Abstract
In vivo imaging is increasingly being utilized in studies investigating stem cell-based treatments for neurological disorders. Direct labeling is used in preclinical and clinical studies to track the fate of transplanted cells. To further determine cell viability, experimental studies are able to take advantage of reporter gene technologies. Structural and functional brain imaging can also be used alongside cell imaging as biomarkers of treatment efficacy. Furthermore, it is possible that new imaging techniques could be used to monitor functional integration of stem cell-derived cells with the host nervous system. In this review, we examine recent developments in these areas and identify promising directions for future research at the interface of stem cell therapies and neuroimaging.
Introduction
Stem cell-based therapies promise to revolutionize the treatment of neurological diseases. By homing to sites of injury and differentiating into mature neurons, glia or oligodendrocytes, stem cells have the ability to treat a wide range of disorders. However, many issues in regard to both safety and efficacy need to be resolved before their true potential can be realized. Imaging is likely to play a key role in addressing these problems.
There are currently many ongoing and completed clinical trials in the area of cell therapies for neurological diseases, but most have so far shown only modest improvements in patient outcome [1,2]. Systematic reviews of stem cell therapies for experimental stroke [3] and experimental traumatic spinal cord injury [4] concluded that these therapies are likely to confer functional benefits, as quantified by neurobehavioral outcomes. A large proportion of the beneficial effects reported from cell-based therapies are likely to be due to transplanted cells releasing growth factors and cytokines, which stimulate endogenous repair mechanisms [5], rather than due to their differentiation into fully functioning neurons. Nevertheless, the integration of stem cell-derived neurons into host circuitry is what makes these therapies so promising, and a better understanding of this process will help lead to the next generation of stem cell-based therapies. Along these lines, studies in Parkinson's disease models have demonstrated the capacity of engrafted stem-cell derived neurons to generate synapses with host neurons [6] and exhibit spontaneous action potentials in brain slices [7]. This has also been demonstrated using human embryonic stem (ES) cell-derived neural stem cells [8] and human adult neuroepithelial-like stem cells in rodent models of stroke [9].
In both preclinical and clinical studies, imaging technologies are essential for monitoring the fate of transplanted cells over time. Techniques such as direct labeling and reporter gene technology (by genetically engineering stem cells before transplantation) enable the migration, survival, and viability of cells to be tracked upon transplantation (Figure 1a). Imaging can also be used as a biomarker for functional improvement (Figure 1b) or to detect adverse effects such as tumor growth [10], teratoma development from nondifferentiated cells [11], and inflammation due to immune rejection.
Figure 1.
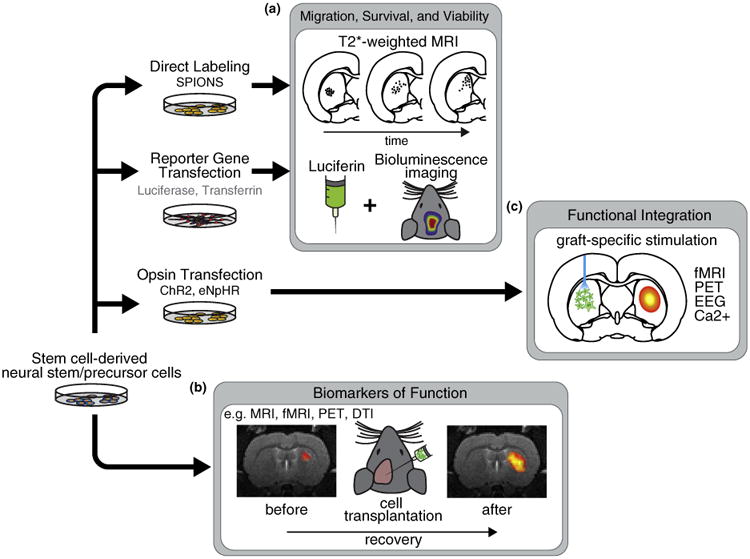
Imaging cell-based therapies for neurological disorders. (a) Cell migration and viability can be assessed by direct labeling, for example, with super paramagnetic iron oxide nanoparticles (SPIONs), or the insertion of reporter genes, for example, luciferase for bioluminescence. (b) In the long-term, imaging can be used as a biomarker of functional improvement. For example, functional magnetic resonance imaging (fMRI) can be combined with electrical, pharmacological or task-related stimuli. PET has been applied to assess the expression of specific receptors or transporters. Diffusion tensor imaging (DTI) has recently been used to assess myelin formation (see section on ‘Biomarkers of function’). (c) Cells can be modified to express light sensitive opsins such as channelrhodopsin-2 (ChR2) or halorhodopsin (eNpHR). Cells are then differentiated and transplanted to the brain. Over time, cells may become integrated with host circuitry and form functional synapses with remote regions. In combination with simultaneous functional imaging or EEG, different stimulation paradigms could be used to interrogate the specific dynamics of newly formed circuits.
Direct labeling
Tracking the fate of stem cells in vivo can be performed by using direct labeling or via the introduction of reporter genes. Direct labeling techniques are based on labeling the cells before transplantation with imaging agents such as radiotracers for nuclear imaging [12] and superpara-magnetic iron oxide nanoparticles (SPIONs) [13] or polymeric conjugates of gadolinium [14] for detection with magnetic resonance imaging (MRI). Of these methods, direct labeling with iron oxide is the preferred technique for human trials [15,16], since radioisotopes with short half-life cannot be tracked over extended periods and there are significant safety issues associated with using longer-lived radioisotopes in humans.
Despite these clinical barriers, nuclear imaging of radio-labeled cells has shown promising results in animal studies. For example, a recent study demonstrated that nuclear imaging had greater sensitivity compared to clinical field-strength MRI for detecting radiolabeled stem cells co-labeled with iron oxide in the canine heart [17]. However, nuclear imaging has limited spatial resolution. Therefore for smaller animals, partial volume effects (which occur when the object is smaller than the imaging resolution) will be more evident, thereby reducing this sensitivity. While partial volume effects are also a concern for MRI, the use of high magnetic field strengths for preclinical studies permits higher resolution for small animal MRI compared to clinical imaging. As a result, the sensitivity of detecting iron oxide labeled cells using high-field MRI could be comparable to the nuclear imaging modalities.
Despite the benefits of labeling cells with iron oxide, a major disadvantage of this contrast agent is that it causes signal dropouts, rather than signal enhancement on MRI images. This can be problematic for quantification, as it means there is often not a clear relationship between iron concentration and signal intensity. Although research is in its infancy, new contrast agents designed to enhance the MRI signal could help to circumvent these issues [18]. A second disadvantage of using iron oxide is that it is not possible to distinguish between iron particles within the transplanted cells and those particles which have been taken up by macrophages [19].
As recently demonstrated in a proof-of-concept study by Thu et al. [20•], another method is to directly label cells with self-assembling nanocomplexes, which are aggregates of iron oxide nanoparticles. These nanocomplexes — which are formed by combining three US Food and Drug Administration (FDA)-approved compounds — may facilitate the rapid translation of more sensitive iron labeling to the clinic. Nevertheless, further studies will be required to determine whether cell labeling with these nanocomplexes is feasible for human studies.
Reporter genes
Direct labeling techniques suffer from a lessening of signal over time as the imaging agent inevitably gets broken down. In order to circumvent these issues, stem cells can be genetically modified to express reporter genes. Tracking cells via the insertion of reporter genes also has the advantage of revealing information regarding cell viability. The most commonly used reporter genes are the luciferases for bioluminescence imaging [21,22]. Bioluminescence suffers from limited tissue penetration and poor spatial resolution but offers excellent sensitivity when used in small animals. Other reporter gene strategies include the use of ferritin-type reporter genes which can be used to sequester paramagnetic iron for detection on MRI images [23]. Currently, the sensitivity of ferritin-type reporter genes is limited compared to both bioluminescence and the direct labeling methods [24]. At this time, reporter genes for nuclear imaging (which are typically based on the herpes simplex virus type 1 thymidine kinase (HSV1-tk) or the sodium iodide symporter genes) have limited application in the brain due to their low rates of diffusion across the intact blood-brain barrier [25,26]. However, recent efforts have combined direct labeling with reporter gene methods to provide complementary information. For example, Boehm-Sturm et al. directly labeled ES cells with perfluorocarbon nanoparticles, which enabled 19F magnetic resonance imaging to be performed alongside bioluminescence to image stem cell graft location and survival after an ischemic injury [27]. While reporter genes are not yet deemed to be safe for use in clinic, recent advances may help to overcome these hurdles (for further information see Nguyen et al. [28]).
Biomarkers of function
Biomarkers of treatment response can be invaluable when assessing the effectiveness of cell-based treatments. Functional neuroimaging can not only be used to achieve this, but can also offer insights into possible mechanisms for cognitive improvements. Positron emission tomography (PET), for example, is a powerful functional imaging technique that can assess receptor distribution, metabolic activity [29] or rates of ligand uptake. The 6-[18F]fluoro-l-3,4-dihydroxyphenylalanine ([18F]FDOPA) tracer has been used to image increases in presynaptic dopamine synthesis [30] after transplantation of predifferentiated dopamine-synthesizing neural stem cells in a non-human primate model of Parkinson's disease. [18F]FDOPA was also used to detect improvements in dopaminergic function after Parkinson's disease patients were transplanted with fetal mesencephalic tissue [31]. Another ligand specific to the dopaminergic system is the PET tracer [11C]2β-carbomethoxy-3β-(4-fluorophenyl) tropane ([11C]CFT), which targets the presynaptic dopamine transporter. This tracer was tested in the unilateral 6-hydroxydopamine (6-OHDA) Parkinson's disease rat model after embryonic stem cell implantation [32]. Nine weeks after the ES cell engraftment, there was a dramatic difference in ligand binding in the striatum (75–90% of the intact hemisphere) relative to the sham surgery (<25% of the intact hemisphere). This same study used intravenous iron oxide nanoparticles combined with MRI to measure cerebral blood volume (CBV) during infusion of amphetamine. Amphetamine, which causes dopamine release, leads to measurable increases in CBV that are absent in the lesioned striatum. The response to amphetamines was partially restored in rats with surviving ES cell-grafts after excluding those with teratoma-like structures, thus demonstrating a plausible mechanism for the behavioral improvements in the implanted animals.
Functional MRI (fMRI), which measures the blood oxygen level dependent (BOLD) signal as a marker of neural activity, can also be used as a biomarker of functional improvement in response to cell-based therapies. The fMRI response can be evoked by direct modulation of the nervous system using pharmacological agents, various forms of sensory stimuli, or even cognitive tasks. In rodents, electrical stimulation of the forepaw or hindpaw is a common experimental paradigm and results in an observable BOLD signal change in the primary somatosensory cortex. This has been used as an outcome measure in a study focusing on intraspinal neural stem cell grafts [33] and another study which looked at intravenous administration of mesenchymal stem cells after stroke [34•]. Both of these investigations found an enhanced BOLD signal change in the treated group compared to controls. This therefore suggests that fMRI can be used alongside cognitive and behavioral tests to assess the efficacy of cell-based therapies.
Another MRI acquisition technique, diffusion tensor imaging (DTI), provides information about white matter microstructure. DTI is based on observing the magnitude and direction of the diffusion of water molecules. Within white matter tracts, the diffusion of water is restricted by cell membranes, which can be detected using DTI. As Gupta et al. have recently shown, this makes it ideal as a non-invasive marker of myelin development after neural stem cell engraftment in patients with hypomyelination [35••]. Conventional structural MRI can be combined with more advanced analysis methods such as cortical thickness measures and voxel-based morphometry (VBM) [36] to monitor patient outcome after cell-based therapy. In a phase 2 randomized controlled clinical trial, patients with multiple system atrophy who were intra-arterially administered mesenchymal stem cells showed less extensive cortical thinning and loss of VBM-assessed gray matter density than controls [37••,38]. Moreover, in the placebo group, cortical thinning of the frontal and posterior temporal areas correlated with the cognitive assessment score.
Functional integration
Current cell imaging techniques can be used to evaluate the degree of cell retention and assess cell viability. As discussed in the previous section, functional and structural imaging can be used as biomarkers for monitoring therapeutic interventions. One issue with using biomarkers or surrogate endpoints is that because they are indirect measures, there may be numerous mechanisms which can lead to biomarker changes [39]. For this reason, they can often be difficult to interpret and therefore multiple different markers are often needed to help elucidate therapeutic mechanisms of action. Currently available imaging techniques have limited potential for assessing the functional integration of stem cell derived neurons into neural networks.
In order to assess the functional integration of stem cell derived neurons into the host neural circuitry, it might be possible to perform in vivo imaging while directly modulating engrafted cells. Preliminary work in this area using electrophysiological techniques has been fundamental in demonstrating that engrafted stem cells form working synapses during the process of functional integration. For example, patch-clamp recordings can be performed in slice preparations on cells transfected to express fluorescent reporter genes [7–9,40,41]. However, to understand how engrafted cells causally influence downstream targets, new imaging approaches will be needed to measure these types of responses in the living animal. This could be achieved by directly modulating the activity of engrafted cells during non-invasive functional readouts such as electroencephalography (EEG), fMRI or PET.
While electrical stimulation has traditionally been used to interrogate neural circuits in vivo, this technique is unable to distinguish between host tissue and engrafted cells. As a result, the measured outcome may not be attributable solely to the engrafted cells' activity. Recent developments in the field of optogenetics have overcome this barrier and shown that stem cell-derived neurons can be selectively controlled both in vitro [42–46] and in vivo [47••] in animal models. Using this technique, stem cells are transduced to express a light-sensitive ion channel such as channelrho-dopsin-2 (ChR2) [48] or halorhodopsin [49], so that their activity — but not the activity of host cells — can be selectively modulated with light. Bryson et al. [47••] demonstrated that ChR2 motor neurons derived from ES cells can reinnervate denervated muscles after sciatic nerve ligation and that these neurons can be selectively stimulated to cause muscle contractions. It remains to be seen whether a similar approach can be achieved in models of neurological disorders. For example, engrafted cells could be silenced using halorhodopsins to reveal the degree to which they contribute to cognitive improvements. While the prospect of selectively controlling engrafted cells is astounding in itself, its combination with imaging is expected to provide great insight into the functional integration process. Already, technical developments have enabled optogenetic stimulation of native cells to be performed simultaneously with large-scale readouts of brain activity using fMRI [50–54]. Thus, combining selective optical stimulation of engrafted cells with whole-brain imaging will likely be an important direction for future research (Figure 1c). This type of imaging can provide important information on the functional engagement of engrafted cells with host circuitry, and — given its non-invasive nature — can be used to assess its relationship with cognitive outcomes.
Conclusions
Stem cell therapies for neurological disorders have rapidly been translated to the clinic. Despite this, only a few studies have shown that engrafted cells can integrate into existing neural networks and the mechanisms of functional improvement remain largely unknown. Imaging of stem cells using direct labeling methods and reporter genes has led to important discoveries regarding cell migration, retention and survival. At the same time, structural and functional neuroimaging can be used as biomarkers for therapy monitoring. New imaging techniques are needed to more closely investigate the mechanisms by which stem cell therapies can improve neurological function.
Acknowledgments
This work was supported by the NIH/NIBIB R00 Award (4R00EB008738), Okawa Foundation Research Grant Award, NIH Director's New Innovator Award (1DP2OD007265), the NSF CAREER Award (1056008), and the Alfred P. Sloan Research Fellowship. The authors would like to thank Johannes Riegler for helpful comments and suggestions.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Morales PL, Revilla A, Ocana I, Gonzalez C, Sainz P, McGuire D, Liste I. Progress in stem cell therapy for major human neurological disorders. Stem Cell Rev. 2013;9:685–699. doi: 10.1007/s12015-013-9443-6. [DOI] [PubMed] [Google Scholar]
- 3.Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, Macleod MR. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. Int J Stroke. 2012;7:582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- 4.Antonic A, Sena ES, Lees JS, Wills TE, Skeers P, Batchelor PE, Macleod MR, Howells DW. Stem cell transplantation in traumatic spinal cord injury: a systematic review and meta-analysis of animal studies. PLoS Biol. 2013;11:e1001738. doi: 10.1371/journal.pbio.1001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 7.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, Wu JC, Steinberg GK. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, Monni E, Tornero D, Ahlenius H, Ladewig J, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30:1120–1133. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 10.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seminatore C, Polentes J, Ellman D, Kozubenko N, Itier V, Tine S, Tritschler L, Brenot M, Guidou E, Blondeau J, et al. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke. 2010;41:153–159. doi: 10.1161/STROKEAHA.109.563015. [DOI] [PubMed] [Google Scholar]
- 12.Wolfs E, Struys T, Notelaers T, Roberts SJ, Sohni A, Bormans G, Van Laere K, Luyten FP, Gheysens O, Lambrichts I, et al. 18F-FDG labeling of mesenchymal stem cells and multipotent adult progenitor cells for PET imaging: effects on ultrastructure and differentiation capacity. J Nucl Med. 2013;54:447–454. doi: 10.2967/jnumed.112.108316. [DOI] [PubMed] [Google Scholar]
- 13.Hoehn M, Kustermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Focking M, Arnold H, Hescheler J, Fleischmann BK, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modo M, Beech JS, Meade TJ, Williams SC, Price J. A chronic 1 year assessment of MRI contrast agent-labelled neural stem cell transplants in stroke. Neuroimage. 2009;47 (Suppl. 2):T133–T142. doi: 10.1016/j.neuroimage.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Zhou L, Xing Wu F. Tracking neural stem cells in patients with brain trauma. Int N Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]
- 17.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo WS, Lee JH, Sun X, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, McConnell MV, Nishimura DG, et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat Mater. 2006;5:971–976. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Suzuki Y, Huang M, Cao F, Xie X, Connolly AJ, Yang PC, Wu JC. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26:864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Thu MS, Bryant LH, Coppola T, Jordan EK, Budde MD, Lewis BK, Chaudhry A, Ren J, Varma NR, Arbab AS, et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat Med. 2012;18:463–467. doi: 10.1038/nm.2666. This report details the synthesis of iron oxide nanocomplexes (aggregates) using clinically approved iron oxide nanoparticles (ferumoxytol), heparin and protamine. The preliminary in vivo data suggest that these nanocomplexes could provide added sensitivity over ferumoxytol alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Y, Walczak P, Bulte JW. Comparison of red-shifted firefly luciferase Ppy RE9 and conventional Luc2 as bioluminescence imaging reporter genes for in vivo imaging of stem cells. J Biomed Opt. 2012;17:016004. doi: 10.1117/1.JBO.17.1.016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernau K, Lewis CM, Petelinsek AM, Benink HA, Zimprich CA, Meyerand ME, Suzuki M, Svendsen CN. In vivo tracking of human neural progenitor cells in the rat brain using bioluminescence imaging. J Neurosci Methods. 2014;228:67–78. doi: 10.1016/j.jneumeth.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iordanova B, Ahrens ET. In vivo magnetic resonance imaging of ferritin-based reporter visualizes native neuroblast migration. Neuroimage. 2012;59:1004–1012. doi: 10.1016/j.neuroimage.2011.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vande Velde G, Rangarajan JR, Toelen J, Dresselaers T, Ibrahimi A, Krylychkina O, Vreys R, Van der Linden A, Maes F, Debyser Z, et al. Evaluation of the specificity and sensitivity of ferritin as an MRI reporter gene in the mouse brain using lentiviral and adeno-associated viral vectors. Gene Ther. 2011;18:594–605. doi: 10.1038/gt.2011.2. [DOI] [PubMed] [Google Scholar]
- 25.Min JJ, Gambhir SS. Gene therapy progress and prospects: noninvasive imaging of gene therapy in living subjects. Gene Ther. 2004;11:115–125. doi: 10.1038/sj.gt.3302191. [DOI] [PubMed] [Google Scholar]
- 26.Daadi MM, Hu S, Klausner J, Li Z, Sofilos M, Sun G, Wu JC, Steinberg GK. Imaging neural stem cell graft-induced structural repair in stroke. Cell Transplant. 2013;22:881–892. doi: 10.3727/096368912X656144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boehm-Sturm P, Aswendt M, Minassian A, Michalk S, Mengler L, Adamczak J, Mezzanotte L, Lowik C, Hoehn M. A multi-modality platform to image stem cell graft survival in the naive and stroke-damaged mouse brain. Biomaterials. 2014;35:2218–2226. doi: 10.1016/j.biomaterials.2013.11.085. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visnyei K, Tatsukawa KJ, Erickson RI, Simonian S, Oknaian N, Carmichael ST, Kornblum HI. Neural progenitor implantation restores metabolic deficits in the brain following striatal quinolinic acid lesion. Exp Neurol. 2006;197:465–474. doi: 10.1016/j.expneurol.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Muramatsu S, Okuno T, Suzuki Y, Nakayama T, Kakiuchi T, Takino N, Iida A, Ono F, Terao K, Inoue N, et al. Multitracer assessment of dopamine function after transplantation of embryonic stem cell-derived neural stem cells in a primate model of Parkinson's disease. Synapse. 2009;63:541–548. doi: 10.1002/syn.20634. [DOI] [PubMed] [Google Scholar]
- 31.Piccini P, Lindvall O, Bjorklund A, Brundin P, Hagell P, Ceravolo R, Oertel W, Quinn N, Samuel M, Rehncrona S, et al. Delayed recovery of movement-related cortical function in Parkinson's disease after striatal dopaminergic grafts. Ann Neurol. 2000;48:689–695. [PubMed] [Google Scholar]
- 32.Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 34•.Suzuki J, Sasaki M, Harada K, Bando M, Kataoka Y, Onodera R, Mikami T, Wanibuchi M, Mikuni N, Kocsis JD, et al. Bilateral cortical hyperactivity detected by fMRI associates with improved motor function following intravenous infusion of mesenchymal stem cells in a rat stroke model. Brain Res. 2013;1497:15–22. doi: 10.1016/j.brainres.2012.12.028. This paper shows that intravenous administration of mesenchymal stem results in improved motor function compared to the control group and also that a bilateral fMRI response to electrical forepaw stimulation appears to be a more powerful predictor of functional improvement than structural MRI. The mechanisms for this are largely unknown. [DOI] [PubMed] [Google Scholar]
- 35••.Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, et al. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. The aim of this study was to evaluate the safety of neural stem cells in patients with Pelizaeus-Merzbacher disease with hypomyelination of the central nervous system. A secondary aim was to detect evidence of myelin formation after the surgical procedure. Diffusion tensor imaging was performed at 6 time points over the course of a year. The study included 4 subjects and there was evidence of increased fractional anisotropy, which is suggestive of myelin development. This study exemplifies the use of imaging markers for therapy response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashburner J, Friston KJ. Voxel-based morphometry — the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 37••.Sunwoo MK, Yun HJ, Song SK, Ham JH, Hong JY, Lee JE, Lee HS, Sohn YH, Lee JM, Lee PH. Mesenchymal stem cells can modulate longitudinal changes in cortical thickness and its related cognitive decline in patients with multiple system atrophy. Front Aging Neurosci. 2014:6. doi: 10.3389/fnagi.2014.00118. This paper presents the 1-year follow-up data from a phase 2 randomized controlled trial of mesenchymal stem cell treatment in patients with multiple system atrophy. The results reveal more extensive cortical thinning in the placebo group compared to the treatment group. This study highlights the power of conventional T1-weighted MRI for therapy monitoring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee PH, Lee JE, Kim HS, Song SK, Lee HS, Nam HS, Cheong JW, Jeong Y, Park HJ, Kim DJ, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- 39.Cross DJ, Minoshima S. Perspectives on assessment of stem cell therapy in stroke by 18F-FDG PET. J Nucl Med. 2013;54:668–669. doi: 10.2967/jnumed.112.118380. [DOI] [PubMed] [Google Scholar]
- 40.Wernig M, Benninger F, Schmandt T, Rade M, Tucker KL, Bussow H, Beck H, Brustle O. Functional integration of embryonic stem cell-derived neurons in vivo. J Neurosci. 2004;24:5258–5268. doi: 10.1523/JNEUROSCI.0428-04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J, Bali C, Orduz D, Bilheu A, Herpoel A, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Stroh A, Tsai HC, Wang LP, Zhang F, Kressel J, Aravanis A, Santhanam N, Deisseroth K, Konnerth A, Schneider MB. Tracking stem cell differentiation in the setting of automated optogenetic stimulation. Stem Cells. 2011;29:78–88. doi: 10.1002/stem.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tonnesen J, Parish CL, Sorensen AT, Andersson A, Lundberg C, Deisseroth K, Arenas E, Lindvall O, Kokaia M. Functional integration of grafted neural stem cell-derived dopaminergic neurons monitored by optogenetics in an in vitro Parkinson model. PLoS ONE. 2011;6:e17560. doi: 10.1371/journal.pone.0017560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang SJ, Weng CH, Xu HW, Zhao CJ, Yin ZQ. Effect of optogenetic stimulus on the proliferation and cell cycle progression of neural stem cells. J Membr Biol. 2014;247:493–500. doi: 10.1007/s00232-014-9659-7. [DOI] [PubMed] [Google Scholar]
- 45.Weick JP, Johnson MA, Skroch SP, Williams JC, Deisseroth K, Zhang SC. Functional control of transplantable human ESC-derived neurons via optogenetic targeting. Stem Cells. 2010;28:2008–2016. doi: 10.1002/stem.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, Fleischmann BK, Sasse P. Optogenetic control of heart muscle in vitro and in vivo. Nat Methods. 2010;7:897–900. doi: 10.1038/nmeth.1512. [DOI] [PubMed] [Google Scholar]
- 47••.Bryson JB, Machado CB, Crossley M, Stevenson D, Bros-Facer V, Burrone J, Greensmith L, Lieberam I. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science. 2014;344:94–97. doi: 10.1126/science.1248523. This report describes the implantation of channelrhodopsin-2 expressing embryonic stem cell-derived motor neurons into partially denervated branches of the sciatic nerve in adult mice. The implanted cells innervated hind-limb muscles, which permitted optical control of muscle function. This work highlights how optogenetic technology can be used to specifically stimulate engrafted cells and observe the functional response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 49.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, Fenno LE, Ramakrishnan C, Deisseroth K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desai M, Kahn I, Knoblich U, Bernstein J, Atallah H, Yang A, Kopell N, Buckner RL, Graybiel AM, Moore CI, et al. Mapping brain networks in awake mice using combined optical neural control and fMRI. J Neurophysiol. 2011;105:1393–1405. doi: 10.1152/jn.00828.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang Z, Lee JH. High-throughput optogenetic functional magnetic resonance imaging with parallel computations. J Neurosci Methods. 2013;218:184–195. doi: 10.1016/j.jneumeth.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JH. Informing brain connectivity with optogenetic functional magnetic resonance imaging. Neuroimage. 2012;62:2244–2249. doi: 10.1016/j.neuroimage.2012.01.116. [DOI] [PubMed] [Google Scholar]
- 54.Lee JH. Tracing activity across the whole brain neural network with optogenetic functional magnetic resonance imaging. Front Neuroinform. 2011;5:21. doi: 10.3389/fninf.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]


