This paper reviews the phase III trial results of antigen-specific immunotherapeutic approaches in nonsmall-cell lung cancer and explores in-depth the potential reasons behind their failure and discuss strategies for the future.
Keywords: active immunotherapy, vaccines, nonsmall-cell lung cancer, immune checkpoint, tumor-mediated immunosuppression
Abstract
Vaccines that rely on active specific stimulation of the host immune system have the potential to trigger durable antitumor responses with minimal toxicity. However, in nonsmall-cell lung cancer (NSCLC), several large phase III trials of vaccines reported within the last year have yielded disappointing results. Compared with placebo, belagenpumatucel-L (an allogenic tumor cell vaccine), tecemotide (a peptide vaccine targeting MUC-1) and melanoma-associated antigen-A3 (a protein-based vaccine) did not improve outcomes in NSCLC. The lack of clinically significant outcomes, despite their ability to prime and expand tumor antigen-specific T cells could at least partly be attributed to the inability of vaccine-induced T-cell responses to overcome the tumoral mechanisms of immune escape which limit the clonal expansion of T cells following vaccination. A number of such mechanisms have been recognized including reduced antigen presentation, antigenic loss, cytokines, immunosuppressive cells and immune checkpoints. Strategies aimed at modulating the immune checkpoints have shown promise and are on the verge of revolutionizing the therapeutic landscape of metastatic NSCLC. Overcoming immune tolerance and improving the activation of antitumor T cells via combinatorial approaches may represent a new and more promising therapeutic application for active immunotherapies in NSCLC.
introduction
The significant and durable responses induced by antibodies blocking the programmed cell death-1 (PD-1) checkpoint have led to a renewed interest in immunotherapy for nonsmall-cell lung cancer (NSCLC) [1, 2]. These results are particularly encouraging given the many unsuccessful attempts at immunotherapy in NSCLC over the last several years. In general, these have included active immunotherapies which rely on the ability of the patient's own immune system to mount an immune response specific to tumor-associated antigens, passive immunotherapy which uses exogenous lymphocytes or antibodies to mediate an immune response and nonspecific immune stimulation which should be effective regardless of the tumor antigen which stimulates the immune response [3, 4].
Active specific stimulation of the host immune system has the potential to cause durable antitumor responses with minimal toxicity. This promise of antigen-specific immunotherapy has borne out in prostate cancer where the use of sipuleucel-T, an autologous active cellular immunotherapy prolonged overall survival (OS) among men with metastatic castration-resistant prostate cancer [5]. However, in NSCLC, several agents whose large phase III trial results have been reported within the last year have yielded no significant benefit. Given the dire need for better therapies and the cost of drug development, it is imperative to try to understand these failures. In this article, we will review the phase III trial results of recently reported antigen-specific immunotherapeutic approaches in NSCLC, explore the potential reasons behind their failure and discuss strategies for the future.
antigen-specific immunotherapeutic approaches in NSCLC
belagenpumatucel-L
Belagenpumatucel-L (Lucanix) is an allogeneic tumor cell vaccine, which consists of four irradiated NSCLC cell lines that have been modified with transforming growth factor-β2 (TGF-β2) antisense gene plasmid. TGF-β inhibits T-cell, B-cell and dendritic cell activation, induces immunosuppressive T regulatory (Treg) cells and inhibits immune effector cell activation [6]. In a phase II study of patients with low-volume disease, belagenpumatucel-L was well tolerated, induced antibody-mediated response to vaccine human leukocyte antigens (HLA) and demonstrated a dose-dependent improvement in survival and response [7].
A phase III trial compared the efficacy of belagenpumatucel-L with placebo as a maintenance therapy in patients with stages IIIA (T3, N2 only), IIIB and IV NSCLC without progression after up to six cycles of first-line platinum-based chemotherapy (which had to be completed 4–17 weeks before randomization) [8]. Belagenpumatucel-L (2.5 × 107 cells/injection intradermally) or placebo were administered every month for 18 months followed by additional two quarterly injections. The primary end point was OS. Maintenance belagenpumatucel-L did not result in improvement in OS over placebo [median OS 20.3 months with belagenpumatucel-L (n = 270) and 17.8 months with placebo (n = 262); P = 0.59]. Of interest, however, in a preplanned subgroup analysis, among patients who received prior radiation therapy and enrolled within 12 weeks, belagenpumatucel-L resulted in significantly improved OS [median OS 40.1 months with belagenpumatucel-L (n = 43) and 10.3 months with placebo (n = 36); P = 0.014].
tecemotide
Tecemotide (Liposomal BLP25; L-BLP25) is a peptide vaccine, which targets the exposed core peptide of MUC-1, a membrane-associated glycoprotein differentially overexpressed and aberrantly glycosylated in cancer cells [9, 10]. Tecemotide consists of the MUC1-derived 25-aminoacid BLP25 lipopeptide, the immunoadjuvant monophosphoryl lipid A and three liposome-forming lipids. Tecemotide was well tolerated and induced T-cell responses to MUC1 in phase I and II studies [11–13].
A phase III trial compared the efficacy of tecemotide with placebo (2 : 1 randomization) as a maintenance therapy in patients with unresectable stage III NSCLC who had responded to or had stable disease after primary chemoradiotherapy (which had to be completed within 4–12 weeks before randomization) [14]. One dose of cyclophosphamide (300 mg/m2 i.v., maximum dose 600 mg) or placebo was administered before treatment. Eight consecutive weekly subcutaneous injections of tecemotide or placebo were followed in the absence of progressive disease by maintenance tecemotide or placebo every 6 weeks until disease progression. The primary end point was OS. Maintenance tecemotide did not result in improvement in OS over placebo {median OS 25.6 months with tecemotide (n = 829) and 22.3 months with placebo (n = 410) [hazard ratio (HR) 0.88, 0.75–1.03; P = 0.123]}. In a preplanned subgroup analysis, however, among patients who received concurrent chemoradiotherapy, OS was significantly longer with tecemotide than placebo {median OS 30.8 months [95% confidence interval (CI) 25.6–36.8] with tecemotide (n = 538) and 20.6 months (95% CI 17.4–23.9) with placebo (n = 268)}. However, in patients who received previous sequential chemoradiotherapy, OS was worse in patients in the tecemotide group [median OS 19.4 months (95% CI 17.6–23.1; n = 291) and 24.6 months (95% CI 18.8–33.0) with placebo (n = 142) (HR 1.12, 0.87–1.44; P = 0.38)]. Based on these results, an ongoing trial is studying the effect of tecemotide or placebo on OS of patients with unresectable stage III NSCLC with either stable disease or objective response following primary concurrent chemoradiotherapy (ClinicalTrials.gov Identifier: NCT02049151).
melanoma-associated antigen-A3 vaccine
Melanoma-associated antigen-A3 (MAGE-A3) vaccine is a protein-based vaccine consisting of the recombinant antigen ProtD-MAGE-A3/His (a fusion protein containing Protein D, a lipoprotein present on the surface of haemophilus influenzae B, MAGE-A3 protein and a polyhistidine tail) and a proprietary immunological adjuvant. MAGEs are tumor-specific shared antigens which are differentially overexpressed in many cancers including NSCLC. In a phase II trial of patients with completely resected, MAGE-A3-expressing early-stage NSCLC, humoral and cellular immune responses to MAGE-A3 and statistically nonsignificant improvements in disease-free intervals were observed [15, 16].
A phase III trial compared the efficacy of MAGE-A3 vaccine with placebo (2 : 1 randomization) in patients with completely resected MAGE-A3-expressing stage IB, II or IIIA NSCLC. Up to four cycles of adjuvant chemotherapy could be administered at the investigators' discretion. Thirteen doses of the vaccine were administered intramuscularly over 27 months. The primary objectives were disease-free survival (DFS) in the overall population and in those who did not receive adjuvant chemotherapy (co-primary end points). The trial enrolled 2312 MAGE-A3-positive patients (33% of patients screened had MAGE-A3-expressing tumors). The study was terminated by an independent data monitoring committee as MAGE-A3 vaccine did not significantly extend DFS compared with placebo either in the overall MAGE-A3-positive population or in those MAGE-A3-positive patients who did not receive chemotherapy [17].
considerations for active immunotherapy in NSCLC
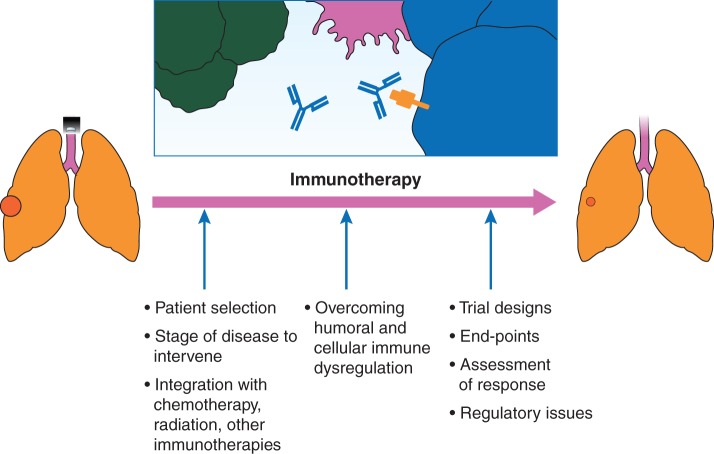
While a number of factors are important in clinical translation of successful active immunotherapy (Figure 1), we will discuss some which are more relevant in the context of the above described negative large phase III trials.
Figure 1.
Important considerations in clinical translation of successful active immunotherapy.
humoral and cellular immune dysregulation in lung cancer
In the first step of an adaptive immune response, effector T cells recognize antigenic peptides of tumor cells presented by antigen-presenting cells (APC) in the context of major histocompatibility complex (MHC) class I or class II molecules expressed on the APC surface. Additional co-stimulatory signals mediated through constitutively expressed co-stimulatory molecules on the T cell and the APC are also necessary for T-cell activation. The presence of both signals trigger intracellular events resulting in the activation and interleukin (IL)-2-dependent clonal proliferation of T cells. Expansion of T cells in sufficient numbers results in recognition and elimination of tumor cells. However, immune responses are dysregulated in cancer.
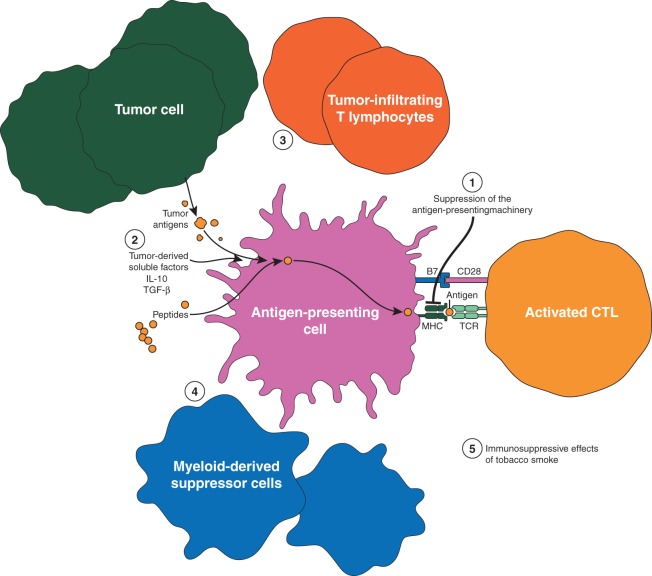
A number of mechanisms are employed by tumors to escape the host immune response and promote immune tolerance. These are perhaps the most important hurdles that need to be overcome for successful antigen-specific immunotherapy in NSCLC. The better understood immune resistance mechanisms in NSCLC are outlined in Figure 2.
Figure 2.
Mechanisms of humoral and cellular immune dysregulation in lung cancer. Tumor antigens are presented by antigen-presenting cells in the context of major histocompatibility complex class I or class II molecules are recognized by the T-cell receptors (TCR). Additional co-stimulatory signals are mediated through constitutively expressed co-stimulatory molecules on the T cell and the APC (e.g. B7-CD28) are also necessary for T-cell activation. The presence of both signals trigger intracellular events resulting in the activation and interleukin (IL)-2-dependent clonal proliferation of T cells. Some of the mechanisms employed by tumors to escape the host immune response and promote immune tolerance are represented: (1) Suppression of antigen-presenting machinery, (2) Soluble factors released by the tumor (examples include interleukin 10, and transforming growth factor-β), (3) Tumor-infiltrating T lymphocytes, (4) Myeloid-derived suppressor cells, (5) The immunosuppressive effects of tobacco smoke.
Suppression of antigen-presenting machinery is one of several mechanisms of immune escape. Multiple molecular mechanisms can lead to altered HLA expression within lung cancer. These include deficiencies in expression of antigen-processing genes [18–21], and haplotype loss of HLA class I antigens [22–24]. In small retrospective studies, absence HLA class I expression was associated with poor prognosis suggesting that downregulation of HLA class I expression may play a critical role in immune surveillance of patients with NSCLC [25, 26]. The reversibility of some of the aberrations in antigen processing by interferon (IFN)-γ indicates that it is possible to overcome the suppression of antigen-presenting machinery and may be of therapeutic relevance [21, 27]. Considering the critical role of antigen presentation in immune recognition of tumor cells, these mechanisms may be of potential therapeutic importance.
In addition to reduced antigen presentation, immune inhibitory cytokines secreted by the tumor cells can impair T-cell survival and help them avoid T-cell-mediated immune responses. Soluble factors derived from NSCLC cell-line supernatants have been described to markedly enhanced apoptosis of activated T cells [28]. TGF-β enables tumor evasion of immune surveillance through various mechanisms most of which converge on the impairment of tumor cell killing by immune effector cells [29]. In addition to inhibiting proliferation and differentiation of normal bronchial epithelial cells, TGF-β mediates conversion of CD4 + CD25− T cells to Tregs [30, 31]. Serum TGF-β levels are elevated in patients with lung cancer compared with normal individuals. Elevated plasma levels of TGF-β confer a poorer prognosis for patients with lung cancer [32]. IL-10 is a potent immunosuppressive cytokine that promotes lung cancer growth by suppressing T-cell and macrophage function and enabling tumors to escape immune detection [33–35].
Yet another mechanism of immunosuppression involves immune checkpoints which are molecules expressed on the surface of T lymphocytes and modulates the immune response to antigens via inhibitory or stimulatory signaling to T cells. Two most extensively studied immune inhibitory checkpoints in NSCLC are cytotoxic T-lymphocyte antigen-4 (CTLA-4) and PD-1. Activation of both receptors causes downregulation and inhibition of immune responses. PD-1 functions primarily in peripheral tissues where T cells may encounter the immunosuppressive PD-1 ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), which are expressed by tumor cells, stromal cells or both [36]. CTLA-4 mediates immune inhibitory signals which are distinct from PD-1 [37]. Clinical trial results of antibody-mediated blocking of CTLA-4 and PD-1 pathways indicate that this strategy is feasible and effective in NSCLC [1, 38].
A number of cells in the tumor microenvironment including Tregs, myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages have immunosuppressive properties. Tumor-infiltrating lymphocytes which are CD4 + CD25+, the activated phenotype of Tregs, mediate potent inhibition of autologous T-cell proliferation and prevent the host from mounting an immune response to tumor antigens [39]. Tregs of a similar phenotype (CD4+CD25+) with marked immunosuppressive activity are elevated in peripheral blood of NSCLC patients [40]. MDSCs are a heterogeneous population of cells of myeloid origin that are characterized by their immature state and ability to suppress T-cell responses [41]. In lung cancer, antibody-mediated MDSC depletion increased APC activity and augmented the activity of effector T cells leading to reduced tumor growth and enhanced therapeutic vaccination responses [42]. The prognostic significance of MDSCs in the tumor microenvironment is not established in NSCLC.
A number of metabolic enzymes including those associated with the catabolism of the amino acids arginine and tryptophan are associated with the suppressive activity of myeloid cells. Indoleamine 2,3-dioxygenase-1 (IDO1) is an enzyme which is expressed by a subset of dendritic cells that catalyzes the degradation of the amino acid tryptophan to kynurenine. IDO1 is thought to be an important regulator of the immunosuppressive mechanisms responsible for tumor escape from host immune surveillance and blockade of IDO activity increases the ability of tumor-bearing mice to reject tumors [43].
In summary, a number of mechanisms including reduced antigen presentation, antigenic loss, cytokines, immune checkpoints, immunosuppressive cells and enzymes are employed by tumors to escape the host immune response and promote immune tolerance.
trial design
With the benefit of hindsight, the negative results of these large phase III trials (with a combined accrual of over 4000) should come as no surprise. All three trials were initiated based on results of negative or at best inconclusive phase II data (Table 1) and post hoc analysis of small subgroups which showed positive results.
Table 1.
Phase II and phase III studies of selected antigen-specific immunotherapeutic approaches in nonsmall-cell lung cancer
| Investigational agent | Phase of study | N | Patients | Primary end point | Primary end point outcome |
Significance of differences between treatment group and control group | |
|---|---|---|---|---|---|---|---|
| Treatment group | Control group | ||||||
| Tecemotide | Randomized phase II (Butts and Maksymiuk et al. [12]) | 171 | IIIB or IV NSCLC SD or OR after first-line chemotherapy or chemoradiation |
OS | 17.2 m | 13 m | NS |
| Randomized, double-blind placebo-controlled phase III (Butts and Socinski et al. [14]) | 1513 | IIIA (T3, N2 only), IIIB and IV SD or OR after first-line chemotherapy or chemoradiation |
OS | 25.6 m | 22.3 m | NS | |
| Belagenpumatucel-L | Randomized, dose-variable phase II (Nemunaitis et al. [7]) | 75 | II, IIIA, IIIB and IV; low tumor burden Completed conventional therapy |
OS | Dose-related improvements in survival in three treatment armsa | NA | No control arm |
| Randomized, double-blind placebo-controlled phase III (Giaccone et al. [8]) | 532 | IIIA (T3, N2 only), IIIB and IV SD or OR after primary platinum-based chemoradiotherapy |
OS | 20.3 | 17.8 | NS | |
| Melanoma-associated antigen-A3 vaccine | Randomized phase II (Vansteenkiste [15]) | 182 | Completely resected IB/II MAGE-A3-expressing tumor | DFI | HR 0.74 (95% CI 0.44–1.20) P = 0.107b | NA | NS |
| Randomized, double-blind placebo-controlled phase III (release 2014) | 2312 | Completely resected IB, II, or IIIA MAGE-A3-expressing tumor |
DFS | Not available | Not available |
NS | |
aThree doses (1.25, 2.5 or 5.0 × 107 cells/injection) of belagenpumatucel-L were studied in three cohorts of 25, 26 and 24 patients each.
bHR in favor of the MAGE-A3 group.
For example, a randomized, open-label, phase II trial failed to show significant improvement in OS of patients who received tecemotide over those who received best supportive care. In the small subset of patients with stage IIIB-LR (locoregional) disease (n = 65), those who received tecemotide had a 17.3-month improvement in median OS (30.6 versus 13.3 months) [12].
In another instance, the phase III trial of belagenpumatucel-L was initiated based on a dose-related improvement in survival and response in the phase II trial. However, the phase II trial itself had small numbers of patients in the individual treatment arms (∼20 patients each in the three cohorts) who had low-volume disease. Furthermore, the phase II trial did not have a control arm [7, 8].
In a third instance, the randomized, placebo-controlled phase II trial of MAGE-A3 vaccine, which led to the larger phase III trial, had a limited sample size. With 182 patients, and an estimated power of 50% to detect a difference of 10% in absolute recurrence after 30 months, the study was unlikely to demonstrate improvements in efficacy. A related issue, emphasized by the phase II–III transition of this drug is the lack of adequate follow-up. Trends of activity observed in earlier analysis were not confirmed with more mature follow-up data [15, 16]. A number of factors including commercial pressures and misguided enthusiasm of investigators based on early trends may explain these failures.
While it is true that investigators would not initiate a trial if they did not think it had a reasonable chance of a statistically significant and clinically meaningful benefit, some have argued that the investigators frequently use overly optimistic assumptions of treatment benefits [44]. Unfortunately, this may have been true in the transition from phase II to phase III trials of antigen-specific immunotherapies in NSCLC.
future of antigen-specific immunotherapy in NSCLC
The failure of vaccines in NSCLC, despite their ability to prime and expand tumor antigen-specific T cells, could at least partly be attributed to the inability of vaccine-induced T-cell responses to overcome the tumoral mechanisms of immune escape. These mechanisms probably limit the clonal expansion of T cells following vaccination.
Many of the immunosuppressive mechanisms discussed above are potentially amenable to therapeutic modulation. Low doses of cyclophosphamide have been shown to selectively decrease circulating Tregs and suppress their inhibitory functions leading to a restoration of peripheral T-cell proliferation and innate killing activity [45]. Other drugs including chemotherapies and signal transduction inhibitors have also been shown to selectively target immunosuppressive cells in the tumor microenvironment [46, 47]. Metabolic enzymes and cytokines involved in the induction of tumor immune tolerance can also be inhibited pharmacologically [42, 48]. MDSC differentiation can be blocked in a number of ways including cyclooxygenase inhibitors, which prevent the production of prostaglandin [49].
Recent studies have demonstrated that immune checkpoints can be successfully modulated [1, 2]. An anti-PD-1 antibody, nivolumab was evaluated in a phase I trial in patients with advanced previously treated cancers [1]. Doses of 1, 3 and 10 mg/kg were administered i.v. once every 2 weeks with immune response assessment every 8 weeks. In the NSCLC expansion cohort, across all doses and histologies (squamous and nonsquamous), the objective response rate was 17% (22 of 129 patients) and median response duration 17 months [50]. Median OS was 9.2–14.9 months and 1-year OS rates 32% to 56%. In March 2015, nivolumab was approved by the FDA for use in patients with metastatic squamous cell lung cancer with progression on or after platinum-based chemotherapy. Its efficacy was established in a phase III, open-label, study that randomized previously treated patients (n = 272) with advanced squamous cell lung cancer to receive nivolumab 3 mg/kg i.v. every 2 weeks or docetaxel 75 mg/m2 i.v. every 3 weeks. OS, the primary end point of the trial, was prolonged by 3.2 months at the median in patients who received nivolumab compared with those who received docetaxel. Several other agents targeting PD-1 pathway are in clinical development, including pembrolizumab (MK-3475, anti PD1), MEDI4736 (anti-PDL1), BMS-936559 (anti-PDL1) and MPDL-3280 (anti-PDL1). Despite the promise of immune checkpoint inhibitors, it is clear that responses are limited, restricted presumably to patients with a pre-existing tumor-reactive T-cell response. Investigations of ways to select patients (e.g. PDL-1 expression in the tumor or infiltrating immune cells or both) are underway. There is growing interest in modulating the multiple immune inhibitory and co-stimulatory pathways in the tumor microenvironment by combining inhibitors of the PD-1 pathway with other immune checkpoints antibodies, including antagonist antibodies to KIR, LAG-3 and CTLA-4.
Antigen-specific vaccines offer an opportunity to potentially extend the responses with immune checkpoint inhibitors to a greater percentage of patients. A recent study showed that tumors resistant to anti PD-1 antibodies could be eradicated by combining them with vaccines containing tumor-specific peptides with high MHC-binding affinity [51]. In the study, melanomas that contained a high percentage of dysfunctional endogenous PD-1+ tumor-specific CD8+ T cells were treated with a PD-1 inhibitor and an exogenous tumor-specific antigen using attenuated Salmonella typhimurium. The combination rescued the endogenous tumor-specific CD8+ T-cell response and resulted in tumor regressions. A combinatorial strategy of vaccines and immune checkpoint inhibitors could rescue T cells which become dysfunctional after infiltrating long-established suppressive tumors, thereby overcoming one of the major obstacles to clinical benefit from vaccines. Most of these strategies are still in preclinical evaluation in NSCLC. While there is strong rationale to combine vaccines with other immunomodulatory strategies, important considerations in clinical testing of these combinations include determining the sequence of administration of drugs, and metrics of response assessment.
While the above-discussed approaches aim to overcome tumor-mediated immunosuppression, other approaches seek to enhance cellular immune responses through a number of different mechanisms. These include induction of immunogenic cell death with radiotherapy [52] and combination with adoptive T-cell transfer to prime T cells and amplify antitumor T-cell responses [53]. Immunogenic cell death is different from apoptotic cell death in the generation of specific molecular signals that are sensed by APC which stimulate their maturation and ability to cross-present tumor-derived antigens to T cells [54]. In addition to immunogenic cell death, radiation causes MHC I upregulation and release of antigens which are taken up by dendritic cells and presented to T cells that in turn migrate back to the tumor and provide local control, thus serving as an intrinsic vaccine priming adaptive immunity [55]. The ongoing process of killing of tumor cells by cytotoxic T lymphocytes sustains release of more tumor antigens and possibly promotes antigenic spread, i.e. the activation of a broader T-cell repertoire. Antigenic spread has been reported in some patients with prostate cancer who were treated with the combination of a vaccine and local radiotherapy [56].
Possible beneficial effects observed in subsets of patients on active immunotherapy trials indicates the need for better patient selection [8, 14]. While it is generally believed that these therapies are most active in patients with minimal volume of disease, no predictive markers have been identified to date. Better measures are needed to assess tumor-specific immune responses and understand the relationship between immune induction and clinical responses. The failure of phase III trials which were initiated based on ‘promising’ phase II trials also indicate the need to temper our optimism, particularly when making the expensive leap from phase II to phase III trials.
Finally, a better understanding of the immune dysregulation specific to NSCLC is needed. The immune evasion mechanisms in lung cancer are likely different from other tumors [57] due to the proinflammatory and immunosuppressive effects of tobacco smoke. Chronic inhalation of cigarette smoke is known to alter a wide range of immunological functions, including innate and adaptive immune responses [58]. In the context of active immunotherapy, the effect of smoking on T-cell responsiveness and proliferative capacity are important considerations. In animal models, chronic exposure to cigarette smoke affects T-cell responsiveness and decreases T-cell proliferative and T-cell dependent antibody responses [59]. Yet there are limited data on the effects of cigarette smoke on immune dysregulation in lung cancer patients. Challenges to this field of study include the multipartite nature of cigarette smoke and the significant variability in smoking patterns which makes it difficult to study its effect in experimental systems [60]. Recent data indicating that smoking-associated NSCLC may respond better to immune checkpoint blockade [61] also suggests the distinctive influence of tobacco smoke on the tumor microenvironment. To our knowledge, clinical reports of active specific immunostimulatory agents have not assessed the effect if any of smoking on the clinical or immune outcomes.
Heterogeneity within NSCLC between the primary tumor and metastatic sites and between tumors from different patients is well described [62]. However, our understanding of the association between oncogenes and immune escape and the differential influences of different oncogenic drivers on the immune milieu are still preliminary [63]. A study of PD-L1 expression by immunohistochemistry in surgically resected NSCLC samples showed a significant association between PD-L1 expression and the presence of EGFR mutations independent of other clinical factors studied [64]. In preclinical models, EGFR mutation-positive NSCLC may preferentially use PD-1/PD-L1-mediated mechanisms to evade immune surveillance [63]. In mouse models of lung cancer, tumors with different oncogenic drivers were characterized by distinct immune infiltrates [65]. Taken together, these data suggest the potentially distinctive effects on the immune microenvironment in individual genetic subsets of NSCLC. Further understanding of how NSCLCs with different genetic backgrounds shape the tumor immune mileu will help refine the use of active specific immunotherapy in NSCLC.
In conclusion, despite their ability to prime and expand tumor antigen-specific T cells, large phase III trials of several active specific immunostimulatory agents have yielded disappointing results in NSCLC. Several important issues need to be addressed to fully harness the therapeutic potential of antitumor immune responses induced by active immunotherapy. Strategies aimed at overcoming immune tolerance and improving the activation of antitumor T cells via combinatorial approaches may represent a new and more promising therapeutic application for active immunotherapies in NSCLC.
funding
This work was supported by the Intramural program, National Cancer Institute, National Institutes of Health. No grant numbers apply.
disclosure
The authors have declared no conflicts of interest.
references
- 1.Topalian SL, Hodi FS, Brahmer JR et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Drake CG, Wollner I et al. . Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol 2012; 13: e301–e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepherd FA, Douillard JY, Blumenschein GR Jr. Immunotherapy for non-small cell lung cancer: novel approaches to improve patient outcome. J Thorac Oncol 2011; 6: 1763–1773. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Higano CS, Shore ND et al. . Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–422. [DOI] [PubMed] [Google Scholar]
- 6.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 2010; 10: 415–424. [DOI] [PubMed] [Google Scholar]
- 7.Nemunaitis J, Dillman RO, Schwarzenberger PO et al. . Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol 2006; 24: 4721–4730. [DOI] [PubMed] [Google Scholar]
- 8.Giaccone G, Bazhenova L, Nemunaitis J et al. . A phase III study of belagenpumatucel-L therapeutic tumor cell vaccine for non-small cell lung cancer (NSCLC). In Presented at 2013 European Cancer Congress; 27 September–1 October 2013; Late-Breaking Abstract 2. Amsterdam, The Netherlands. [Google Scholar]
- 9.North S, Butts C. Vaccination with BLP25 liposome vaccine to treat non-small cell lung and prostate cancers. Expert Rev Vaccines 2005; 4: 249–257. [DOI] [PubMed] [Google Scholar]
- 10.Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non-small cell lung cancer. Clin Cancer Res 2007; 13: s4652–s4654. [DOI] [PubMed] [Google Scholar]
- 11.Butts C, Murray N, Maksymiuk A et al. . Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 2005; 23: 6674–6681. [DOI] [PubMed] [Google Scholar]
- 12.Butts C, Maksymiuk A, Goss G et al. . Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 2011; 137: 1337–1342. [DOI] [PubMed] [Google Scholar]
- 13.Butts C, Murray RN, Smith CJ et al. . A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer 2010; 11: 391–395. [DOI] [PubMed] [Google Scholar]
- 14.Butts C, Socinski MA, Mitchell PL et al. . Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 59–68. [DOI] [PubMed] [Google Scholar]
- 15.Vansteenkiste J, Zielinski M, Linder A et al. . Final results of a multi-center, double-blind, randomized, placebo-controlled phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC). J Clin Oncol 2007; 25 (suppl 18): abstr 7554. [Google Scholar]
- 16.Vansteenkiste J, Zielinski M, Linder A et al. . Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 2013; 31: 2396–2403. [DOI] [PubMed] [Google Scholar]
- 17. Update on phase III clinical trial of investigational MAGE-A3 antigen-specific cancer immunotherapeutic in non-small cell lung cancer. GSK Press Release. 2 April 2014.
- 18.Chen HL, Gabrilovich D, Tampe R et al. . A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat Genet 1996; 13: 210–213. [DOI] [PubMed] [Google Scholar]
- 19.Singal DP, Ye M, Qiu X. Molecular basis for lack of expression of HLA class I antigens in human small-cell lung carcinoma cell lines. Int J Cancer 1996; 68: 629–636. [DOI] [PubMed] [Google Scholar]
- 20.Johnsen A, France J, Sy MS, Harding CV. Down-regulation of the transporter for antigen presentation, proteasome subunits, and class I major histocompatibility complex in tumor cell lines. Cancer Res 1998; 58: 3660–3667. [PubMed] [Google Scholar]
- 21.Restifo NP, Esquivel F, Kawakami Y et al. . Identification of human cancers deficient in antigen processing. J Exp Med 1993; 177: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baba T, Hanagiri T, Ichiki Y et al. . Lack and restoration of sensitivity of lung cancer cells to cellular attack with special reference to expression of human leukocyte antigen class I and/or major histocompatibility complex class I chain related molecules A/B. Cancer Sci 2007; 98: 1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiraki A, Kaneshige T, Kiura K et al. . Loss of HLA haplotype in lung cancer cell lines: implications for immunosurveillance of altered HLA class I/II phenotypes in lung cancer. Clin Cancer Res 1999; 5: 933–936. [PubMed] [Google Scholar]
- 24.Sugaya M, Takenoyama M, Osaki T et al. . Establishment of 15 cancer cell lines from patients with lung cancer and the potential tools for immunotherapy. Chest 2002; 122: 282–288. [DOI] [PubMed] [Google Scholar]
- 25.Passlick B, Izbicki JR, Simmel S et al. . Expression of major histocompatibility class I and class II antigens and intercellular adhesion molecule-1 on operable non-small cell lung carcinomas: frequency and prognostic significance. Eur J Cancer 1994; 30A: 376–381. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi E, Yamazaki K, Torigoe T et al. . HLA class I antigen expression is associated with a favorable prognosis in early stage non-small cell lung cancer. Cancer Sci 2007; 98: 1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traversari C, Meazza R, Coppolecchia M et al. . IFN-gamma gene transfer restores HLA-class I expression and MAGE-3 antigen presentation to CTL in HLA-deficient small cell lung cancer. Gene Therapy 1997; 4: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 28.Batra RK, Lin A, Sharma S et al. . Non-small cell lung cancer-derived soluble mediators enhance apoptosis in activated T lymphocytes through an I kappa B kinase-dependent mechanism. Cancer Res 2003; 63: 642–646. [PubMed] [Google Scholar]
- 29.Torre-Amione G, Beauchamp RD, Koeppen H et al. . A highly immunogenic tumor transfected with a murine transforming growth factor type beta 1 cDNA escapes immune surveillance. Proc Natl Acad Sci USA 1990; 87: 1486–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masui T, Wakefield LM, Lechner JF et al. . Type-beta transforming growth-factor is the primary differentiation-inducing serum factor for normal human bronchial epithelial-cells. Proc Natl Acad Sci USA 1986; 83: 2438–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu VC, Wong LY, Jang T et al. . Tumor evasion of the immune system by converting CD4(+) CD25(-) T cells into CD4(+) CD25(+) T regulatory cells: role of tumor-derived TGF-beta. J Immunol 2007; 178: 2883–2892. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa Y, Takanashi S, Kanehira Y et al. . Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 2001; 91: 964–971. [PubMed] [Google Scholar]
- 33.Sharma S, Stolina M, Lin Y et al. . T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol 1999; 163: 5020–5028. [PubMed] [Google Scholar]
- 34.Spagnoli GC, Juretic A, Schultzthater E et al. . On the relative roles of interleukin-2 and interleukin-10 in the generation of lymphokine-activated killer-cell activity. Cell Immunol 1993; 146: 391–405. [DOI] [PubMed] [Google Scholar]
- 35.Huang M, Sharma S, Mao JT, Dubinett SM. Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhance peripheral blood lymphocyte IL-10 transcription and protein production. J Immunol 1996; 157: 5512–5520. [PubMed] [Google Scholar]
- 36.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura H, Nose M, Hiai H et al. . Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11: 141–151. [DOI] [PubMed] [Google Scholar]
- 38.Lynch TJ, Bondarenko I, Luft A et al. . Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012; 30: 2046–2054. [DOI] [PubMed] [Google Scholar]
- 39.Woo EY, Yeh H, Chu CS et al. . Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 2002; 168: 4272–4276. [DOI] [PubMed] [Google Scholar]
- 40.Wolf AM, Wolf D, Steurer M et al. . Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003; 9: 606–612. [PubMed] [Google Scholar]
- 41.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9: 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srivastava MK, Zhu L, Harris-White M et al. . Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One 2012; 7:e40677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Shin N, Koblish HK et al. . Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010; 115: 3520–3530. [DOI] [PubMed] [Google Scholar]
- 44.Gan HK, You B, Pond GR, Chen EX. Assumptions of expected benefits in randomized phase III trials evaluating systemic treatments for cancer. J Natl Cancer Inst 2012; 104: 590–598. [DOI] [PubMed] [Google Scholar]
- 45.Sistigu A, Viaud S, Chaput N et al. . Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol 2011; 33: 369–383. [DOI] [PubMed] [Google Scholar]
- 46.Finke JH, Rini B, Ireland J et al. . Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res 2008; 14: 6674–6682. [DOI] [PubMed] [Google Scholar]
- 47.Locher C, Conforti R, Aymeric L et al. . Desirable cell death during anticancer chemotherapy. Ann N Y Acad Sci 2010; 1209: 99–108. [DOI] [PubMed] [Google Scholar]
- 48.Garrison K, Hahn T, Lee WC et al. . The small molecule TGF-beta signaling inhibitor SM16 synergizes with agonistic OX40 antibody to suppress established mammary tumors and reduce spontaneous metastasis. Cancer Immunol Immunother 2012; 61: 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res 2007; 67: 4507–4513. [DOI] [PubMed] [Google Scholar]
- 50.Brahmer JR HL, Gandhi G et al. . Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC): Survival and clinical activity by subgroup analysis. J Clin Oncol 2014; 32 (5s suppl): abstr 8112. [Google Scholar]
- 51.Binder DC, Engels B, Arina A et al. . Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer Immunol Res 2013; 1: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golden EB, Demaria S, Schiff PB et al. . An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013; 1: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kandalaft LE, Tanyi J, Chiang C et al. . Autologous whole-tumor antigen vaccination in combination with adoptive T cell therapy for patients with recurrent ovarian cancer. J Immunother Cancer 2013; 1(Suppl 1): 220. [Google Scholar]
- 54.Tesniere A, Panaretakis T, Kepp O et al. . Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 2008; 15: 3–12. [DOI] [PubMed] [Google Scholar]
- 55.Tang C, Wang X, Soh H et al. . Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014; 2: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nesslinger NJ, Ng A, Tsang KY et al. . A viral vaccine encoding prostate-specific antigen induces antigen spreading to a common set of self-proteins in prostate cancer patients. Clin Cancer Res 2010; 16: 4046–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prado-Garcia H, Romero-Garcia S, Aguilar-Cazares D et al. . Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clin Dev Immunol 2012; 2012: 741741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002; 2: 372–377. [DOI] [PubMed] [Google Scholar]
- 59.Kalra R, Singh SP, Savage SM et al. . Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive Ca(2+) stores. J Pharmacol Exp Ther 2000; 293: 166–171. [PubMed] [Google Scholar]
- 60.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 2009; 9: 377–384. [DOI] [PubMed] [Google Scholar]
- 61.Herbst RS, Soria JC, Kowanetz M et al. . Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Fujimoto J, Zhang J et al. . Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014; 346: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akbay EA, Koyama S, Carretero J et al. . Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013; 3: 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azuma K, Ota K, Kawahara A et al. . Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014; 25: 1935–1940. [DOI] [PubMed] [Google Scholar]
- 65.Xu C, Fillmore CM, Koyama S et al. . Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 2014; 25: 590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]