In a retrospective analysis, maintenance rituximab after autologous stem cell transplantation in patients with mantle cell lymphoma appears to improve progression-free and overall survival.
Keywords: mantle lymphoma, autologous transplantation, rituximab, maintenance
Abstract
Background
High-dose therapy and autologous stem cell transplantation (ASCT) improves outcomes for patients with mantle cell lymphoma (MCL), but relapse ultimately occurs in most patients. Recently presented interim results from a phase III prospective trial suggest maintenance rituximab (MR) after ASCT for MCL improves progression-free survival (PFS). The maturation of these data and any benefit of MR on overall survival (OS) remain to be defined.
Patients and methods
In this retrospective study, we examined a cohort of consecutive patients with MCL that underwent ASCT for MCL at our center and evaluated their outcomes according to whether they received MR after ASCT (n = 50) or did not (n = 107). MR was treated as a time-dependent covariate to account for variation in timing of its initiation.
Results
MR was associated with an improved PFS [hazard ratio (HR) 0.44; confidence interval (CI) (0.24–0.80), P = 0.007] and overall survival (OS; HR 0.46; CI 0.23–0.93, P = 0.03) following a multivariate adjustment for confounding factors with a median follow-up of ∼5 years. Grade 4 neutropenia was increased (34% versus 18%, P = 0.04) in the MR group, but no effect on the rate of mortality unrelated to relapse was observed.
Conclusions
These data support that MR after ASCT for MCL confers a benefit in PFS and additionally suggest it may improve OS. General application of this strategy will require confirmation of benefit in prospective randomized trials.
introduction
Mantle cell lymphoma (MCL), a mature B-cell non-Hodgkin lymphoma (B-NHL), typically presents with disseminated nodal and extra-nodal involvement, and a variable but aggressive course. Treatment strategies depend on patient age, condition, and preference [1]. Patients fit to receive intensive treatment may benefit from multi-agent immunochemotherapy followed by consolidation with high-dose therapy and autologous stem cell transplantation (ASCT) [2–4]. Despite an objective response rate (ORR) exceeding 90% in recent series [5, 6], patients almost inevitably relapse and not all are candidates for intensive therapy, necessitating improvements in standard treatment [4].
Increasingly, data support a role for the administration of maintenance therapy for improving outcomes after induction. In patients with MCL unable to receive ASCT consolidation, maintenance rituximab (MR) after induction immunochemotherapy has been shown to extend progression-free survival (PFS) [7, 8] and overall survival (OS) [9, 10]. For patients with MCL that undergo ASCT, however, the value of MR after ASCT remains to be established, though limited retrospective data have shown a benefit in PFS [11] and recently presented interim data from a prospective phase III trial indicate an improvement in PFS, but not OS [12]. In this article, we analyze the impact of MR following ASCT in a sequential cohort of patients with MCL treated at our institutions.
methods
study cohort
We retrospectively studied consecutive patients with MCL who underwent ASCT at the University of Washington Medical Center (UWMC), the Fred Hutchinson Cancer Research Center (FHCRC), and the Veterans Affairs Puget Sound Health Care System (Seattle, Washington) between November 1995 and May 2011. Data were collected from comprehensive medical record review and institutional databases. Follow-up was updated as of November 2014. MR was defined as rituximab administered after ASCT in the absence of disease progression. Patients who underwent tandem autologous/allogeneic stem cell transplantation (n = 2) or had inadequate data to assess receipt of MR (n = 5) were excluded from analysis. All authors had access to the primary clinical data. The Institutional Review Board of FHCRC approved data collection and analysis.
treatment and definitions
Transplant conditioning regimens were determined based on patient age, comorbidities, remission status, and prior therapies and categorized as chemotherapy-only or radiation-based. Chemotherapy-only regimens consisted of busulfan, melphalan, and thiotepa as well as carmustine, etoposide, cytarabine and melphalan . Radiation-based regimens included fractionated total body irradiation (TBI) combined with cyclophosphamide with or without etoposide or high-dose radiolabeled antibody-based regimens either alone or in combination with cyclophosphamide and etoposide or combined with fludarabine. Rituximab maintenance regimens are described below in the Results section.
Response to chemotherapy was defined as chemosensitive if a CR or a partial remission (PR) had been achieved with the chemotherapy immediately before ASCT according to standard criteria [13, 14]. Simplified MCL prognostic index (sMIPI) scores were calculated using data from diagnosis and before ASCT [15].
statistical methods
Patient characteristics and treatments were compared using a χ2 analysis or Fisher's exact test, as appropriate. Continuous variables were compared using the Wilcoxon rank-sum test. PFS and OS were calculated from the date of ASCT, where PFS was defined as a lack of relapse, progression, or death from any cause.
To account for the variability in time to its initiation after ASCT, MR was treated as a time-dependent covariate. Statistical significance of differences in event rates between the MR and no-MR groups was evaluated with the Cox proportional hazards regression model. The number of events of the outcome in PFS and OS was sufficiently small to require a selection process for covariate inclusion. Covariates were selected according to clinical relevance and by using a series of univariate models to pare down the possible variables that would be useful, then backwards selection was employed to determine which variables would be included in the model. Two-sided P-values less than 0.05 were considered statistically significant.
Treating MR as a time-dependent covariate prohibits the precise graphical depiction of the data since patients move from the no-MR group to the MR group at different points after ASCT [16]. Survival curves were therefore approximated using Kaplan–Meier (KM) plots generated with a day 100 landmark; patients who died or experienced progression of disease before day 100 after ASCT were not included in the KM plots and patients who did not begin MR before day 100 remained in the no-MR group. Statistical differences in the survival curves were estimated using the Cox proportional hazards regression model.
results
patient characteristics
One-hundred and fifty-seven patients met the above criteria and were evaluated in this study. Their demographics and characteristics are summarized in Table 1. The median age at ASCT was 57 years old (range 35–71) and 134 (85%) were men. MR was administered to 50 patients (32%) and the remaining 107 patients (68%) received no MR. Patients in the MR and no-MR groups were similar with respect to gender, age, blastoid-variant histology, and the presence of B-symptoms at diagnosis. All patients in the MR group had received rituximab before ASCT, whereas 13 of the 107 patients in the no-MR group had no prior rituximab (P = 0.01). Patients in the MR group were more likely to have received high-dose cytarabine as a component of induction chemoimmunotherapy (P = 0.02) and to have undergone ASCT during first remission (P < 0.001) and in complete remission (CR) (P = 0.002), and to have received conditioning without radiation (P = 0.001). The groups were well matched for sMIPI score at the time of diagnosis (P = 0.38) and at ASCT (P = 0.61). Patients who received MR underwent ASCT more recently than those patients that did not (P = 0.009).
Table 1.
Demographic and clinical characteristics of study cohort, stratified by receipt of maintenance rituximab
| Variable | All (n = 157) | MR (n = 50) | no-MR (n = 107) | P-valuea |
|---|---|---|---|---|
| Male (%) | 134 (85) | 41 (82) | 93 (87) | 0.47 |
| Median age at ASCT (range) | 58 (35–71) | 58 (38–71) | 58 (35–70) | 0.43 |
| B-symptoms at diagnosis (%) | 39 (25) | 16 (32) | 23 (21) | 0.17 |
| Blastoid variant (%, of evaluable)b | 12 (9) | 2 (4) | 10 (11) | 0.16 |
| sMIPI at diagnosisb (%, of evaluable) | ||||
| 0–2 | 37 (31) | 14 (36) | 23 (28) | |
| ≥3 | 84 (69) | 25 (64) | 59 (72) | 0.38 |
| Chemosensitive disease (%) | 144 (92) | 49 (98) | 95 (89) | 0.06 |
| Rituximab before ASCT (%) | 144 (92) | 50 (100) | 94 (88) | 0.01 |
| HiDAC with induction (%) | 58 (37) | 25 (50) | 33 (31) | 0.02 |
| sMIPI at ASCTb (%, of evaluable) | ||||
| 0–2 | 79 (51) | 27 (54) | 52 (50) | |
| ≥3 | 76 (49) | 23 (46) | 53 (50) | 0.61 |
| ASCT in the first remission (%) | 98 (62) | 42 (84) | 56 (52) | <0.001 |
| Disease status at ASCT (%) | ||||
| CR | 74 (47) | 34 (68) | 40 (37) | |
| PR | 68 (43) | 13 (26) | 55 (51) | |
| SD/Relapse | 15 (10) | 3 (6) | 12 (11) | 0.002 |
| Transplant conditioning (%) | ||||
| Chemotherapy-only | 35 (22) | 19 (38) | 16 (15) | 0.001 |
| Radiation-based | 122 (78) | 31 (62) | 91 (85) | |
| Year of ASCT (%) | ||||
| 1995–2003 | 47 (30) | 8 (16) | 39 (37) | 0.009 |
| 2004–2011 | 110 (70) | 42 (84) | 68 (64) | |
MR, maintenance rituximab; ASCT, autologous stem cell transplant; HiDAC, high-dose cytarabine; sMIPI, simplified mantle cell lymphoma international prognostic index; CR, complete remission; PR, partial remission; SD, stable disease.
aP-values are calculated from comparing the MR and no-MR groups.
bData are not available for all patients.
maintenance rituximab regimens
The decision to administer MR was made by the treating physician on an individual basis (n = 150) or as a part of two separate phase II protocols (n = 7). MR was given according to the following dosing schedules: weekly dosing for 4 weeks every 6 months for two to four courses (n = 15), weekly dosing for a single 4-week course (n = 8), and every 3-month dosing for two to eight doses (n = 7); multiple different dosing schedules were used in the remaining cases (n = 20). A median of eight (range 1–16) doses of MR was administered at a dose of 375 mg/m2. MR was initiated at a median of 77 days after ASCT (range 27–287, standard deviation 56 days) and the last dose was administered at a median of 271 days after ASCT (range 55–1074).
toxicities of maintenance rituximab
Grade 4 neutropenia was observed in 16 of 47 assessable patients (34%) in the MR group, and 16 of 87 assessable patients (18%) in the no-MR group (P = 0.04). Granulocyte colony stimulating factor (GCSF) was administered for neutropenia in 15 of 47 assessable patients (32%) in the MR group, and 10 of 85 assessable patients (12%) in the no-MR group (P = 0.005). Mortality unrelated to MCL relapse (NRM) occurred in four patients (7%) in the MR group and nine patients (9%) in the no-MR group (P = 0.77) at a median time of 840 days (range 7–2730) after ASCT, and no instances of NRM were predated by documented severe neutropenia.
association of maintenance rituximab with PFS and OS
Direct unadjusted comparison of PFS and OS between the MR and non-MR groups indicated a PFS (HR 0.48; CI 0.29–0.82, P = 0.007) and OS (HR 0.43; CI 0.23–0.80, P = 0.008) benefit was associated with MR (Supplementary Table S1, available at Annals of Oncology online). Since the decision to deliver post-transplant MR was non-randomized, we evaluated these end points in the context of the baseline features of each treatment arm. Following multivariable analysis adjusting for potentially confounding variables, the association of MR with a significantly prolonged PFS (HR 0.44; CI 0.24–0.80, P = 0.007) (Table 2) was retained. Multivariable analysis was similarly carried out to account for imbalances potentially impacting OS, and MR retained its association with improved OS (HR 0.46; CI 0.23–0.93, P = 0.03) (Table 2) with a median follow-up after ASCT for surviving patients of 5.0 years (range 0.02–18.1).
Table 2.
Multivariate analysis of factors imbalanced between the MR and no-MR groups
| Variable | PFS (91 events) |
OS (67 events) |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| MR | 0.44 (0.24–0.80) | 0.007 | 0.46 (0.23–0.93) | 0.03 |
| Year of ASCTa | 1.16 (1.06–1.27) | 0.001 | 1.03 (0.93–1.14) | 0.50 |
| Chemosensitive disease | 0.43 (0.22–0.87) | 0.02 | 0.41 (0.19–0.88) | 0.02 |
| ASCT in 1st Remission | 0.43 (0.26–0.71) | <0.001 | 0.36 (0.20–0.65) | <0.001 |
| CR at ASCT | 0.49 (0.28–0.85) | 0.01 | 0.51 (0.27–0.96) | 0.02 |
| Chemo-only conditioning | 0.76 (0.44–1.30) | 0.32 | 0.74 (0.40–1.39) | 0.36 |
| Rituximab before ASCT | 0.73 (0.26–2.01) | 0.54 | 0.51 (0.20–1.34) | 0.17 |
| sMIPI ≥3 at ASCT | 2.49 (1.43–4.33) | 0.001 | ||
| B symptoms at diagnosis | 2.42 (1.40–4.20) | 0.002 | ||
The Cox proportional hazard model for PFS included the following variables: the year of ASCT, receipt of rituximab before ASCT, disease status at ASCT, chemosensitivity, whether ASCT was carried out in 1st CR, the conditioning regimen, presence of B-symptoms at diagnosis, and the sMIPI score at the time of ASCT. The Cox proportional hazard model for OS included the following variables: the year of ASCT, receipt of rituximab before ASCT, disease group at ASCT, chemosensitivity of disease, whether ASCT was carried out in the first CR, and the transplant conditioning regimen. Note the relatively smaller number of events registered for OS compared with PFS precluded inclusion in the model of the presence of B-symptoms at diagnosis and the sMIPI score at the time of ASCT.
PFS, progression-free survival; OS, overall survival; MR, maintenance rituximab; ASCT, autologous stem cell transplant; sMIPI, simplified mantle cell lymphoma international prognostic index; CR, complete remission.
aContinuous variable.
As the study spanned a range of time during which the treatment options for MCL evolved to include novel agents such as proteasome inhibitors and immunomodulators, we conducted additional analyses to identify whether the OS benefit with MR might reflect the use of these newer treatment options. Patients who relapsed after ASCT were entered into a Cox proportional hazard model of OS after relapse. The same covariates as shown in Table 2 were included. There was no difference in OS after relapse (HR 1.3 for MR group, P = 0.75), implicating time to progression (TTP) after ASCT as the key factor influencing OS. Additionally, as the cohort of patients that underwent ASCT between 2004 and 2011 reflects a population treated according to more modern algorithms, it was examined independently with an interaction analysis for OS. This showed no association of the time period of ASCT with the effect of MR on OS (P = 0.36).
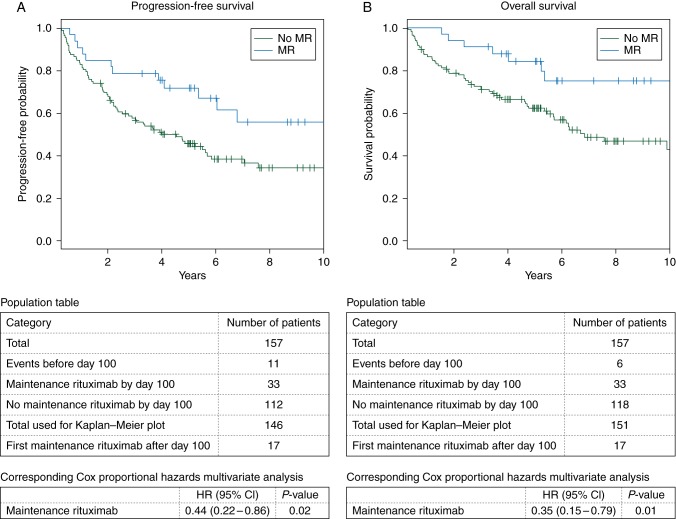
With the limitations described above for graphically representing estimates of survival in time-dependent covariate analyses, PFS and OS at 3 years were 79% and 91%, respectively, in the MR group and 55% and 69%, respectively, in the no-MR group (Figure 1).
Figure 1.
Kaplan–Meier plots using landmark of day 100 after autologous stem cell transplant depict the effect of MR on PFS (A) and OS (B). The population tables describe the number of patients accounted for in each group. Statistical differences in the survival curves were estimated using the Cox proportional hazards regression model according to the corresponding population table.
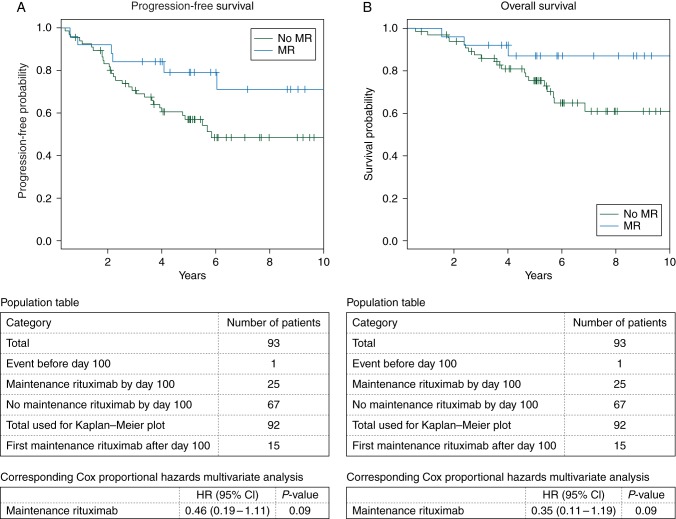
Since ASCT consolidation is now thought most beneficial to patients with MCL in first CR (CR1) or first PR (PR1), interaction analyses were carried out for this subgroup, as well. These showed no relationship between remission status of disease at ASCT (P = 0.26 for PFS and 0.92 for OS) or whether ASCT was carried out in the first remission (P = 0.49 for PFS and 0.93 for OS) and the effect of MR on survival outcomes, indicating that the benefit of MR in this setting is not impacted by remission status and holds true for this most clinically relevant setting. Kaplan–Meier plots of the CR1/PR1 subgroup using a day 100 landmark are shown in Figure 2. Though the limitations of plotting these time-dependent covariate data reduce the power of the observations when shown graphically, the impact of MR in this subgroup is best described by the interaction analyses that suggest its benefit meets statistical significance.
Figure 2.
Kaplan–Meier plots using landmark of day 100 after autologous stem cell transplant (ASCT) depict the effect of MR on PFS (A) and OS (B) in the subgroup of patients who underwent ASCT in first complete remission or first partial remission. The population tables describe the number of patients accounted for in each group. Statistical differences in the survival curves were estimated using the Cox proportional hazards regression model according to the corresponding population table.
discussion
MR after ASCT in patients with MCL has to-date shown potential though not conclusive benefit, though all previously published prospective studies have included small numbers of representative patients and have been non-comparative [17–20]. The data presented here suggest that MR after ASCT for patients with MCL results in an improvement in both PFS and OS. The relatively large number of patients evaluated (n = 157), long follow-up (median = 5 years), comparative nature, and detailed information allow for adjustment for many potential imbalances inherent to the retrospective analysis. The relative effect-size for each end point after multivariable adjustment (HR 0.44; CI 0.24–0.80, P = 0.007 for PFS; HR 0.46; CI 0.23–0.93, P = 0.03 for OS) are comparable with those demonstrated in a prospective randomized study comparing MR versus maintenance interferon alfa in patients with MCL that received R-CHOP induction therapy (HR 0.41; CI 0.26–0.65, P = 0.0001 for remission duration; HR 0.48; CI 0.23–0.97, P = 0.04 for OS) but were not fit enough to subsequently undergo consolidative ASCT [10].
The benefit to OS shown here for patients receiving MR after ASCT is in contrast to a retrospective analysis from Dietrich et al. that reported in a multivariate analysis of a cohort of 72 patients with MCL, 22 of whom received MR after ASCT, PFS was improved (HR 0.23; CI 0.06–0.80, P = 0.02) but OS was unchanged (HR 0.61; CI 0.14–2.65, P = 0.51) [11]. Le Gouill et al. recently presented interim analysis from the LyMa study (NCT00921414) designed to assess the impact of 3 years of MR after ASCT in patients with MCL and showed a statistically significant improvement in 2-year event-free survival (EFS) (93.2% with MR; CI 68.9%–96.6% versus 81.5% with no MR; CI 72.7%–87.7%, P = 0.015) but no effect, to date, on OS (2-year OS 93.4% with MR; CI 86.6–96.9 versus 93.9% with no MR; CI 86.7–97.3) [12]. Further maturation of these data is therefore awaited.
The mechanism for MR's efficacy in this population remains to be elucidated. The lack of interaction between MR effect and disease status at transplant (P = 0.26 for PFS and 0.92 for OS) argues against conversion to CR as the driving etiology, though minimal residual disease was not taken into account in this analysis. Alternatively, MR may postpone clinical disease relapse in those patients in whom molecular relapse has occurred [21]. MR after ASCT was associated with a twofold increased risk of severe neutropenia (34% from 18%), a well-described phenomenon with, to-date, a poorly understood pathogenesis [22, 23]. Previously, a wide range (3.6%–54%) of severe neutropenia has been reported from studies investigating MR after ASCT in B-NHL [17–19], though our data are the first to quantify this toxicity compared with patients not receiving MR to account for underlying marrow damage from prior cytotoxic therapy. Reassuringly, the rates of NRM in the MR and no-MR groups were similar, suggesting that the increased incidence of neutropenia could be effectively managed and should not preclude the use of MR for this population, though it does mandate close clinical and laboratory follow-up and adds to the complexity and cost of this strategy. It is also likely that the prolonged B-cell depletion from this approach will delay successful immunization of patients after ASCT [24].
Despite the noted observations of this study, the retrospective, non-randomized design must be acknowledged before immediately applying this strategy to all MCL patients completing ASCT. MR was administered in a variety of dosing schedules and times after ASCT, adding to the heterogeneity of the findings but, arguably, also enhancing the applicability of these data to general practice. Our data encompassed a wide span of time during which additional effective therapies in MCL were introduced and may have improved OS in patients with progression of disease after ASCT, though analyses investigating the impact of MR did show that the improvement in OS appears to be primarily consequent to delayed progression of disease. Though the multivariate adjustment accounted for some of the variation in characteristics between the two populations, the number of PFS and OS events did restrict the input of potential confounders into the models. As such, these data should primarily be used to set the stage for confirmation of benefit of MR after ASCT in MCL via prospective randomized trials, with maturation of the data from the aforementioned LyMa trial anticipated. In the meantime, this study adds to the growing body of evidence in support of using MR in MCL and is the first to suggest a survival advantage for its use after ASCT.
funding
This work was supported by research funding from NCI P01 CA44991, K12 CA076930, K24 CA184039, and Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Cancer Center Support Grant P30 CA015704, and gifts from Frank and Betty Vandermeer, Don and Debbie Hunkins, the Mary Aileen Wright Memorial Fund, and The David and Patricia Giuliani Family Foundation. A.K.G. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society. SAG is supported by NIH 5T32 HL007093.
disclosure
AKG additionally received research funding from the Lymphoma Research Foundation, Leukemia and Lymphoma Society, NCCN, GlaxoSmithKline, Merck, Bristol Myers Squibb, Gilead, Seattle Genetics, Teva, Eli Lilly, Piramal, Spectrum, Biogen-Idec, Pfizer, Abbott, BioMarin, Janssen, Millennium, and private donations. AKG has received honoraria and served as a consultant for Millennium, Seattle Genetics, Compliment, American College of Physicians, Pfizer, Gilead, Spectrum, and National Marrow Donor Program. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We thank all the patients who participated in this study.
references
- 1.Dreyling M, European Mantle Cell Lymphoma Network. Mantle cell lymphoma: biology, clinical presentation, and therapeutic approaches. Am Soc Clin Oncol Educ Book 2014: 191–198. [DOI] [PubMed] [Google Scholar]
- 2.Dreyling M, Lenz G, Hoster E et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood 2005; 105: 2677–2684. [DOI] [PubMed] [Google Scholar]
- 3.Geisler CH, Kolstad A, Laurell A et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 2008; 112: 2687–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrmann A, Hoster E, Zwingers T et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol 2009; 27: 511–518. [DOI] [PubMed] [Google Scholar]
- 5.Delarue R, Haioun C, Ribrag V et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood 2013; 121: 48–53. [DOI] [PubMed] [Google Scholar]
- 6.Geisler CH, Kolstad A, Laurell A et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol 2012; 158: 355–362. [DOI] [PubMed] [Google Scholar]
- 7.Forstpointner R, Unterhalt M, Dreyling M et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006; 108: 4003–4008. [DOI] [PubMed] [Google Scholar]
- 8.Kahl BS, Longo WL, Eickhoff JC et al. Maintenance rituximab following induction chemoimmunotherapy may prolong progression-free survival in mantle cell lymphoma: a pilot study from the Wisconsin Oncology Network. Ann Oncol 2006; 17: 1418–1423. [DOI] [PubMed] [Google Scholar]
- 9.Kluin-Nelemans HC, Doorduijn JK. Treatment of elderly patients with mantle cell lymphoma. Semin Hematol 2011; 48: 208–213. [DOI] [PubMed] [Google Scholar]
- 10.Kluin-Nelemans HC, Hoster E, Hermine O et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012; 367: 520–531. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich S, Weidle J, Rieger M et al. Rituximab maintenance therapy after autologous stem cell transplantation prolongs progression-free survival in patients with mantle cell lymphoma. Leukemia 2014; 28: 708–709. [DOI] [PubMed] [Google Scholar]
- 12.Le Gouill S, Thieblemont C, Oberic L et al. Rituximab maintenance versus wait and watch after four courses of R-DHAP followed by autologous stem cell transplantation in previously untreated young patients with mantle cell lymphoma: first interim analysis of the phase III prospective lyma trial, a LYSA study. In: American Society of Hematology Annual Conference: Blood 2014; 124 (21), Abstract 146. [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Horning SJ, Coiffier B et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244. [DOI] [PubMed] [Google Scholar]
- 15.Hoster E, Dreyling M, Klapper W et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008; 111: 558–565. [DOI] [PubMed] [Google Scholar]
- 16.Delgado J, Pereira A, Villamor N, López-Guillermo A, Rozman C. Survival analysis in hematologic malignancies: recommendations for clinicians. Haematologica 2014; 99: 1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brugger W, Hirsch J, Grunebach F et al. Rituximab consolidation after high-dose chemotherapy and autologous blood stem cell transplantation in follicular and mantle cell lymphoma: a prospective, multicenter phase II study. Ann Oncol 2004; 15: 1691–1698. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg LA, Maloney D, Bensinger W. Immunotherapy with rituximab/interleukin-2 after autologous stem cell transplantation as treatment for CD20+ non-Hodgkin’s lymphoma. Clin Lymphoma Myeloma 2006; 7: 135–139. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz SM, Negrin RS, Blume KG et al. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood 2004; 103: 777–783. [DOI] [PubMed] [Google Scholar]
- 20.Mangel J, Buckstein R, Imrie K et al. Immunotherapy with rituximab following high-dose therapy and autologous stem-cell transplantation for mantle cell lymphoma. Semin Oncol 2002; 29: 56–69. [DOI] [PubMed] [Google Scholar]
- 21.Andersen NS, Pedersen LB, Laurell A et al. Pre-emptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol 2009; 27: 4365–4370. [DOI] [PubMed] [Google Scholar]
- 22.Arai Y, Yamashita K, Mizugishi K et al. Risk factors for late-onset neutropenia after rituximab treatment of B-cell lymphoma. Hematology 2015; 20: 196–202. [DOI] [PubMed] [Google Scholar]
- 23.Kato H, Yamamoto K, Matsuo K et al. Clinical impact and predisposing factors of delayed-onset neutropenia after autologous hematopoietic stem-cell transplantation for B-cell non-Hodgkin lymphoma: association with an incremental risk of infectious events. Ann Oncol 2010; 21: 1699–1705. [DOI] [PubMed] [Google Scholar]
- 24.Issa NC, Baden LR. Current issues in vaccines for adult patients with hematologic malignancies. J Natl Compr Canc Netw 2012; 10: 1447–1454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.