuleucel-T: results of a phase I study in immunologic and clinical activity in HER-2 overexpressing breast cancer. J Clin Oncol. 2007;25(24):3680–7. doi: 10.1200/JCO.2006.10.5718. [DOI] [PubMed] [Google Scholar]
- Peacey M, Wilson S, Baird MA, Ward VK. Versatile RHDV virus-like particles: incorporation of antigens by genetic modification and chemical conjugation. Biotechnol Bioeng. 2007;98(5):968–77. doi: 10.1002/bit.21518. [DOI] [PubMed] [Google Scholar]
- Peethambaram PP, Melisko ME, Rinn KJ, Alberts SR, Provost NM, Jones LA, Sims RB, Lin LR, Frohlich MW, Park JW. A phase I trial of immunotherapy with lapuleucel-T (APC8024) in patients with refractory metastatic tumors that express HER-2/neu. Clin Cancer Res. 2009;15(18):5937–44. doi: 10.1158/1078-0432.CCR-08-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechocki MP, Pilon SA, Kelly C, Wei WZ. Degradation signals in ErbB-2 dictate proteasomal processing and immunogenicity and resist protection by cis glycine-alanine repeat. Cell Immunol. 2001a;212(2):138–49. doi: 10.1006/cimm.2001.1853. [DOI] [PubMed] [Google Scholar]
- Piechocki MP, Pilon SA, Wei WZ. Complementary antitumor immunity induced by plasmid DNA encoding secreted and cytoplasmic human ErbB-2. J Immunol. 2001b;167(6):3367–74. doi: 10.4049/jimmunol.167.6.3367. [DOI] [PubMed] [Google Scholar]
- Pilon SA, Piechocki MP, Wei WZ. Vaccination with cytoplasmic ErbB-2 DNA protects mice from mammary tumor growth without anti-ErbB-2 antibody. J Immunol. 2001;167(6):3201–6. doi: 10.4049/jimmunol.167.6.3201. [DOI] [PubMed] [Google Scholar]
- Poloso NJ, Nagarajan S, Bumgarner GW, Selvaraj P. Development of therapeutic vaccines by direct modification of cell membranes from surgically removed human tumor tissue with immunostimulatory molecules. Vaccine. 2001;19(15-16):2029–38. doi: 10.1016/s0264-410x(00)00424-2. [DOI] [PubMed] [Google Scholar]
- Poloso NJ, Nagarajan S, Mejia-Oneta JM, Selvaraj P. GPI-anchoring of GM-CSF results in active membrane-bound and partially shed cytokine. Mol Immunol. 2002;38(11):803–16. doi: 10.1016/s0161-5890(02)00005-6. [DOI] [PubMed] [Google Scholar]
- Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, Hung G, Robinson RA, Harris C, El-Naggar A, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15(8):2894–904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23(50):5751–9. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- Quan FS, Sailaja G, Skountzou I, Huang C, Vzorov A, Compans RW, Kang SM. Immunogenicity of virus-like particles containing modified human immunodeficiency virus envelope proteins. Vaccine. 2007;25(19):3841–50. doi: 10.1016/j.vaccine.2007.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, Porcedda P, Boggio K, Smorlesi A, Lollini PL, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165(9):5133–42. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Morrison BJ, Burke JD, Park JM, Terabe M, Janik JE, Forni G, Berzofsky JA, Morris JC. Vaccination by genetically modified dendritic cells expressing a truncated neu oncogene prevents development of breast cancer in transgenic mice. Cancer Res. 2004;64(21):8022–8. doi: 10.1158/0008-5472.CAN-03-3442. [DOI] [PubMed] [Google Scholar]
- Selvaraj P, Dustin ML, Silber R, Low MG, Springer TA. Deficiency of lymphocyte function-associated antigen 3 (LFA-3) in paroxysmal nocturnal hemoglobinuria. Functional correlates and evidence for a phosphatidylinositol membrane anchor. J Exp Med. 1987;166(4):1011–25. doi: 10.1084/jem.166.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharamurthy R, Bozeman EN, Patel J, Kaur R, Meganathan J, Selvaraj P. Immunotherapeutic strategies for cancer treatment: a novel protein transfer approach for cancer vaccine development. Med Res Rev. 2012;32(6):1197–219. doi: 10.1002/med.20237. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405(1):165–75. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsan S, Patel JM, Bozeman EN, Imasuen IE, He S, Daniels D, Selvaraj P. Allogeneic tumor cell vaccines: the promise and limitations in clinical trials. Hum Vaccin Immunother. 2014;10(1):52–63. doi: 10.4161/hv.26568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegerstedt K, Lindencrona JA, Curcio C, Andreasson K, Tullus C, Forni G, Dalianis T, Kiessling R, Ramqvist T. A single vaccination with polyomavirus VP1/VP2Her2 virus-like particles prevents outgrowth of HER-2/neu-expressing tumors. Cancer Res. 2005;65(13):5953–7. doi: 10.1158/0008-5472.CAN-05-0335. [DOI] [PubMed] [Google Scholar]
- Thery JC, Spano JP, Azria D, Raymond E, Penault Llorca F. Resistance to human epidermal growth factor receptor type 2-targeted therapies. Eur J Cancer. 2014;50(5):892–901. doi: 10.1016/j.ejca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Wei WZ, Shi WP, Galy A, Lichlyter D, Hernandez S, Groner B, Heilbrun L, Jones RF. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int J Cancer. 1999;81(5):748–54. doi: 10.1002/(sici)1097-0215(19990531)81:5<748::aid-ijc14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Zhang F, Schmidt WG, Hou Y, Williams AF, Jacobson K. Spontaneous incorporation of the glycosyl-phosphatidylinositol-linked protein Thy-1 into cell membranes. Proc Natl Acad Sci U S A. 1992;89(12):5231–5. doi: 10.1073/pnas.89.12.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Li M, Chen C, Yao Q. SHIV virus-like particles bind and activate human dendritic cells. Vaccine. 2004;23(2):139–47. doi: 10.1016/j.vaccine.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cubas R, Li M, Chen C, Yao Q. Virus-like particle vaccine activates conventional B2 cells and promotes B cell differentiation to IgG2a producing plasma cells. Mol Immunol. 2009;46(10):1988–2001. doi: 10.1016/j.molimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang L, Liu Y, Chen X, Liu Q, Jia J, Yang T, Qiu S, Ma G. Immune responses to vaccines involving a combined antigen-nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials. 2014;35(23):6086–97. doi: 10.1016/j.biomaterials.2014.04.022. [DOI] [PubMed] [Google Scholar]


