Abstract
Background
The effect of increased age on the induction of oral tolerance by low-dose antigen feeding and its effect on the response to antigen airway challenge in aged mice have not been well characterized.
Objective
To determine whether oral tolerance can be induced in aged mice and its impact on the development of allergic airway inflammation.
Methods
Younger (6 weeks old) and aged (18 months old) mice were fed ovalbumin (OVA) prior to sensitization to induce antigen tolerance. Serum antigen-specific immunoglobulins (Igs), bronchoalveolar lavage fluid (BALF), lung histology, enumeration of CD4 + Foxp3+ Treg cells, and airway hyperresponsiveness (AHR) were determined after the final antigen challenge.
Results
Feeding antigen to aged mice prior to sensitization induced oral tolerance as determined by a decrease in antigen-specific IgE and IgG1; however, the effect was greater in younger mice. Induction of oral tolerance was associated with a greater increase in airway Treg cells in the younger mice. Despite these differences, oral tolerance significantly suppressed features of asthma in aged mice, including BALF total cell and eosinophil numbers, cytokine production, and AHR.
Conclusions
Aged mice developed oral tolerance to antigen, which suppressed several features of allergic airway inflammation.
Keywords: aging, asthma, murine model, oral tolerance
Introduction
Tolerance is defined as inhibiting the immune response to an antigen by previous exposure to the antigen (1). The mechanisms underlying tolerance induction depend upon the antigen dose (low or high) (2–5) and its route of administration (oral, nasal, or intravenous) (6–9). Feeding low-dose antigen induces expansion of regulatory cells and/or downregulation of Th2 like cytokines, whereas high-dose antigen feeding induces anergy or deletion of antigen-specific T-cells. Oral tolerance (using low and high dose antigen) has been well studied in younger mouse models (10, 11). Few studies have investigated tolerance level in aged mice; however, most have focused upon induction of oral tolerance with high dose of antigen and have suggested that induction of tolerance is impaired in these mice (12, 13). Additionally, in the younger mice, oral tolerance to antigen decreases several features of the allergic airway response including airway hyperresponsiveness (AHR), airway eosinophilia, and mucus deposition (9, 14), but, whether this attenuates the response in aged mice has not been investigated.
Although antigen sensitization most likely plays a more significant role in younger patients with asthma, recent data indicate as many as 75% of adults >65 years of age with asthma are sensitized to at least one antigen (15–21) and it may increase disease severity (19). In some of these older patients, antigen sensitization developed later in life and prior to a late onset of asthma. Furthermore, between 50% and 66% of asthma deaths occur in patients >65 years of age (22–24). This demonstrates a major unmet need in this population, which can be addressed through more effectively understanding the underlying airway pathology, with the ultimate goal being to decrease this higher rate of morbidity and mortality in patients over 65 years of age. These observations support the relevance of allergen challenge studies in older subjects, human or animal, as a model, to gain insight into mechanisms of allergic inflammation with aging. The purpose of this study was to (1) address if there are defects in the induction of oral tolerance by feeding low-dose antigen, and, if so, (2) does it impact the development of allergic airway disease in aged mice, which may be of importance to some patients with later onset asthma.
Materials and Methods
Mice and Reagents
Younger (6 weeks old) female BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Aged (18 months old) female BALB/c mice were obtained from the National Institutes of Aging (NIA, Bethesda, MD, USA). The aged groups of mice represent 20.1 and 60.3 human years, respectively, based upon the 24-month life span of BALB/c mice and the life expectancy of 80.4 years in a human female (source National Center for Health Statistics, www.cdc.gov/nchs). The ages were chosen to represent early and later adulthood. (In preliminary experiments, we used antigen sensitized mice older than 22 months but were unable to obtain data from these animals; >50% died during antigen challenge and many of those who survived developed spontaneous tumors, making AHR measurements unobtainable). Mice were maintained in the animal facility at Mount Sinai School of Medicine following standard guidelines for laboratory animal care (25) and with institutional permission for animal handling. Mice were housed in the same facility to normalize gut flora. (Preliminary data on lung histology, lung cytokine expression, and airway function revealed no statistical differences between antigen-sensitized and -challenged mice purchased from Jackson Laboratories, who were allowed to age in our facilities, and similarly, antigentreated and aged mice obtained from the NIA)
Oral Antigen Administration
Oral tolerance to ovalbumin (OVA) was induced by intragastric (i.g.) feeding with 1 mg of OVA (Grade VI; Sigma-Aldrich, St. Louis, MO, USA), dissolved in 250 µl water for 5 consecutive days (days 1–5; Figure 1) to both 6-week-old and 18-month-old mice (n = 5–10/age group). Mice fed OVA prior to sensitization and challenge are labeled as “OVA-fed/OVA-mice.”
Figure 1.
Experimental protocol. Mice (6-week-olds and 18-month-olds) were sensitized intraperitoneally (i.p) with 100 µg OVA absorbed with 2 mg alum in 0.4 ml PBS on days 8 and 16. Ten days after the last sensitization dosing, mice were anesthetized i.p. with ketamine and xylazine and challenged intratracheally (i.t.) for a total of three times at weekly intervals with 100 µg OVA in 0.05 ml PBS, determined to be optimal for distribution to both lobes of the lung (26). One subset of mice from each age group were pre-fed with intragastric (i.g.) OVA (1 mg/mouse/day) for 5 consecutive days prior to sensitization to induce tolerance (OVA-fed/OVA); another subset were nonfed, positive controls (non-fed/OVA). Twenty-four (BALF and lung lymphocyte isolation) or 72 h (AHR, histology, serum immunoglobulins, BALF cellularity) after the final OVA challenge mice were sacrificed. (Time points for our protocol were selected based upon our previous work) (27, 28–30).
Antigen Sensitization and Bronchial Challenges
Mice were antigen sensitized intraperitoneally (i.p.) with 100 µg OVA (Grade VI, Sigma) absorbed with 2 mg alum (Pierce Biotechnology Inc., Rockford, IL, USA) in 0.4 ml phosphate buffered saline (PBS) twice at a week interval (Figure 1). Ten days after the final sensitization, mice were anesthetized i.p. with ketamine and xylazine and challenged intratracheally (i.t.) with 100 µg OVA in 0.05 ml PBS at weekly intervals for 3 weeks. I.t. antigen challenge was performed as previously described (31). Mice sensitized and challenged to, but not pre-fed, OVA, are labeled as “OVA-mice” in the text. (Preliminary data demonstrated no significant difference in bronchoalveolar lavage fluid (BALF) cell counts and cytokine profiles in mice pre-fed saline prior to OVA sensitization and challenge and non-fed mice receiving OVA sensitization and challenge). Control mice were age-matched, naïve mice.
Determination of Serum OVA-Specific Immunoglobulins
Sera were obtained from each group of mice 72 h after final OVA challenge and stored at −80°C. Plates were coated with monoclonal rat anti-mouse IgE (Pharmingen, San Diego, CA, USA), followed by incubation with serum at a 1:10 dilution overnight at 4°C. OVA-specific IgE was detected with digoxigenin (DIG)-labeled OVA (prepared with DIG-3-O-methylcarbonyl-ε-aminocaproic acid-N-hydroxysuccinimide ester (Roche, Indianapolis, IN, USA)) and OVA (1 mg/ml) rocked 2 h at room temperature (RT), separated over PD-10 desalting columns (GE Healthcare, Pittsburgh, PA, USA) and protein content verified with a Bradford Assay, followed by horseradish peroxidase (HRP)-labeled anti-DIG Fab fragments (Roche). ELISA was developed using TMB substrate and read at wavelengths of 450/570 nm. For the measurement of OVA-specific IgG2a and IgG1, plates were coated with OVA and then blocked and washed as above. Serum samples (diluted 1:1000 for IgG2a, 1:5000 for IgG1) were added to the plates in duplicate. HRP enzyme-linked goat anti-mouse IgG2a or IgG1 monoclonal antibodies (Southern Biotech, Birmingham, AL, USA) were added for 90 min at RT and the reactions developed and read as described above for IgE. Results were expressed in optical density (O.D.), as done by other groups (32, 33).DIG-labeled OVA prepared as follows: A 1-mg/ml OVA solution was combined with a 20-mg/ml DIG-3-O-methylcarbonyl-ε-aminocaproic acid-N-hydroxysuccinimide ester (Roche) solution and the tubes rocked 2 h at RT before separation on the PD-10 desalting columns (GE Healthcare). Columns were washedwith5ml PBS 5× (25 ml total) and the flow through was discarded. The OVA-DIG-3-O solution was then added, and once again, the flow through was discarded. Column was washed 2× with 500 µl PBS, and the flow through was discarded after the first wash; however for the second wash, the fraction was collected in a 1.5-ml eppendorf tube. This was repeated for a total of six fractions (500 µl each). The fractions were numbered 1–6 in the order in which they were eluted off the column. Fractions were then tested using Bradford assay to determine the protein content. The fractions with the highest protein present were pooled and the other fractions were discarded.
Lung Lymphocyte Isolation and Analysis
Lungs were removed from the mice 24 h after the final OVA challenge and immediately placed in the complete RPMI 1640 media, kept at 4°C and cut into small pieces. Lung pieces were transferred to digestion buffer containing collagenase D and DNase I (Roche) and incubated at 37°C with constant shaking for 1 h. The samples were filtered through a 70-µm cell strainer to remove tissue fragments, centrifuged, washed, and red blood cells lysed in hypotonic buffer. After washing, the cells were layered over Lympholyte M (Cedarlane Laboratories, Burlington, NC, USA). Lymphocytes were isolated after centrifugation, washed, and placed in staining buffer. Cellular staining was performed using the antibodies of interest at 4°C for 30 min. Dead cells were excluded using a Fixable Live/ Dead Cell Staining Kit (Pacific Blue; Invitrogen, Carlsbad, CA, USA). Cells were labeled with murine CD4(FITC), CD45(Alexa700), and CD25(PerCP-Cy5.5) (in a subset of the experiments) followed by intracellular staining for Foxp3(APC) after fixing/permeabilizing cells overnight (Fixation/Permeabilization Working Solution from Foxp3 Staining Buffer Set; eBioscience, San Diego, CA, USA). All antibodies were purchased from eBioscience. Samples were analyzed using an LSR II Flow Cytometer (BD Biosciences, San Jose, CA, USA). Data analysis was done using FlowJo software (Tree Star, Inc., Ashland, OR, USA). To quantify regulatory T-cells, live cells were gated on CD45 followed by CD4 and Foxp3. We verified in a subset of experiments that the CD4 + Foxp3+ cells were CD25high. Regulatory T-cells were expressed as a percentage of total CD4+ T-cells in the lung digests.
BALF Preparation and Cell Differential Counts
BALF was collected either 24 h (cytokine determination) or 72 h (cell count and differential) after the final antigen challenge. To collect BALF, the chest was opened, and the lungs were lavaged with 1.0 ml cold PBS, which was then placed into chilled tubes. The cell pellet was resuspended in PBS and total cell numbers were determined using a hemocytometer. Cytospin preparations were made with a cytocentrifuge and then stained with Diff-Quick (Dade Diagnostics of PR, Aguada, PR, USA). Cell differential counts were obtained by counting at least 500 cells per slide by light microscopy. The supernatant was transferred and stored at −80°C for cytokine measurement.
Lung Histology
Lung tissue was surgically removed and separated from the trachea 72 h after final OVA challenge. The left lobe was fixed in neutral buffered formaldehyde, and 5-µm paraffin sections were stained with periodic acid-Schiff (PAS) for the evaluation of goblet cells. Approximately 7–10 randomly selected bronchioles per animal were evaluated, and PAS staining graded on a scale of 0–4 (0, no staining; 1, <50% stained; 2, 50% stained; 3, >50% stained; and 4, completely stained) for each bronchiole (34). In addition, lung sections were stained with hematoxylin and eosin (H&E). Perivascular (PV) and peribronchial (PB) inflammation was graded using a published scoring method (0, no inflammatory cells noted around vessel or bronchi; 1, occasional inflammatory cells around the vessel or bronchi; 2, a thin layer (1–5 inflammatory cells) surrounding the vessel or bronchi; 3, a thick layer (>5 inflammatory cells surrounding the vessel or bronchi) (35). At least eight randomly selected sections of the lung tissue were examined. The mean scores for PV and PB H&E and PAS staining for each animal was calculated and the values were reported as mean per group.
Cytokine Determination
The levels of IL-4, IL-5, IL-6, IL-10, IL-13, IFN-γ, and eotaxin from BALF were determined by using the Th1/Th2 multiplex panel of Bio-Plex mouse cytokine assay (Bio-Rad Laboratories, Irvine, CA, USA) according to the manufacturer’s instructions. TGF-β levels were determined by ELISA (R&D systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Values were expressed in pg/ml.
Measurement of AHR
Seventy-two hours following final OVA challenge, airway responsiveness to acetylcholine (ACh) was measured (25). Mice were anesthetized i.p. with pentobarbital (80 mg/kg) and ventilated with a tracheal cannula (18 gage) at the rate of 120 breaths per minute and a constant tidal volume of air (0.2 mL) with an RSP1002 pressure-controlled respirator system (Kent Scientific, Torrington, CT, USA). Muscle paralysis was induced by intravenous injection of decamethonium bromide (25 mg/kg). Airway pressure was measured with a pressure transducer through a port in the trachea. A stable baseline was recorded for at least 2 min, and ACh (50 µg/kg) was injected into the inferior vena cava (IVC) and the recording continued for 4 min. The airway pressure changes were viewed and recorded for 4 min with VENTP software respiratory data acquisition system (Kent Scientific, Torrington, CT, USA). The time-integrated changes in peak airway pressure referred to as the airway pressure–time index (APTI) (cm H2O/s) were calculated and served as the measurement of airway responsiveness.
Statistical Analysis
Data are expressed as means ± SEMs. Statistical analyses were performed using the Student’s unpaired two-tailed t-test for comparison between the two groups for normally distributed data or the Mann–Whitney U-test for nonparametric data. A p-value <.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism® Software (La Jolla, CA, USA).
Results
Differences in the Effects of Oral Tolerance on Antigen Sensitization in Younger (6 Weeks Old) and Aged (18 Months Old) Mice
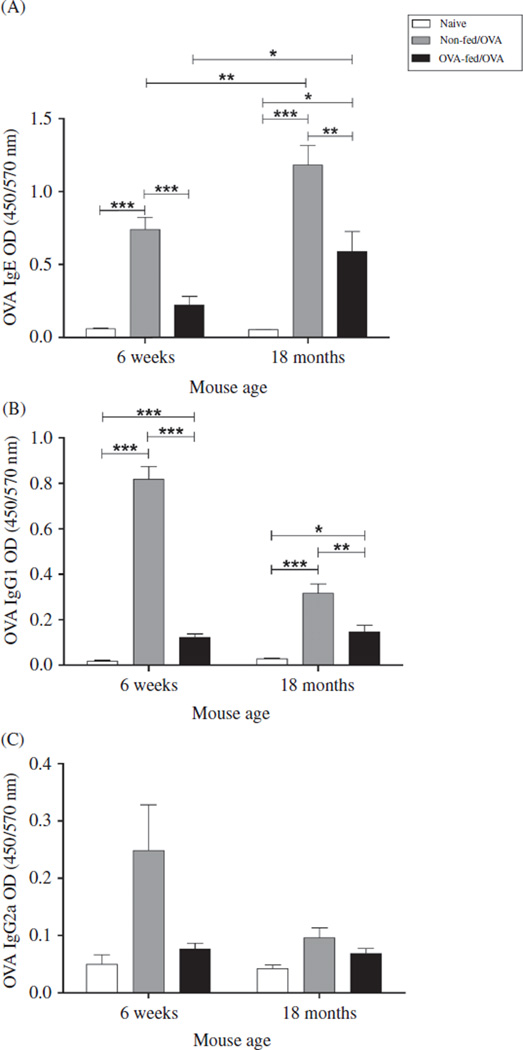
All antigen-sensitized and -challenged mice (“OVA-mice”) developed elevated serum IgE to OVA (Figure 2A). Interestingly, 18-month OVA-mice exhibited significantly higher antigen-specific serum IgE compared with 6-weekold OVA-mice (p = .008), representing a significantly increased delta OD for IgE from naïve to OVA-mice in the aged compared with the younger mice (p = .0072). To investigate whether the allergic response to antigen challenge in sensitized mice was less susceptible to regulation in aged mice, we fed OVA prior to sensitization to induce tolerance. Aged mice achieved antigen tolerance as demonstrated by a significantly decreased serum IgE to OVA after feeding (Figure 2, p = .005), a decrease of 50% based upon the delta OD between the two groups of mice. The induction of oral tolerance, however, was significantly (p < .0001) greater in the younger mice as demonstrated by a greater decrease in serum IgE after feeding; 70%, based upon delta OD. These data demonstrate that aged mice develop a greater IgE response to antigen compared to younger mice and that orally administered OVA suppressed antigen-specific IgE response to a greater extent in the younger mice, suggesting that with age, there is less responsiveness to regulation by oral tolerance.
Figure 2.
Effect of age and OVA feeding on specific antigen-induced immunoglobulin production. Sera were collected 48 h after the final OVA challenge and antigen-specific (A) IgE, (B) IgG1, and (C) IgG2a were determined by ELISA (Serum dilutions: IgG1-1:5000, IgG2a-1:1000, IgE-1:10). Data expressed as mean ± SEM from combined data from two independent experiments. (Total numbers of mice: n = 8/group, 6-week non-fed/OVA, 6- week OVA-fed/OVA, 18-month OVA-fed/OVA; n = 7, 18-month non-fed/OVA; n = 4/group 6-week, and 18-month naïve mice). *p < .05, **p < .01, ***p < .001.
As an additional measure of sensitization and modification by tolerance, OVA-specific serum IgG1 and IgG2a were measured. Both 6-week-old and 18-month-old mice developed significantly elevated IgG antibody responses after sensitization. However, in contrast to IgE, both OVA-IgG1 and OVA-IgG2a were lower in the aged mice, reaching significance only with IgG1 (p < .0001, Figure 2B and C). This represented a significantly increased delta OD for IgG1 from naïve to OVA-mice in the younger compared with the aged mice (p <.001). OVA-specific IgG1 decreased significantly with OVA feeding in the younger OVA-mice (p < .001), but to a lesser extent in aged OVA-mice (p = .002), translating to a decrease of 85.2% and 53.8% in the younger and the aged mice, respectively. OVA-specific IgG2a was decreased, but not significantly with OVA feeding in the younger OVA-mice (Figure 2C) and was not affected in aged OVA-mice (Figure 2C). Taken together, these results indicate that, although antigen sensitization can be modified in the aged mice, the effects of oral tolerance are achieved to a greater degree in the younger mice.
The Effect of Oral Tolerance on Lung Treg Cells
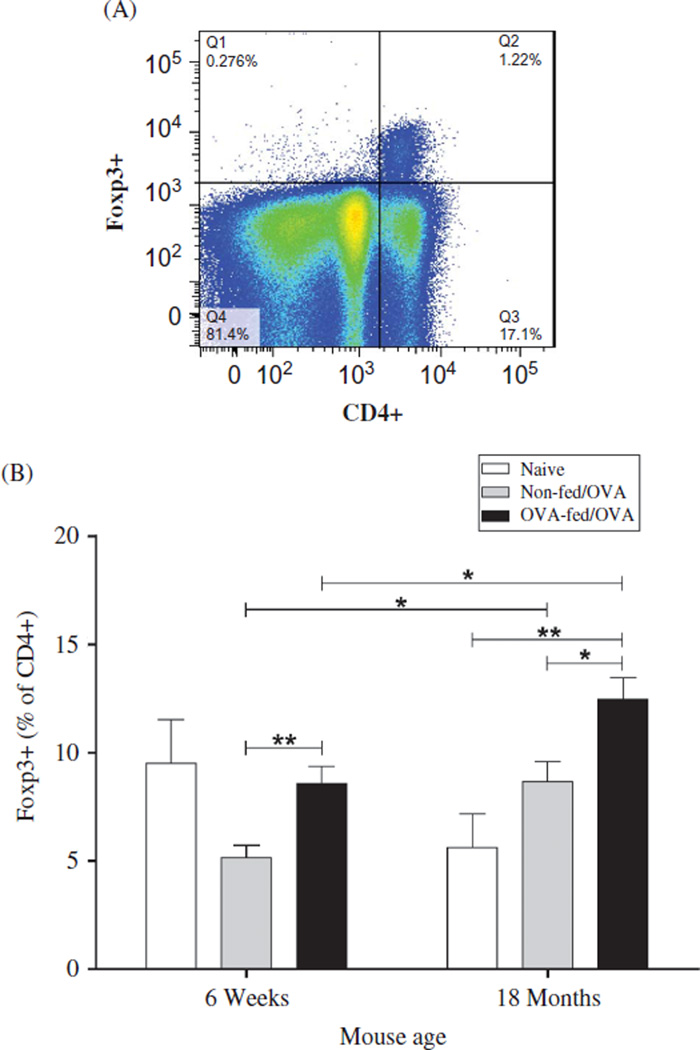
To measure the impact of sensitization and tolerance on Treg cells within the lung tissue, we quantified CD4+ Foxp3+ cells by flow cytometry and expressed them as the percentage of the total CD4+ population. Aged antigensensitized and -challenged mice had a significantly greater percentage of lung Treg cells compared with similarly treated younger mice (p=.016) (Figure 3B). Combined data from three independent experiments demonstrated that OVA feeding prior to sensitization, increased the percentage of Foxp3+ cells significantly in both the aged (p = .02) and the younger OVA-mice; however, the effect was greater in the younger OVA-mice (p = .009). In the younger mice, compared to age-matched naïve controls, the percentage of airway Treg cells was suppressed after OVA sensitization and challenge and subsequently, reversed by OVA feeding. In contrast, antigen sensitization and challenge of aged mice increased the percentage of Treg cells compared with naïve age-matched controls. Absolute Foxp3+cell numbers from one of three independent experiments demonstrated a similar trend: 6-week OVA-mice (2646 cells), 6-week OVA-fed/OVA-mice (14,386 cells), 18-month OVA-mice (18,311 cells), and 18-month OVA-fed/ OVA-mice (23,491 cells).
Figure 3.
Quantification of lung tissue CD4 + Foxp3+ cells. Twenty-four hours after the final antigen challenge, lung lymphocytes were isolated and stained with Pacific Blue-conjugated Live/Dead, and live cells were gated on CD45 followed by CD4 and Foxp3. Regulatory T-cells were expressed as a percentage of total CD4+ T-cells in the lung digests. (A) Representative gating strategy from an 18-month non-fed/OVA mouse. (B) The proportion of CD4+ T-cells that are Foxp3 +. Data are expressed as means ± SEM from combined data from three independent experiments. (Total numbers of mice: n = 13/group, 6-week non-fed/OVA, 6-week OVA-fed/ OVA; n = 8/group, 18-month OVA-fed/OVA, 18-month non-fed/OVA; n = 7, 6-week naïve; and n = 6, 18-month naïve mice). *p < .05, **p < .01.
Differences in the Effects to Oral Tolerance on Cellular Airway Inflammation
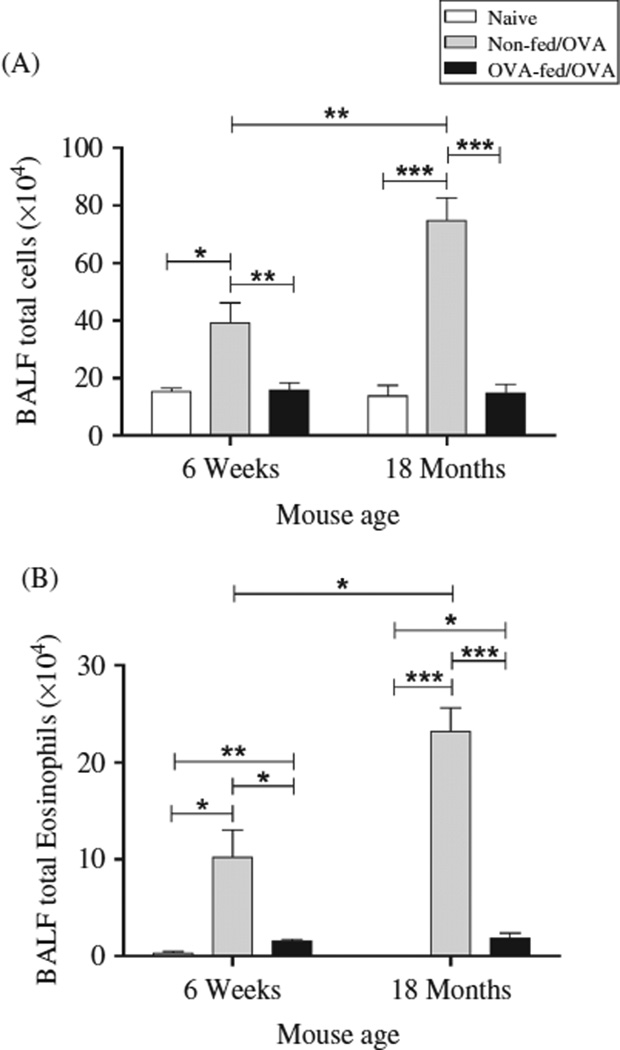
In BALF collected 72 h after the final antigen challenge, the aged (18 months old) OVA-sensitized mice had a greater increase in the total number of BALF leukocytes (74.8 ± 7.8 × 104) and eosinophils (23.2 ± 2.4 × 104) compared to respective numbers in the BALF from the younger (6 weeks old) OVA-mice (39.2 ± 6.9 × 104, (p = .008) and 10.2 ± 2.8 × 104, (p = .012) (Figure 4A and B). In addition, there was an increase in total BALF lymphocytes (9.1 ± 0.8 × 104 vs. 3.5 ± 0.5 × 104, p < .0001), macrophages (36.6 ± 9.6 × 104 vs. 23.5 ± 4.0 × 104, p = .16), and polymorphonuclear leukocytes (PMNs; 5.8 ± 3.2 × 104 vs. 2.0 ± 0.5 × 104, p=.12) in the aged mice compared with the younger mice, respectively. There was no significant difference in the total BALF cell count, as well as eosinophil, lymphocyte, macrophage, and PMN numbers between the younger and the aged mice prior to sensitization, i.e., naïve mice.
Figure 4.
Effect of age and oral OVA administration on antigen-induced BALF inflammation. Forty-eight hours after the final OVA challenge, BALF was collected for total cell differential count. Data expressed as mean ± SEM from combined data from two independent experiments (n = 5–7/group). (A) Total BALF cell count. (B) Total BALF eosinophil count. (Total numbers of mice: n = 10, 6-week non-fed/OVA; n = 9, 6-week OVA-fed/OVA; n = 11, 18-month OVA-fed/OVA; n = 5, 18-month non-fed/OVA; n = 6, 6-week naïve; and n = 5, 18-month naïve mice). *p < .05, **p < .01, ***p < .001.
Feeding OVA prior to antigen sensitization reduced the total BALF cell count and eosinophilia in both relative and absolute numbers. Total BALF cell numbers were significantly decreased in the younger (p = .008) but to a greater extent in the aged (p < .0001) OVA-mice (Figure 4A) as reflected by a decrease in total BALF cells of 59.8 ± 6.6% and 80.3 ± 4.1% in the younger and the aged mice, respectively. Additionally, OVA feeding prior to antigen sensitization and challenge significantly reduced BALF eosinophil numbers in both the younger (p = .011) and the aged OVA-mice (p < .0001) (Figure 4B). This represented an absolute eosinophil decrease of 8.7± 0.22 × 104 in the younger mice and 21.3 ± 0.52 × 104 in the aged mice.
Effect of Oral Tolerance on Antigen-Induced Pulmonary Tissue Inflammation and Mucus Metaplasia
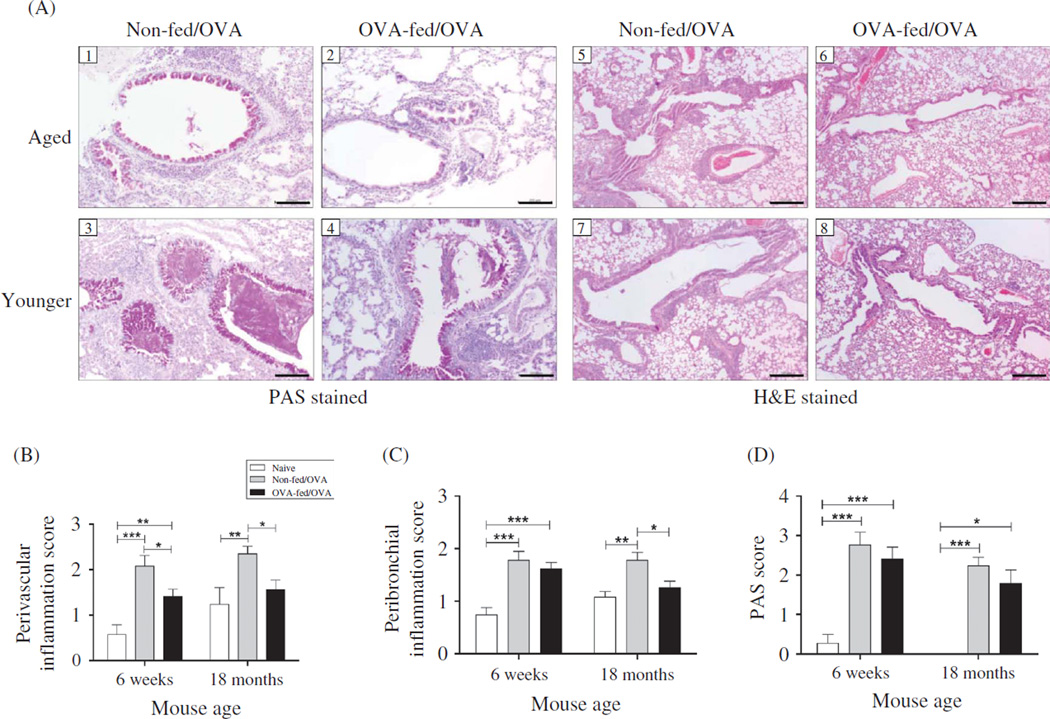
Lung sections were collected 72 h after the final OVA-challenge and then fixed and stained with H&E. As shown in representative histologic sections, there were notable decreases in the inflammatory cells surrounding the bronchi and vasculature in mice that were fed OVA antigen prior to sensitization and challenge (Figure 5A). To quantify these changes, the peribronchial (PB) and perivascular (PV) inflammation were scored. Both the younger and the aged mice demonstrated an increase in PB and PV scores compared with age-matched naïve controls (Figure 5B and C). There was no significant difference in PB inflammation between the aged and the younger non-fed/OVA-mice (Figure 5C). Of note, prior to antigen exposure, the lung tissue in aged naïve mice exhibited greater inflammation, but it did not reach significance (PV, p = .13; PB, p = .1). After oral tolerance induction, PV inflammation decreased in both the younger (p = .03) and the aged (p < .007) mice (Figure 5B and C). PB inflammation was not affected by oral tolerance in the younger mice (p = .43), but was decreased in the aged mice (p = .014).
Figure 5.
Effect of age and oral OVA administration on lung tissue inflammation and goblet cell metaplasia. Forty-eight hours after the final OVA challenge, lung tissue was surgically removed and fixed. (A, 1–4) Representative PAS stained lung sections from mice demonstrating reduced mucus (deep purple) with OVA feeding. (A, 5–8) Representative H&E stained lung sections demonstrating reduced cellular infiltration surrounding bronchi (PB) and vessels (PV) with OVA feeding in both aged and younger mice. H&E stained tissue was graded for both (B) perivascular (PV) and (C) peribronchial (PB) inflammation, as described in Methods. (D) Bronchiolar mucus metaplasia was accessed by PAS tissue staining, as described in Methods. (Total numbers of mice: n=10, 6-week non-fed/OVA, 6-week OVA-fed/OVA, 18-month non-fed/OVA; n = 14, 18-month non-fed/OVA; n = 7, 6-week naïve; and n = 5, 18-month naïve mice). *p < .05, **p < .01, ***p < .001. Scale bars: PAS-200 µm, H&E-500 µm.
Lung tissue was also stained with PAS to measure goblet cell hyperplasia (Figure 4A). There was significantly increased PAS staining in bronchioles in all OVA-mice compared with aged-matched naïve mice, in which there was none to minimal PAS epithelial cell staining. There was no significant difference between PAS staining of younger and aged OVA-mice. PAS staining of lung tissue from the younger and the aged mice was not significantly attenuated with oral tolerance (Figure 5D). These results suggest that oral tolerance has minimal effects on attenuation of goblet cell hyperplasia in both ages of mice.
The Effect of Oral Tolerance on Airway Cytokine Generation Following Antigen Sensitization and Challenge in Young and Aged Mice
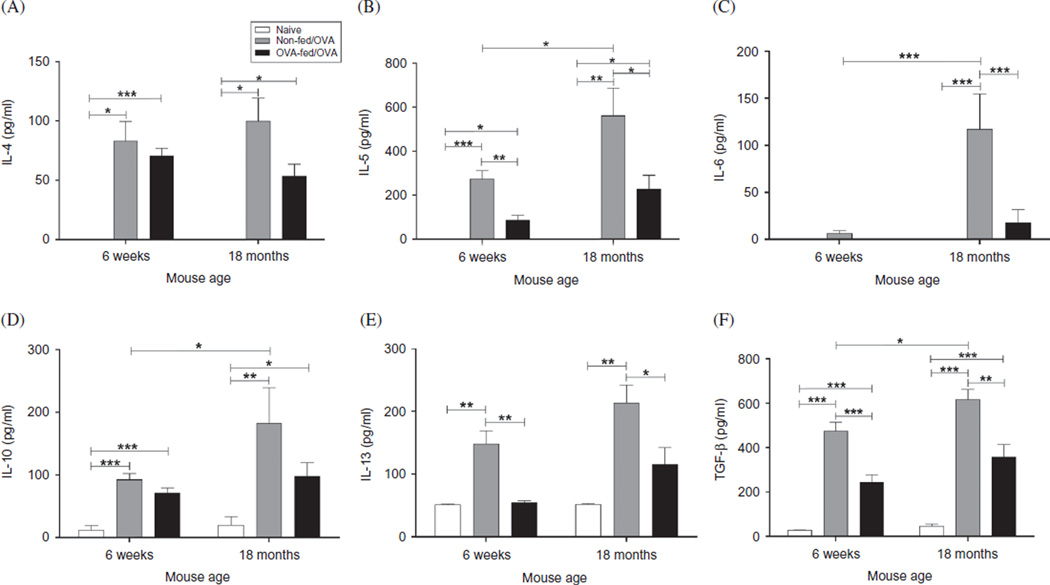
In BALF supernatant collected 24 h after the final OVA challenge, aged OVA-mice had significantly increased generation of IL-5 (p = .04), IL-6 (p < .001), IL-10 (p = .02), and TGF-β (p = .028) compared with the younger OVA-mice (Figure 6). In addition, the aged OVA-mice had increased eotaxin (184.1 ± 88.2 ng/ml) and IFN-γ (11.6 ± 5.1 ng/ml) compared with the younger OVA-mice (eotaxin: 50.7 ± 14.2 ng/ml and IFN-γ: none detected), but these differences did not reach statistical significance. Although antigen sensitization and challenge also provoked increases in IL-4 and IL-13, there was no significant difference between the two age groups (Figure 6). Feeding antigen to both the younger and the aged mice prior to sensitization resulted in a global decrease in BALF cytokine expression, which was more pronounced in the younger mice. Although both the younger and the aged mice had significant reduction in IL-5, IL-13, and TGF-β, the effect was greater in the younger OVA-fed/OVA-mice (Figure 6B, D and E). Additionally, in both the aged and the younger OVA-mice, feeding antigen significantly decreased eotaxin expression to 20.0 ± 0.02 ng/ml (p < .05) and 10.0 ± 1.9 ng/ml (p < .05), respectively.
Figure 6.
Characterization of BALF cytokine expression. Twenty-four hours after the final OVA challenge, BALF was collected, centrifuged, and cytokine expression in supernatant measured for (A) IL-4, (B) IL-5, (C) IL-6, (D) IL-10, (E) IL-13, and (F) TGF-β. Data were means ± SEM (n = 5–7/group). * p < .05, **p < .01, ***p < .001. ND, none detected.
AHR Is Suppressed after Antigen Sensitization and Challenge in Aged Mice
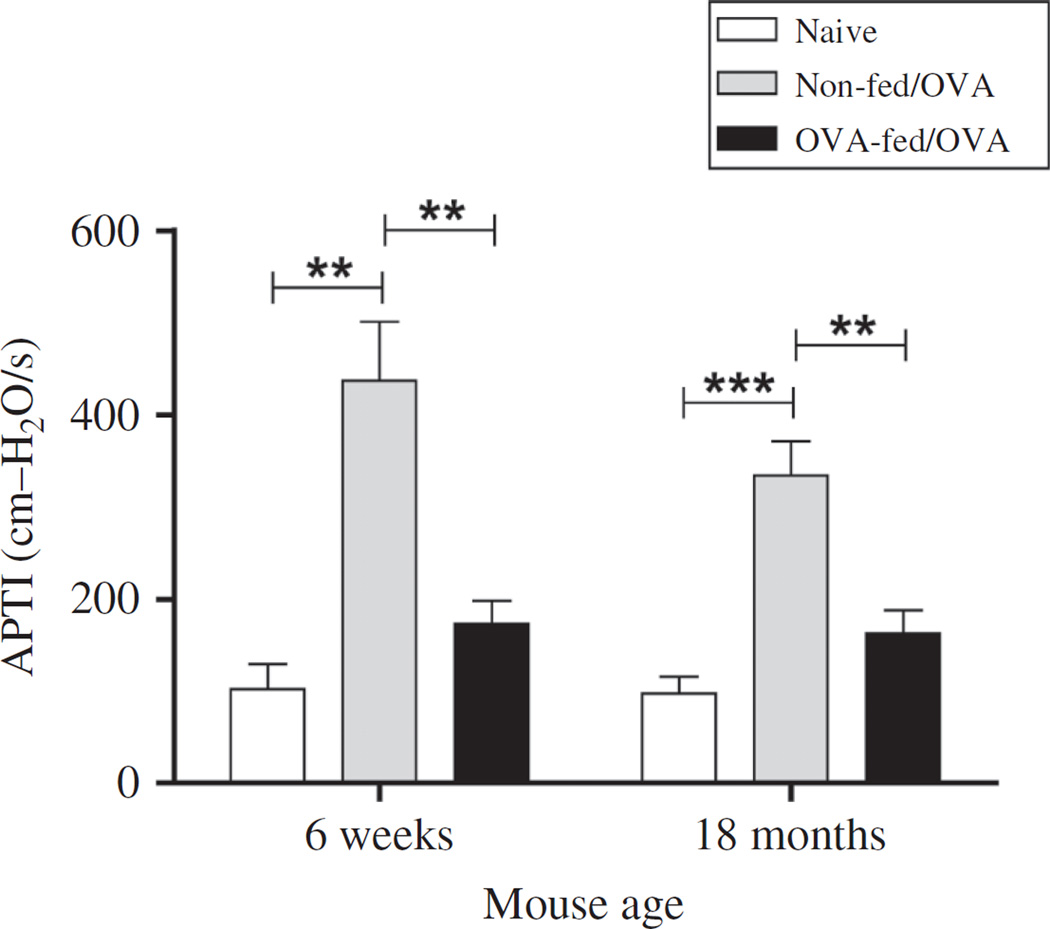
To determine the physiologic effects of antigen sensitization and challenge on AHR, we measured APTI after Ach injection 72 h after the final OVA challenge (Figure 7). Despite increased airway inflammation in the aged mice, APTI levels were greater in the younger (436.8 ± 63.9 cm H2O/s) compared with the aged (333.9 ± 37.18 cm H2O/s) OVA-mice, but these differences did not reach significance (p = .16). Feeding OVA prior to antigen sensitization significantly decreased APTI in both the younger (173.4 ± 25.1 cm H2O/s, p = .002) and the aged (162.7 ± 25.3 cm H2O/s, p = .002) OVA-mice. These values translated to a 60.3% decrease in APTI for the younger mice and a 51.3% decrease in the aged mice. There was no significant difference in APTI between OVA-fed/OVA-mice and age-matched naïve controls in both the younger and the aged mice.
Figure 7.
Effect of age and oral OVA administration on antigen-induced airway hyperresponsiveness (AHR). Forty-eight hours after the final OVA challenge, AHR was determined by APTI after acetylcholine injection. Data were means ± SEM from combined data from two independent experiments (n = 5–7/group). (Total numbers of mice: n = 7, 6-week non-fed/OVA, 6-week OVA-fed/OVA; n = 9/group, 18-month non-fed/OVA, 18-month nonfed/ OVA; n = 5, 6-week naïve; and n = 6, 18-month naïve mice). **p < .01, ***p < .001.
Discussion
In this study, we demonstrated that feeding low-dose antigen to aged mice induces oral tolerance. This was shown by a decrease in antigen-specific IgE and IgG1 in both the younger and the aged mice fed OVA prior to sensitization; however, the decrease was greater in the younger mice. Additionally, antigen feeding prior to sensitization increased the expression of airway Treg cells when compared with antigen sensitization alone, but again, this change was greater in the younger mice. Despite these age-related differences in the induction of oral tolerance, feeding antigen attenuated several features of the allergic airway response in both ages of mice (Table 1.).
Table 1.
Summery of Ag feeding in young and aged mice.
| IgE | IgG1 | AHR | BALF total cell | BALF Eo | Mucus met | H&E PV | H&E PB | IL-4 | IL-5 | IL-10 | IL-13 | TGF-β | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response to OT: | Y | ↓↓↓ | ↓↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓↓ | ↓ | ↓↓ | ↓ | ↓ | ↓↓↓ | ↓ | ↓↓↓ | ↓↓ |
| A | ↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓↓ | ↓↓↓↓ | ↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | |
| Response to Sens: | Y | ↑↑↑ | ↑↑↑↑ | ↑↑ | ↑ | ↑↑↑↑ | ↑↑↑ | ↑↑ | ↑ | ↑↑↑↑↑ | ↑↑↑↑↑↑ | ↑↑ | ↑ | ↑↑↑↑ |
| A | ↑↑↑↑ | ↑↑↑ | ↑↑ | ↑↑ | ↑↑↑↑↑ | ↑↑↑↑↑ | ↑ | ↑ | ↑↑↑↑↑ | ↑↑↑↑↑↑↑ | ↑↑↑ | ↑↑ | ↑↑↑ |
Note: Y, young; A, aged; OT, oral tolerance; MM, mucus metaplasia; Eo, eosinophils.
Oral tolerance is characterized by immunologic unresponsiveness after oral delivery of antigen. The mechanisms of tolerance are dependent upon antigen dosing; low dose typically generates antigen-specific regulatory cells, producing TGF-β and/or IL-10, whereas higher dose produces unresponsiveness of lymphocyte function via anergy/deletion (2–5). Most work on the effect of age on induction of oral tolerance has focused upon high-dose OVA-feeding (20 mg OVA) and has demonstrated in several mouse strains that by approximately 24 weeks of age (6 months), induction of tolerance is lost (12, 13). This loss was hypothesized to be related to age-related loss of peyer’s patches (36). However, in aged B6D2F1 mice, which are highly susceptible to induction of oral tolerance, continuous feeding of high-dose OVA decreased antigen-specific IgG1, and overcame the refractory nature to tolerance induction in aged mice (37). Few studies are published on the effect of low-dose antigen feeding and oral tolerance induction in aged mice. Waskabayashi et al. reported that feeding aged (19 months old) and younger (3 months old) BDF1 male mice low-dose (0.25 mg) OVA for 5 consecutive days decreased serum OVA-specific IgE and IgG1, although to a greater extent in the younger mice (38). However, DTH reaction, measured by swelling of the ear after OVA injection, was only suppressed in the younger mice (38). Our study differs in that we used a low-dose, but higher than 0.25 mg, OVA, a different mouse strain (BALB/c), and measured functional consequences of the allergic airway response after the induction of oral tolerance.
To determine whether oral tolerance could be induced by feeding low-dose antigen to aged mice, we measured antigen-specific IgE and IgG1, after the final antigen challenge. Similar to our previous results (27), antigen sensitization produced a significantly elevated antigen-specific IgE in aged mice compared with the younger mice. Thus, in a murine model of asthma, not only may sensitization occur at a later age in life, but it also results in an increased expression of antigen-specific IgE that is greater than that in the younger mice. In mice, IgG1 frequently parallels the IgE response to antigen. However, we noted that unlike IgE, IgG1 to OVA was significantly decreased in the aged compared with the younger mice after antigen sensitization. The reported effect of aging on serum immunoglobulin levels is not consistent (39). Some studies in aged mice suggest that antigen sensitization increases both total and antigen-specific IgG (40). Other groups have demonstrated that total IgG is unchanged with age, but that OVA-specific IgG decreases, potentially to the activation of memory B-cells and decreased class switching with aging. (41, 42). We found that feeding low-dose OVA for 5 consecutive days significantly decreased OVA-specific IgE and IgG1 in both the younger and the aged mice, although to a greater extent in the younger mice.
Several investigators have demonstrated that the induction of oral tolerance with low-dose antigen feeding increases Treg cells (43, 44), but whether this also occurs in aged mice has not been addressed. Treg cells are reported to increase with age in mice (45–47) and humans (48–50) and appear to maintain their suppressive function (45, 47–49, 51). The percentage ofCD4+Foxp3+airway cells after antigen sensitization and challenge was significantly increased in aged mice compared with the younger mice. Consistent with the changes in serum IgE and IgG1, we demonstrated that, although antigen feeding increased Treg cells in both ages of mice, the change was greater in the younger mice, suggesting that although induction of oral tolerance is maintained in aged mice, the effect is reduced compared with younger mice.
To determine the effects of oral tolerance on the allergic airway response in aged mice, we first measured BALF for cell differential count. Despite a greater induction of oral tolerance in the younger mice, the decrease in total BALF cells and eosinophils after an antigen challenge was significantly more in the aged mice. To determine potential mechanisms for decreased BALF eosinophilia, we measured cytokine expression in the BALF 24 h after a final OVA challenge.OVA feeding significantly decreased IL-5 and eotaxin in both the age groups of mice. However, there may be additional mechanisms that decrease BALF eosinophilia in aged mice and require further study. These include examining age-related effects of IL-5 on bone marrow eosinophil precursor growth and eosinophil trafficking to the lung.
OVA feeding did not increase BALF TGF-β or IL-10 despite increased Treg airway cell expression in both the aged and the younger mice. However, prior studies investigating the role of oral tolerance on suppression of the allergic airway response younger mice have reported similar findings (14, 52), including a lack of reversal of antigenspecific unresponsiveness induced by tolerance after administration of anti-TGF-β during antigen challenge (14). Additionally, oral tolerance has been achieved in IL-10-/-mice and OVA feeding prior to sensitization did not increase TGF-β or SMAD2 expression in CD4 + CD25+ spleen or mesenteric lymph node cultures in the younger mice (9). A potential reason for our findings may include that we measured total TGF-β, which may not distinguish between bioactive and inactive forms and that membrane-bound TGF-β cannot be measured in the BALF.
Despite the presence of greater BALF inflammation and eosinophilia in aged mice, AHR was lower than in younger mice. One possibility for the discord between increased inflammation and reduced AHR in aged mice is an increased expression of airway Treg cells with age. Treg cells most likely have a protective role in the allergic airway response, modulating AHR, possibly independent of eosinophil regulation (53–57), and have lower expression in children and adults with asthma (58, 59) compared with normal controls. The role and function of Treg cells in older patients with asthma has not been well defined. However, it was recently reported that numbers of peripheral blood Treg cells were decreased compared with age-matched controls (60), suggesting that Treg cells are also decreased in older patients with asthma similar to younger patients. Additional studies are required to address whether the function of airway Treg cells in an aged mouse model of asthma and in older subjects with asthma is altered. To examine other etiologies to explain differences between AHR and airway inflammation in younger and aged mice, we measured the contraction of tracheal smooth muscle to ACh, a protocol previously used in our laboratory (61). We did not detect significant differences in the contraction of tracheal rings between younger and aged mice (data not shown).
Conclusions
Although the role of atopy is not entirely clear in older patients, several studies suggest that allergic sensitization is not uncommon (15–21) and may be a risk factor for asthma onset after 60 years of age (62–64), as well as contribute to an increase in disease severity (19). We have demonstrated that aged mice develop antigenspecific IgE sensitization with several features of asthma following allergen challenge including BALF eosinophilia, Th2 cytokine expression, mucus metaplasia, and AHR. The present study examined whether immune modulation, via oral tolerance, could alter the development of antigen-induced asthma in an aged mouse model. Our study suggests that although feeding low-dose antigen prior to sensitization has a decreased effect on modulating antigen-specific immunoglobulin levels and Treg cell numbers, it attenuated several features of the allergic airway response. Few trials have examined the role of immunomodulatory treatment for asthma in older patients, but allergen immunotherapy in patients over 54 years of age demonstrated an improvement in the markers of asthma control (65) and two studies using post-marketing data and pooled analysis of previously published data, suggested that anti-IgE had clinical efficacy in patients >50 years of age (66, 67). Further studies are necessary to understand the underlying mechanisms of antigen regulation with increased age and whether this translates to humans. Understanding the differences in immune response that occur with aging may provide new information on approaches to the treatment of older adults with asthma and lead to improved clinical outcomes and a reduction in the high rates of morbidity and mortality often found in this group of patients (68–70). It is our belief that these observations are a first step toward meeting this goal.
Acknowledgments
5K08AI063932 (PJB), ART/JACI Allergy/Immunology Research Support Award (PJB). Partial support by NIH/ NCCAM center grant # 1P01 AT002644725-01 Center for Chinese Herbal Therapy (CHT) for Asthma (XML).
Footnotes
Declaration of Interest
The authors have no relevant interests to declare.
References
- 1.Wu HY, Weiner HL. Oral tolerance. Immunol Res. 2003;28(3):265–284. doi: 10.1385/IR:28:3:265. [DOI] [PubMed] [Google Scholar]
- 2.Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004;4(6):407–419. doi: 10.1038/nri1370. [DOI] [PubMed] [Google Scholar]
- 3.Weiner HL. Oral tolerance: Immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001;3(11):947–954. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- 4.Samsom JN. Regulation of antigen-specific regulatory T-cell induction via nasal and oral mucosa. Crit Rev Immunol. 2004;24(3):157–177. doi: 10.1615/critrevimmunol.v24.i3.10. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376(6536):177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 6.Samsom JN, van Berkel LA, van Helvoort JM, Unger WW, Jansen W, Thepen T, Mebius RE, Verbeek SS, Kroal G. Fc gamma RIIB regulates nasal and oral tolerance: A role for dendritic cells. J Immunol. 2005;174(9):5279–5287. doi: 10.4049/jimmunol.174.9.5279. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald DC, Zhang GX, Yu S, Cullimore ML, Zhao Z, Rostami A. Intravenous tolerance effectively overcomes enhanced pro-inflammatory responses and experimental autoimmune encephalomyelitis severity in the absence of IL-12 receptor signaling. J Neuroimmunol. 2012;247(1–2):32–37. doi: 10.1016/j.jneuroim.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matta B, Jha P, Bora PS, Bora NS. Antigen-specific tolerance inhibits autoimmune uveitis in pre-sensitized animals by deletion and CD4 + CD25+ T-regulatory cells. Immunol Cell Biol. 2010;88(2):187–196. doi: 10.1038/icb.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller AC, Mucida D, Gomes E, Faquim-Mauro E, Faria AM, Rodriguez D, Russo M. Hierarchical suppression of asthma-like responses by mucosal tolerance. J Allergy Clin Immunol. 2006;117(2):283–290. doi: 10.1016/j.jaci.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Berin MC. Mechanisms of allergic sensitization to foods: Bypassing immune tolerance pathways. Immunol Allergy Clin North Am. 2012;32(1):1–10. doi: 10.1016/j.iac.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167(8):4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 12.Faria AM, Garcia G, Rios MJ, Michalaros CL, Vaz NM. Decrease in susceptibility to oral tolerance induction and occurrence of oral immunization to ovalbumin in 20–38-week-old mice. The effect of interval between oral exposures and rate of antigen intake in the oral immunization. Immunology. 1993;78(1):147–151. [PMC free article] [PubMed] [Google Scholar]
- 13.de Faria AM, Ficker SM, Speziali E, Menezes JS, Stransky B, Silva Rodrigues V, Vaz NM. Aging affects oral tolerance induction but not its maintenance in mice. Mech Ageing Dev. 1998;102(1):67–80. doi: 10.1016/s0047-6374(98)00024-4. [DOI] [PubMed] [Google Scholar]
- 14.Russo M, Nahori MA, Lefort J, Gomes E, de Castro Keller A, Rodriguez D, Ribeiro OG, Adriouch S, Gallois V, de Faria AM, Vargaftig BB. Suppression of asthma-like responses in different mouse strains by oral tolerance. Am J Respir Cell Mol Biol. 2001;24(5):518–526. doi: 10.1165/ajrcmb.24.5.4320. [DOI] [PubMed] [Google Scholar]
- 15.Zureik M, Orehek J. Diagnosis and severity of asthma in the elderly: Results of a large survey in 1,485 asthmatics recruited by lung specialists. Respiration. 2002;69(3):223–228. doi: 10.1159/000063624. [DOI] [PubMed] [Google Scholar]
- 16.King MJ, Bukantz SC, Phillips S, Mohapatra SS, Tamulis T, Lockey RF. Serum total IgE and specific IgE to Dermatophagoides pteronyssinus, but not eosinophil cationic protein, are more likely to be elevated in elderly asthmatic patients. Allergy Asthma Proc. 2004;25(5):321–325. [PubMed] [Google Scholar]
- 17.Huss K, Naumann PL, Mason PJ, Nanda JP, Huss RW, Smith CM, Hamilton RG. Asthma severity, atopic status, allergen exposure and quality of life in elderly persons. Ann Allergy Asthma Immunol. 2001;86(5):524–530. doi: 10.1016/S1081-1206(10)62900-6. [DOI] [PubMed] [Google Scholar]
- 18.Burrows B, Barbee RA, Cline MG, Knudson RJ, Lebowitz MD. Characteristics of asthma among elderly adults in a sample of the general population. Chest. 1991;100(4):935–942. doi: 10.1378/chest.100.4.935. [DOI] [PubMed] [Google Scholar]
- 19.Rogers L, Cassino C, Berger KI, Goldring RM, Norman RG, Klugh T, Reibman J. Asthma in the elderly: Cockroach sensitization and severity of airway obstruction in elderly nonsmokers. Chest. 2002;122(5):1580–1586. doi: 10.1378/chest.122.5.1580. [DOI] [PubMed] [Google Scholar]
- 20.Busse PJ, Lurslurchachai L, Sampson HA, Halm EA, Wisnivesky J. Perennial allergen-specific immunoglobulin E levels among inner-city elderly asthmatics. J Asthma. 2010;47(7):781–785. doi: 10.3109/02770903.2010.489140. [DOI] [PubMed] [Google Scholar]
- 21.Ariano R, Panzani RC, Augeri G. Late onset asthma clinical and immunological data: Importance of allergy. J Investig Allergol Clin Immunol. 1998;8(1):35–41. [PubMed] [Google Scholar]
- 22.Bellia V, Pedone C, Catalano F, Zito A, Davi E, Palange S, Forastiere F, Incalzi RA. Asthma in the elderly: Mortality rate and associated risk factors for mortality. Chest. 2007;132(4):1175–1182. doi: 10.1378/chest.06-2824. [DOI] [PubMed] [Google Scholar]
- 23.Moorman JERR, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. Centers for Disease Control and Prevention (CDC). National surveillance for asthma – United States, 1980–2004. MMWR Surveill Summ. 2007;56(SS08):1–14. 8–54. [PubMed] [Google Scholar]
- 24.Diette GBKJ, Dominici F, et al., editors. Why do older adults have higher rates of hospitalization for asthma than younger adults?. American Thoracic Society International Conference; Toronto, Canada. 2000. [Google Scholar]
- 25.NAEPP Working Group Report. Considerations for Diagnosis and Managing Asthma in the Elderly. 1996 Report No/NIH Publication No. 96-3662. [Google Scholar]
- 26.Eyles J, Brimacombe M, Chaulk P, Stoddart G, Pranger T, Moase O. What determines health? To where should we shift resources? Attitudes towards the determinants of health among multiple stakeholder groups in Prince Edward Island, Canada. Soc Sci Med. 2001;53(12):1611–1619. doi: 10.1016/s0277-9536(00)00445-7. [DOI] [PubMed] [Google Scholar]
- 27.Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007;37(9):1392–1403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busse PJ, Zhang TF, Srivastava K, Lin BP, Schofield B, Sealfon SC, Li XM. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116(6):1256–1263. doi: 10.1016/j.jaci.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 29.Busse PJ, Zhang TF, Schofield B, Kilaru S, Patil S, Li XM. Decrease in airway mucous gene expression caused by treatment with anti-tumor necrosis factor alpha in a murine model of allergic asthma. Ann Allergy Asthma Immunol. 2009;103(4):295–303. doi: 10.1016/S1081-1206(10)60528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carson WFt, Guernsey LA, Singh A, Vella AT, Schramm CM, Thrall RS. Accumulation of regulatory T cells in local draining lymph nodes of the lung correlates with spontaneous resolution of chronic asthma in a murine model. Int Arch Allergy Immunol. 2008;145(3):231–243. doi: 10.1159/000109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bochner BS, Busse WW. Allergy and asthma. J Allergy Clin Immunol. 2005;115(5):953–959. doi: 10.1016/j.jaci.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 32.Ci X, Chu X, Wei M, Yang X, Cai Q, Deng X. Different effects of farrerol on an OVA-induced allergic asthma and LPS-induced acute lung injury. PLoS One. 2012;7(4):e34634. doi: 10.1371/journal.pone.0034634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simarro M, Giannattasio G, Xing W, Lundequist EM, Stewart S, Stevens RL, Orduña A, Boyce JA, Anderson PJ. The translational repressor Tcell intracellular antigen-1 (TIA-1) is a key modulator of Th2 and Th17 responses driving pulmonary inflammation induced by exposure to house dust mite. Immunol Lett. 2012;146(1–2):8–14. doi: 10.1016/j.imlet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson N, Kasahara DI, Hristova M, Bernstein R, Janssen-Heininger Y, van der Vliet A. Modulation of NF-{kappa}B and HIF-1 by S-nitrosoglutathione does not alter allergic airway inflammation in mice. Am J Respir Cell Mol Biol. 2011;44(6):813–823. doi: 10.1165/rcmb.2010-0035OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart JM, Noël A, Cataldo DD. Mouse models of asthma: A comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res. 2009;58(12):845–854. doi: 10.1007/s00011-009-0054-2. [DOI] [PubMed] [Google Scholar]
- 36.Kato H, Fujihashi K, Kato R, Dohi T, Hagiwara Y, Kataoka K, Kobayashi R, MgGhee JR. Lack of oral tolerance in aging is due to sequential loss of Peyer’s patch cell interactions. Int Immunol. 2003;15(2):145–158. doi: 10.1093/intimm/dxg011. [DOI] [PubMed] [Google Scholar]
- 37.Faria AM, Maron R, Ficker SM, Slavin AJ, Spahn T, Weiner HL. Oral tolerance induced by continuous feeding: Enhanced up-regulation of transforming growth factor-beta/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J Autoimmun. 2003;20(2):135–145. doi: 10.1016/s0896-8411(02)00112-9. [DOI] [PubMed] [Google Scholar]
- 38.Wakabayashi A, Utsuyama M, Hosoda T, Sato K, Takahashi H, Hirokawa K. Induction of immunological tolerance by oral, but not intravenous and intraportal, administration of ovalbumin and the difference between young and old mice. J Nutr Health Aging. 2006;10(3):183–191. [PubMed] [Google Scholar]
- 39.Bulati M, Buffa S, Candore G, Caruso C, Dunn-Walters DK, Pellicano M, Wu YC, Colonna Romano G. B cells and immunosenescence: A focus on IgG + IgD-CD27- (DN) B cells in aged humans. Ageing Res Rev. 2011;10(2):274–284. doi: 10.1016/j.arr.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Koga T, McGhee JR, Kato H, Kato R, Kiyono H, Fujihashi K. Evidence for early aging in the mucosal immune system. J Immunol. 2000;165(9):5352–5359. doi: 10.4049/jimmunol.165.9.5352. [DOI] [PubMed] [Google Scholar]
- 41.Speziali E, Santiago AF, Fernandes RM, Vaz NM, Menezes JS, Faria AM. Specific immune responses but not basal functions of B and T cells are impaired in aged mice. Cell Immunol. 2009;256(1–2):1–5. doi: 10.1016/j.cellimm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Frasca D, Riley RL, Blomberg BB. Aging murine B cells have decreased class switch induced by anti-CD40 or BAFF. Exp Gerontol. 2007;42(3):192–203. doi: 10.1016/j.exger.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faria AM, Weiner HL. Oral tolerance: Therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13(2–4):143–157. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraal G, Samsom JN, Mebius RE. The importance of regional lymph nodes for mucosal tolerance. Immunol Rev. 2006;213:119–130. doi: 10.1111/j.1600-065X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4 + CD25 + Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81(6):1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- 46.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181(3):1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiu BC, Stolberg VR, Zhang H, Chensue SW. Increased Foxp3(+) Treg cell activity reduces dendritic cell co-stimulatory molecule expression in aged mice. Mech Ageing Dev. 2007;128(11–12):618–627. doi: 10.1016/j.mad.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140(3):540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang KA, Kim HR, Kang I. Aging and human CD4(+) regulatory T cells. Mech Ageing Dev. 2009;130(8):509–517. doi: 10.1016/j.mad.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson DS. Regulatory T cells and asthma. Clin Exp Allergy. 2009;39(9):1314–1323. doi: 10.1111/j.1365-2222.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 51.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4 + CD25 + Foxp3+ T cells and CD4 + CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176(11):6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 52.Nagatani K, Dohi M, To Y, Tanaka R, Okunishi K, Nakagome K, Sagawa K, Tanno Y, Komagata Y, Yamamoto K. Splenic dendritic cells induced by oral antigen administration are important for the transfer of oral tolerance in an experimental model of asthma. J Immunol. 2006;176(3):1481–1489. doi: 10.4049/jimmunol.176.3.1481. [DOI] [PubMed] [Google Scholar]
- 53.Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells. Am J Physiol Lung Cell Mol Physiol. 2009;296(3):L307–L319. doi: 10.1152/ajplung.00521.2007. [DOI] [PubMed] [Google Scholar]
- 54.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4 + CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202(11):1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kearley J, Robinson DS, Lloyd CM. CD4 + CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122(3):617–624. e6. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaefer U, Machida T, Vorlova S, Strickland S, Levi R. The plasminogen activator system modulates sympathetic nerve function. J Exp Med. 2006;203(9):2191–2200. doi: 10.1084/jem.20060077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Köhl J, Belkaid Y, Wills-Karp M. CD4 + CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202(11):1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, Krauss-Etschmann S. Quantitative and functional impairment of pulmonary CD4 + CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119(5):1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 59.Provoost S, Maes T, vanDurme YM, Gevaert P, Bachert C, Schmidt-Weber CB, Brusselle GG, Joos GF, Tournoy KG. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009;64(10):1539–1546. doi: 10.1111/j.1398-9995.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- 60.Vale-Pereira S, Todo-Bom A, Geraldes L, Schmidt-Weber C, Akdis CA. FoxP M-PA. 3, GATA-3 and T-bet expression in elderly asthma. Clin Exp Allergy. 2011;41(4):490–496. doi: 10.1111/j.1365-2222.2010.03640.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhang TF, Srivastava K, Wen MC, Yang N, Cao J, Busse P, Birmingham N, Goldfarb J, Li XM. Pharmacology and immunological actions of a herbal medicine ASHMI (TM) on allergic asthma. Phytother Res. 2010;24(7):1047–1055. doi: 10.1002/ptr.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burrows B, Lebowitz MD, Barbee RA, Cline MG. Findings before diagnoses of asthma among the elderly in a longitudinal study of a general population sample. J Allergy Clin Immunol. 1991;88(6):870–877. doi: 10.1016/0091-6749(91)90243-h. [DOI] [PubMed] [Google Scholar]
- 63.Litonjua AA, Sparrow D, Weiss ST, O’Connor GT, Long AA, Ohman JL., Jr Sensitization to cat allergen is associated with asthma in older men and predicts new-onset airway hyperresponsiveness. The Normative Aging Study. Am J Respir Crit Care Med. 1997;156(1):23–27. doi: 10.1164/ajrccm.156.1.9608072. [DOI] [PubMed] [Google Scholar]
- 64.Parameswaran K, Hildreth AJ, Taylor IK, Keaney NP, Bansal SK. Predictors of asthma severity in the elderly: Results of a community survey in Northeast England. J Asthma. 1999;36(7):613–618. doi: 10.3109/02770909909087299. [DOI] [PubMed] [Google Scholar]
- 65.Asero R. Efficacy of injection immunotherapy with ragweed and birch pollen in elderly patients. Int Arch Allergy Immunol. 2004;135(4):332–335. doi: 10.1159/000082328. [DOI] [PubMed] [Google Scholar]
- 66.Korn S, Schumann C, Kropf C, Stoiber K, Thielen A, Taube C, Buhl R. Effectiveness of omalizumab in patients 50 years and older with severe persistent allergic asthma. Ann Allergy Asthma Immunol. 2010;105(4):313–319. doi: 10.1016/j.anai.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Maykut RJ, Kianifard F, Geba GP. Response of older patients with IgE-mediated asthma to omalizumab: A pooled analysis. J Asthma. 2008;45(3):173–181. doi: 10.1080/02770900701247277. [DOI] [PubMed] [Google Scholar]
- 68.Quadrelli SA, Roncoroni A. Features of asthma in the elderly. J Asthma. 2001;38(5):377–389. doi: 10.1081/jas-100000259. [DOI] [PubMed] [Google Scholar]
- 69.Reed CE. Asthma in the elderly: Diagnosis and management. J Allergy Clin Immunol. 2010;126(4):681–687. doi: 10.1016/j.jaci.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 70.Sin DD, Tu JV. Inhaled corticosteroid therapy reduces the risk of rehospitalization and all-cause mortality in elderly asthmatics. Eur Respir J. 2001;17(3):380–385. doi: 10.1183/09031936.01.17303800. [DOI] [PubMed] [Google Scholar]