ABSTRACT
Hydrogen sulfide (H2S), well known for its toxic properties, has recently become a research focus in bacteria, in part because it has been found to prevent oxidative stress caused by treatment with some antibiotics. H2S has the ability to scavenge reactive oxygen species (ROS), thus preventing oxidative stress, but it is also toxic, leading to conflicting reports of its effects in different organisms. Here, with Shewanella oneidensis as a model, we report that the effects of H2S on the response to oxidative stress are time dependent. When added simultaneously with H2O2, H2S promoted H2O2 toxicity by inactivating catalase, KatB, a heme-containing enzyme involved in H2O2 degradation. Such an inhibitory effect may apply to other heme-containing proteins, such as cytochrome cbb3 oxidase. When H2O2 was supplied 20 min or later after the addition of H2S, the oxidative-stress-responding regulator OxyR was activated, resulting in increased resistance to H2O2. The activation of OxyR was likely triggered by the influx of iron, a response to lowered intracellular iron due to the iron-sequestering property of H2S. Given that Shewanella bacteria thrive in redox-stratified environments that have abundant sulfur and iron species, our results imply that H2S is more important for bacterial survival in such environmental niches than previously believed.
IMPORTANCE Previous studies have demonstrated that H2S is either detrimental or beneficial to bacterial cells. While it can act as a growth-inhibiting molecule by damaging DNA and denaturing proteins, it helps cells to combat oxidative stress. Here we report that H2S indeed has these contrasting biological functions and that its effects are time dependent. Immediately after H2S treatment, there is growth inhibition due to damage of heme-containing proteins, at least to catalase and cytochrome c oxidase. In contrast, when added a certain time later, H2S confers an enhanced ability to combat oxidative stress by activating the H2O2-responding regulator OxyR. Our data reconcile conflicting observations about the functions of H2S.
INTRODUCTION
As an important signaling molecule, along with nitric oxide (NO) and carbon monoxide (CO), hydrogen sulfide (H2S) has been a focus of research in mammals in recent years and has been found to have many beneficial functions (1). The most important one is its cytoprotective effect against oxidative stress (2). Although studies of the physiological roles of H2S in bacteria have lagged behind significantly, it was recently reported that H2S is important in the abilities of certain species, such as Escherichia coli, Bacillus anthracis, Pseudomonas aeruginosa, and Staphylococcus aureus, to survival and grow well in various niches (3). By stimulating protection against reactive oxygen species (ROS), H2S provides bacteria a general defense against antibiotics (3). However, H2S has also long been known for its toxic properties, especially as a growth inhibitor by damaging DNA, by denaturing proteins through disruption of disulfide cross-links, by inactivating the redox centers of metalloenzymes, and by enhancing oxidative stress (4–7). H2S significantly inhibits the growth of several bacteria, including E. coli, Bacillus subtilis, Salmonella enterica serovar Typhimurium, and S. aureus, as well as some marine bacteria (7, 8). In addition, H2S was also found to act as an antifungal agent for several pathogens, including Aspergillus niger and Penicillium italicum, by decreasing the activities and expression of superoxide dismutase (SOD) and catalase (CAT) (8). While the contrasting effects of H2S reported in these studies may be attributed to differences in the concentrations used, it is possible that H2S has distinct impacts on different species of bacteria.
Shewanella bacteria are facultative Gram-negative gammaproteobacteria with a remarkable respiratory versatility and potential for bioremediation of metals, as well as for its use in microbial fuel cells (9, 10). Shewanella species are also gradually emerging as human and animal pathogens, as reports of Shewanella infections have been increasingly reported (11). Owing to these properties, some species, the model species Shewanella oneidensis in particular, have been intensively studied. There is endogenous H2S generation in S. oneidensis (12), but not until recently was its enzymatic foundation determined (13–15). Through anaerobic respiration of inorganic sulfur compounds in the periplasm, including thiosulfate (S2O32−), sulfite (SO32−), tetrathionate (S4O62−), and elemental sulfur (S0), S. oneidensis generates H2S with sulfite reductase SirACD, as well as thiosulfate and polysulfide reductase PsrABC (13, 14, 16). Cysteine degradation via methionine γ-lyase MdeA is the predominant source of endogenous H2S production, with other mechanisms via SO_1095 and SseA being of lesser importance (15). While MdeA and SO_1095 are homologous to P. aeruginosa cystathionine γ-lyase (CSE), SseA is a homologue of 3-mercaptopyruvate sulfurtransferase (3MST) (3, 15, 17).
Endogenous H2S is particularly important for Shewanella species because it is critical to iron reduction (18). In alkaline environments containing multiple electron acceptors, including sulfur and iron species, S. oneidensis first generates H2S (HS−), which in turn reduces iron compounds abiotically. In parallel, in the redox-stratified environments where Shewanella bacteria thrive, ROS are likely to occur. Hence, for these bacteria, H2S may not only function as a reductive chemical for abiotic iron reduction but also play a role in the cellular response to oxidative stress. Like E. coli and many other bacteria, S. oneidensis utilizes OxyR, a LysR family transcriptional regulator, as the predominant regulator mediating the cellular response to H2O2 (19–21). OxyR controls a large number of genes by acting as both an activator and a repressor. Two such genes under OxyR repression, katB and dps, are particularly important in protecting cells from H2O2 damage (19). While KatB is a CAT dominating H2O2 degradation, Dps, as an iron-sequestering protein, plays a role in the control of cellular iron homeostasis, especially when cells are challenged by H2O2.
In this study, we have attempted to understand the effects of H2S on the response of S. oneidensis to H2O2. We found that H2S either aggravates or protects from H2O2 lethality, depending on the time of H2O2 addition. Enhanced killing was due primarily to H2S inactivation of KatB, and such a mechanism appeared to be applicable to some other heme-containing proteins. In contrast, protection against H2O2 induced by pretreatment with H2S depended on the activation of OxyR, the master regulator mediating the cellular response to H2O2 (19–21). This appears to be due to iron influx triggered by lowered intracellular free-iron levels resulting from the iron-sequestering activity of H2S.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are described in Table 1. All chemicals were acquired from Sigma Co. (Shanghai, China) unless otherwise noted. Information about the primers used in this study is available upon request. For genetic manipulation, E. coli and S. oneidensis strains were grown in lysogeny broth (LB; Difco, Detroit, MI) under aerobic conditions at 37 and 30°C, respectively. When needed, the growth medium was supplemented with 2,6-diaminopimelic acid at 0.3 mM, ampicillin at 50 μg/ml, kanamycin at 50 μg/ml, and gentamicin at 15 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | Host strain for plasmids and general use | Lab stock |
| WM3064 | Donor strain for conjugation; ΔdapA | W. W. Metcalf, UIUCa |
| S. oneidensis | ||
| MR-1 | Wild type | ATCC 700550 |
| HG1070 | ΔkatB mutant derived from MR-1 | 19 |
| HG1095 | ΔSO1095 mutant derived from MR-1 | 15 |
| HG1261 | ΔsseA mutant derived from MR-1 | 15 |
| HG1328 | ΔoxyR mutant derived from MR-1 | 19 |
| HG1812 | ΔmdeA mutant derived from MR-1 | 15 |
| HG2364 | ΔccoN mutant derived from MR-1 | 40 |
| HG2903 | ΔcysK mutant derived from MR-1 | 15 |
| HG4056 | ΔmetB mutant derived from MR-1 | 15 |
| ΔcysKC | ΔcysK mutant with copy of cysK integrated into chromosome | 15 |
| Δtriple | ΔmdeA ΔSO1095 ΔsseA mutant derived from MR-1 | This study |
| Δpenta | ΔmdeA ΔSO1095 ΔsseA ΔpsrA ΔsirA mutant derived from MR-1 | This study |
| Plasmids | ||
| pHGM01 | Apr Gmr Cmr suicide vector | 23 |
| pHG101 | Promoterless broad-host-range Kmr vector | 24 |
| pHG102 | pHG101 containing S. oneidensis arcA promoter | 24 |
| pHGEI01 | Integrative lacZ reporter vector | 32 |
| pBBR-Cre | Spr helper plasmid for antibiotic cassette removal | 39 |
| pHGE-PkatB-lacZ | Reporter vector carrying PkatB-lacZ | This study |
| pHGE-Pdps-lacZ | Reporter vector carrying Pdps-lacZ | This study |
| pHGE-PahpC-lacZ | Reporter vector carrying PahpC-lacZ | This study |
| pHGE-PkatG1-lacZ | Reporter vector carrying PkatG1-lacZ | This study |
UIUC, University of Illinois at Urbana-Champaign.
H2O2 sensitivity.
The response of S. oneidensis to H2O2 was assessed as previously described (19). For growth analysis, overnight cultures from a single colony on LB plates were inoculated into fresh LB to an optical density at 600 nm (OD600) of ∼0.01. The cultures were supplemented with chemicals as indicated in Results and the figure legends and monitored for growth by recording OD600 values. To assess susceptibility to H2O2, properly diluted mid-log-phase cultures (OD600 of ∼0.2) were spread onto fresh LB plates (200 μl of culture, approximately 106 CFU). After a bacterial lawn had become visible, paper discs 6 mm in diameter containing 10 μl of 5 M H2O2 were placed on plates, which were incubated for 16 h at 30°C before photography. To determine the effects of chemicals on the survival of S. oneidensis, mid-log-phase cultures were challenged and at various time intervals, samples were serially diluted and plated onto LB. Colonies were counted after overnight incubation at 30°C. All experiments were repeated at least three times.
H2S detection.
To keep levels of H2S from both endogenous and exogenous sources in growing cultures relatively stable, cells for H2S-related physiological assays were grown under aerobic conditions on a shaker at 50 rpm at 30°C. H2S production in S. oneidensis was monitored by a lead acetate detection method and the methylene blue formation assay (3, 15, 22).
Mutagenesis and genetic complementation.
S. oneidensis in-frame deletion strains were constructed by the att-based fusion PCR method (23). In brief, two fragments flanking the target gene were generated by PCR with primers containing attB and gene-specific sequences and joined by a second round of PCR. The fused fragments were introduced into plasmid pHGM01 by site-specific recombination with BP Clonase (Invitrogen) according to the manufacturer's instructions. The resulting vectors were transformed into E. coli WM3064 and then transferred into relevant S. oneidensis strains via conjugation. Mutagenesis constructs integrated into the chromosome were selected by resistance to gentamicin and confirmed by PCR. These transconjugants were grown in LB broth in the absence of NaCl and plated on LB supplemented with 10% sucrose. Gentamicin-sensitive and sucrose-resistant colonies were screened by PCR for deletion of the target gene. All mutations were verified by sequencing the mutated regions.
All mutants from previous studies were successfully complemented by a copy of the corresponding gene on plasmids pHG101 and pHG102 (Table 1) (24, 25). In this study, these complementation vectors were used with similar results.
DNA damage assays.
Measurement of DNA damage was initially performed by quantitative PCR (qPCR) (26). Total genomic DNA was isolated from 10 ml of mid-log-phase cultures with a DNeasy Tissue kit (Qiagen) and quantified with NanoVue (GE Health Care). A fragment of ∼10 kb close to the ccm (encodes the cytochrome c maturation system) region was selected for analysis. qPCR was performed with an ABI 7300 96-well qPCR system (Applied Biosystems) as described previously (27), and DNA damage was calculated by an established method (26). The amount of amplified product from the treated samples was normalized to that from an untreated control. DNA damage was also assessed by measurement of the rate of mutagenesis with the thyA (encodes thymidylate synthase) forward mutagenesis assay scoring resistance to trimethoprim (TMP) (28). TMP is a dihydrofolate reductase inhibitor that suppresses the growth of wild-type cells but not that of mutants lacking thyA as long as thymine is present in the medium. Mid-log-phase cultures were treated with H2O2, H2S, or both and incubated for 20 min. After serial dilution, 250 μl was plated on LB containing 0.2 mg/ml thymine and 0.1 mg/ml TMP and incubated at 30°C.
Enzyme assays.
Mid-log-phase cultures were centrifuged, and cell pellets were washed with 0.2 M phosphate-buffered saline (PBS, pH 7.2), resuspended in PBS to an OD600 of ∼0.5, and lysed by sonication. After centrifugation for 10 min at 10,000 rpm, the supernatants were used as crude enzyme extracts. H2O2 concentrations in aliquots of crude extracts were determined at various time points, as indicated in the relevant figures, by a method described previously (29). The same procedure was used to measure H2O2-degrading activity by extracts and by a commercial heme CAT from bovine liver (Sigma, St. Louis, MO). SOD and peroxidase (POD) activities were measured on the basis of pyrogallol autoxidation and guaiacol as the electron donor, respectively (29, 30). Quantitative analysis of cytochrome c oxidase (Cco) was carried out as described previously (31).
Expression analyses.
β-Galactosidase assays were performed to determine gene expression with the integrative lacZ reporter plasmid pHGEI01 (32). In brief, the sequence of ∼400 bp upstream of a gene of interest was amplified and placed immediately upstream of the full-length E. coli lacZ gene. The resulting vector was maintained in E. coli WM3064 and transferred to S. oneidensis after verification by sequencing. Log-phase cells (OD600, ∼0.2) were harvested by centrifugation, washed with PBS, suspended in lysis buffer (0.25 M Tris HCl, 0.5% Triton X-100, pH 7.5) for 30 min, and assayed with ortho-nitrophenyl-β-d-galactopyranoside as described previously (24). Activity was determined by monitoring color development at 420 nm with a Synergy 2 Multi-Detection microplate reader (M200 Pro; Tecan) and reported in Miller units. In addition, gene expression was determined by qRT-PCR, which was performed with an ABI 7300 96-well qRT-PCR system (Applied Biosystems) essentially as described above.
Quantification of intracellular total and free iron species.
The total cellular iron content of 1-liter cultures was assayed by inductively coupled plasma mass spectrometry (ICP-MS) as described elsewhere (33). For sample preparation, log-phase cells (OD600 of ∼0.2) grown under the conditions indicated in Results or the figure legends were harvested by centrifugation, washed with PBS, and lysed by sonication. The supernatants were analyzed by ICP-MS on an iCAP (Thermo Scientific). The iron content was normalized to the total protein in the lysates. All data were evaluated with at least three independent biological experiments. Measurements of free iron species were performed with a calcein assay as described previously (21, 34).
Other analyses.
Experimental values were subjected to statistical analyses and presented as the mean ± standard deviation (SD). Student's t test was performed for pairwise comparisons of groups.
RESULTS
Endogenous H2S has a negligible role in protecting cells from H2O2.
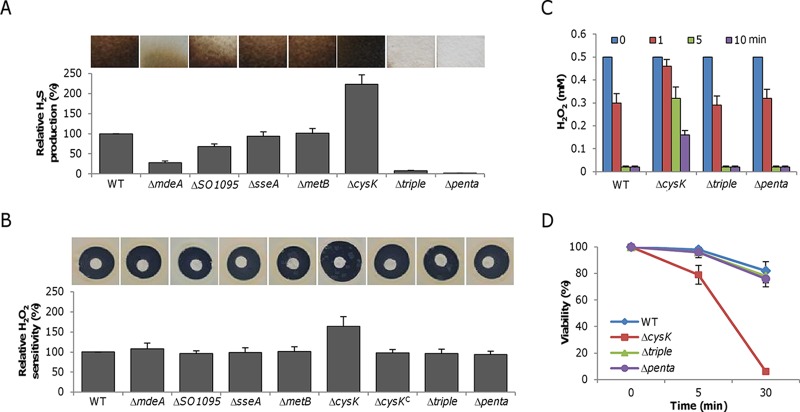
S. oneidensis is able to produce H2S through both cysteine degradation and respiration of sulfur species (13–15). Under aerobic conditions, H2S produced through respiration is negligible, especially when the relevant sulfur species is not added (15). Thus, endogenous H2S results from the activity of the CSE homologues MdeA and SO1095, as well as from the 3MST homologue SseA, with MdeA accounting for >70% (15) (Fig. 1A). In addition, loss of CysK resulted in H2S hyperproduction. To test whether endogenous H2S plays a cytoprotective role against ROS in S. oneidensis, as reported in other bacteria (3), an H2O2 susceptibility assay was done (Fig. 1B). Surprisingly, the wild type and all of the mutant strains with an impaired ability to generate H2S exhibited similar levels of susceptibility to H2O2. In contrast, the ΔcysK mutant, which overproduces H2S, displayed substantially increased sensitivity. The lower sensitivity was restored by cysK expression in trans, indicating that high levels of intracellular H2S sensitize S. oneidensis to H2O2. To further confirm that the loss of H2S generation does not affect resistance to H2O2, we constructed a strain lacking all three of the genes contributing to cysteine degradation (Δtriple mutant). This mutant, which produced very little H2S under aerobic conditions (Fig. 1A), had a level of H2O2 resistance comparable to that of the wild type (Fig. 1B). Moreover, additional removal of the psrA and sirA genes (Δpenta mutant), which completely eliminated the ability to produce H2S (Fig. 1A), did not compromise H2O2 resistance (Fig. 1B).
FIG 1.
Endogenous H2S does not protect bacteria against H2O2. (A) H2S production in various S. oneidensis strains. Lead acetate-soaked paper strips show a brown or black PbS stain as a result of reaction with H2S under aerobic conditions. The relative H2S levels shown were obtained by normalization to the average level of the wild type (WT), which was set to 100%. The Δtriple (ΔmdeA ΔSO1095 ΔsseA) mutant strain lacks the ability to produce H2S via cysteine metabolism, while the Δpenta mutant strain carries additional deletions in the psrA and sirA genes, thus removing the ability to produce H2S through respiration. (B) H2O2 sensitivity assay. Paper disks of 6 mm loaded with 10 μl of 5 M H2O2 were placed on a bacterial lawn and photographed after 16 h at 30°C. The relative H2O2 susceptibilities shown were obtained by normalization to the average level of the wild type, which was set to 100%. The ΔcysKC mutant is a complemented mutant carrying a copy of the cysK gene integrated into the chromosome and produces H2S like the wild type. (C) H2O2 consumption assay. H2O2 at 0.5 mM was added to mid-log-phase cultures (OD600 of ∼0.2, the same afterward), and the H2O2 remaining at the time points indicated was measured. (D) Survival assay. One millimolar H2O2 was added to mid-log-phase cultures. After 5 and 30 min, samples were diluted and plated on LB. Colony counting was done after 24 h. For panels A and B, experiments were performed five times and representative results are shown. In panels B and D, data are reported as the mean ± SD (n = 4).
We also determined the abilities of the ΔcysK, Δtriple, and Δpenta mutant strains to degrade H2O2 and their viability after exposure to H2O2. Cells in the early stationary phase (4 h after the end of exponential growth), when endogenous H2S levels were relatively stable (see Fig. S1 in the supplemental material), were collected for the two assays. Loss of endogenous H2S production did not significantly compromise the ability to scavenge H2O2 (Fig. 2C). However, the ΔcysK mutant strain, consistent with its hypersensitivity to H2O2, degraded H2O2 at a significantly lower rate. With respect to survival, the Δtriple and Δpenta mutant strains resembled the wild type whereas the ΔcysK mutant strain was substantially impaired (Fig. 1D). Overall, these data demonstrate that endogenously generated H2S in S. oneidensis provides little protection from exogenous H2O2 damage; rather, H2S at high concentrations negatively regulates the ability to cope with the stress.
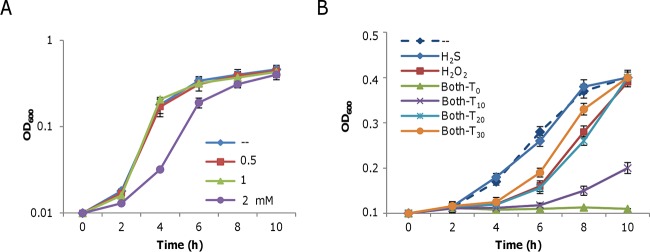
FIG 2.
Effects of H2S, H2O2, and both together on the growth of the S. oneidensis Δpenta mutant. Mid-log-phase cultures were inoculated into LB medium containing the chemicals indicated and incubated statically at 30°C in 24-well plates. (A) Effect of H2S (from NaHS) on growth. (B) Effects of 0.1 mM H2S, 1 mM H2O2, and both on the growth of the Δpenta mutant strain. T0, T10, T20, and T30 represent the addition of H2O2 at the same time as H2S and 10, 20, and 30 min later, respectively. In both panels A and B, -- represents the LB control. Data are reported as the mean ± SD (n = 4).
The impact of H2S on H2O2-treated cells is temporally dependent.
The unexpected findings obtained with H2S-deficient and -overproducing mutants raise questions about the roles that H2S plays in bacteria, since it is apparently not simply acting as a protective molecule. Given that the Δtriple and Δpenta mutant strains responded the same way, the Δpenta mutant strain was used as the H2S-deficient strain. Moreover, comparable results were obtained in all subsequent experiments with the wild-type and Δpenta mutant strains, and H2S concentrations in mutant cultures can be accurately controlled with NaHS as the source of exogenous H2S. For clarity, we present data from the H2S-deficient strain rather than the wild type unless otherwise noted. The impact of H2O2 on the growth and viability of S. oneidensis has been extensively studied recently, showing that it is able to inhibit growth even at concentrations as low as 100 μM, although the MIC is ∼1.25 mM (19). At physiological pH, most H2S exists as HS− (bisulfide ion) and there are only small amounts of H2S and S2−. It should be noted that we mention only H2S for simplicity, but all of the ionic forms are included. Unlike H2O2, H2S did not noticeably inhibit the growth of S. oneidensis at concentrations of up to 1 mM (Fig. 2A).
We then assessed the impact of H2S on growth inhibition by H2O2. H2S and H2O2 were added to mid-log-phase cultures (OD600 of ∼0.2) at the same and different times, and the growth of the cultures was monitored. Strikingly, in the presence of 0.1 mM H2S, the timing of H2O2 addition was found to be important for eliciting a detectable effect on growth (Fig. 2B). When both compounds were added simultaneously (T0), H2S significantly increased the growth-inhibiting effects of 1 mM H2O2. In contrast, when H2O2 was added 30 min after H2S (T30), its effect on growth was protective. The balancing point was about 20 min after the addition of H2S, at which time growth was comparable to that of cultures containing only H2O2. Before 20 min, the general trend was that the later the addition of H2O2, the lower the growth inhibition. These data indicate two distinct time-dependent effects of H2S on the action of H2O2 in S. oneidensis.
H2S promotes H2O2 killing when added promptly after the addition of H2O2.
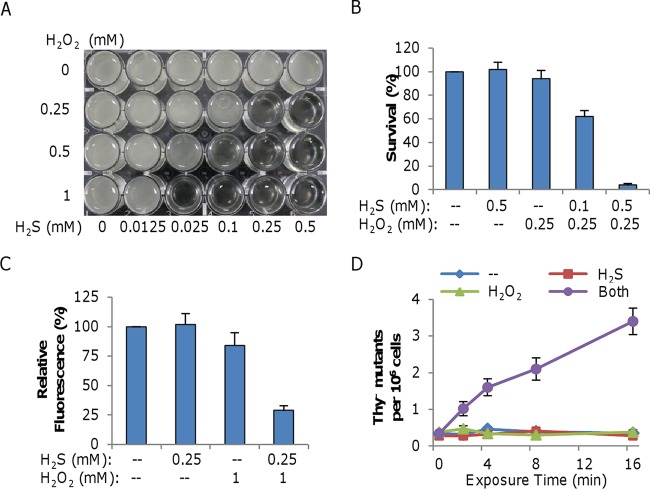
To assess whether H2S aggravates growth inhibition by H2O2 when added promptly, we measured growth in the presence of various concentrations of H2S or H2O2 (Fig. 3A). Similar levels of inhibition could be observed at different combinations; the higher the level of H2S, the lower the level of H2O2. For instance, in the presence of 0.025 mM H2S and 1 mM H2O2, growth was completely prevented and a similar level of inhibition was achieved with 0.25 mM H2S and 0.25 mM H2O2. To determine whether growth inhibition by the simultaneous addition of H2S and H2O2 was due to the bactericidal effect of H2O2, we examined the survival of treated cells. Plating assays showed that cells were readily killed by H2O2 in the presence of H2S (Fig. 3B). After treatment for 20 min, a combination of 0.5 mM H2S and 0.25 mM H2O2 reduced the proportion of viable cells to only ∼4%, whereas killing by either 0.25 mM H2O2 alone or by 1 mM H2S alone was insignificant. These results show that H2S per se is not bactericidal but that it facilitates killing by H2O2.
FIG 3.
H2S promotes H2O2 killing when added promptly after H2O2. (A) Synergistic inhibition of S. oneidensis growth by H2S and H2O2 when added at the same time. Cells at an OD600 of ∼0.01 were inoculated into LB in 24-well plates containing H2S, H2O2, or both at the concentrations indicated and incubated statically at 30°C. The plates were photographed 24 h after inoculation. Experiments were performed five times, and similar results were obtained. (B) Mid-log-phase cells (OD600 of ∼0.2) were treated for 20 min with H2S, H2O2, or both at the concentrations indicated. The treated cultures were diluted, plated on LB, and incubated at 30°C. Survival was calculated as the ratio of the number of colonies in the treated cultures to that in the untreated control. Only plates containing 100 to 300 colonies were counted. (C) H2S-H2O2 treatment generates extensive DNA damage, as determined by qPCR. Mid-log-phase cells were treated with 0.25 mM H2S, 1.0 mM H2O2, or both. Total genomic DNA was extracted, and qPCR was performed with equivalent amounts of template DNA. The relative fluorescence was normalized to that of the untreated control. Data are reported as the mean ± SD (n = 4). (D) H2S-H2O2 treatment generates extensive DNA damage, as determined by thyA mutation analysis. Mid-log-phase cells grown in LB were diluted 100-fold with fresh LB (--) or with LB containing 0.1 mM H2S, 0.25 mM H2O2, or both. At each time point, CAT was added to degrade H2O2, and both total viability and the frequency of thyA mutants were determined. Data are reported as the mean ± SD (n = 4).
In general, DNA damage is the most lethal impact of oxidative stress on viability (35). To test this, chromosomal DNA damage was measured by qPCR, which detects any lesions or strand breaks that block the progression of the PCR DNA polymerase and result in fewer PCR products than with intact DNA (36). A fragment of ∼10 kb near the ccm gene (encodes the cytochrome c maturation system) was used for analysis because this PCR product can be reliably obtained (37). We found that the yield of full-length PCR fragments from the treated samples decreased by approximately 3-fold in comparison to that from the untreated control (Fig. 3C). We also measured H2O2-induced mutagenesis in cells challenged with a combination of 0.1 mM H2S and 0.25 mM H2O2, a condition where a majority of the cells are not killed but mutations could accrue. The number of thyA mutants increased 7-fold in H2S-H2O2-treated cells, whereas neither molecule alone was mutagenic compared to the control (Fig. 3D). On the basis of these results, we conclude that H2S-H2O2-treated cells suffer gross DNA damage.
Reduced CAT activity underlies H2S-induced enhancement of H2O2 killing.
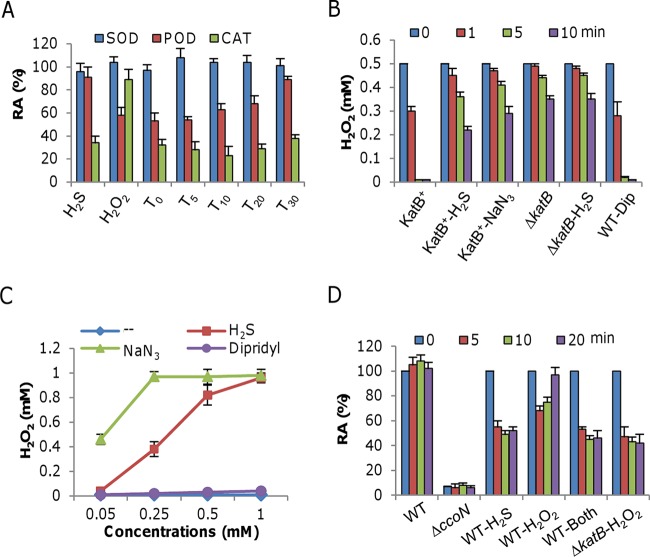
Since H2O2 does not oxidize DNA directly, the observed DNA damage most likely results from the product of the Fenton reaction (35), in which H2O2 reacts with unincorporated iron to produce hydroxyl radicals, extremely strong oxidizing agents that can react with virtually all organic molecules (35). As H2S can bind ferric or ferrous iron to form insoluble FeS or Fe2S3, it is conceivable that the levels of unincorporated iron would not increase. It is possible, therefore, that H2S treatment blocks the degradation of H2O2 and/or other ROS agents, as indicated by the compromised H2O2-scavenging capacity of the cysK mutant (Fig. 1B). S. oneidensis has a full set of ROS-scavenging enzymes, including CAT, POD, and SOD (19, 20). To determine whether H2S affects the activities of these enzymes, exponentially growing cells (OD600 of ∼0.2) were harvested to prepare crude enzyme extracts as described in Materials and Methods. The extracts were treated with H2S and/or H2O2 and assayed 5 min later (Fig. 4A). Consistent with previous observations (19), the SOD activities were relatively constant, implying a negligible role for SOD in the enhanced killing seen. In contrast, the activities of POD and CAT were inhibited by H2S, H2O2, or both, but there were substantial differences. H2S alone had an insignificant effect on POD activity, whereas H2O2 in the presence or absence of H2S caused a reduction to about 60% of the wild-type level. These data indicate that neither SOD nor POD was the target of H2S.
FIG 4.
Effects of H2S, H2O2, and both on selected heme-containing enzymes. (A) Mid-log-phase cells were harvested, washed, and sonicated for the preparation of crude enzyme extracts. H2S at 0.25 mM, H2O2 at 0.5 mM, or the two together were added to crude enzyme extracts at the times indicated, and SOD, POD, and CAT activities were measured. Subscript numbers are the times (in minutes) when H2O2 was added after the addition of H2S. The activities of the treated samples were normalized to the activity of the untreated control and are reported as relative activities (RA). Importantly, the activities of all three enzymes from untreated samples were found to be stable for 30 min. (B) Crude enzyme extracts prepared as described for panel A were treated with the reagents indicated and assayed for H2O2 degradation. Concentrations: H2S, 0.25 mM; NaN3, 0.1 mM; dipyridyl (Dip), 0.5 mM. WT, wild type. (C) Two thousand five hundred units of bovine liver CAT was treated with the chemicals indicated at various concentrations for 5 min and then assayed for the ability to degrade 1 mM H2O2 at 37°C compared to the control (--). After 5 min, the remaining H2O2 concentrations were measured. (D) Cco assay. Crude enzyme extracts prepared as described for panel A were treated with the reagents indicated. The ΔccoN mutant strain, lacking the essential catalytic subunit of the cbb3 oxidase, was used as a negative control, and the ΔkatB mutant strain was also included for reference. All chemicals were added at the same time. Concentrations: H2S, 0.25 mM; H2O2, 0.5 mM. In all panels, data are reported as the mean ± SD (n = 4).
In the case of CAT, the inhibitory effect of H2O2 was rather minor but the addition of H2S resulted in a drastic reduction of activity (Fig. 4A). Given that the CAT KatB is the predominant enzyme for H2O2 degradation in S. oneidensis (19), we hypothesized that the observed enhanced killing by the combination of H2S and H2O2 may be due to the reduced activity of KatB, thus preventing removal of H2O2. This hypothesis was supported by the finding that a katB mutant was unable to respond to H2S addition with respect to its ability to degrade H2O2 (Fig. 4B). To confirm this, we repeated the experiment with the heme-binding agent sodium azide (NaN3), which specifically inhibits heme-containing CAT (38). As shown in Fig. 4B, the presence of NaN3 resulted in extensive reduction of H2O2 degradation, supporting evidence that KatB is directly inhibited by H2S. H2S is an iron-sequestering agent; thus, it is possible that this property plays a role in the inactivation of KatB. To test this, 0.5 mM dipyridyl, a specific iron-chelating agent, was added to the extracts but H2O2 degradation was hardly affected, suggesting that it is unlikely that H2S inactivates KatB through its interaction with unincorporated iron species. As a further confirmation, we examined the effects of H2S, NaN3, and dipyridyl on commercially available heme CAT from bovine liver. Not surprisingly, we found that the activity was drastically inhibited by either H2S or NaN3 but not by dipyridyl (Fig. 4C). NaN3 appears to be a stronger inhibitor than H2S. Moreover, the addition of dipyridyl and H2O2 did not elicit enhanced inhibition of growth (see Fig. S2 in the supplemental material), supporting the in vivo evidence that H2S negatively regulates the activity of heme-containing CAT but this activity is independent of its iron-sequestering ability.
To test whether H2S inhibits other heme-containing proteins, we examined its effect on the cbb3 oxidase. This oxidase is composed of at least three subunits, CcoN, CcoP, and CcoO, and is the primary system for respiration of oxygen and the only enzyme that has Cco activity (39, 40). As shown in Fig. 4D, the activity of the cbb3 oxidase was inhibited by 0.25 mM H2S to approximately 55% of the untreated level after a 5-min treatment. Although H2O2 was also able to inhibit the cbb3 oxidase, the effect was rather modest, with approximately 65% of the activity remaining in the presence of H2O2 at the growth-inhibitory concentration of 1.25 mM (19). Besides, unlike the activity of the enzyme in the H2S-treated samples, that in H2O2-treated samples was restored to the untreated level 20 min after the addition, most likely because of the removal of H2O2. When the experiment was repeated with the katB mutant strain, the activity of the cbb3 oxidase was no longer recoverable. Furthermore, the combination of H2O2 (1.25 mM) and H2S (0.25 mM) failed to further reduce this activity. These data show that H2S is effective in negatively regulating the activity of the two heme-containing proteins KatB and cbb3 oxidase.
H2S induces the OxyR-mediated stress response in S. oneidensis.
In contrast to facilitating killing by H2O2, H2S improves the growth of S. oneidensis under H2O2 stress conditions if pretreatment for 20 min or longer is applied (Fig. 2B), indicating a protective role, as observed in four other bacterial species (3). It is proposed that H2S, as for NO, confers resistance to H2O2-induced oxidative stress by (i) suppressing the damaging Fenton reaction and (ii) improving the antioxidant capacity of bacterial cells involving the major ROS scavengers CAT, POD, and SOD (3, 41). These two mechanisms are in accord with the OxyR-mediated stress response, suggesting that the cytoprotective role of these molecules relies on the OxyR system. To test whether this is true for S. oneidensis, we examined the amounts of ROS-scavenging enzymes after a 30-min treatment with H2S by in-gel staining analysis of proteins separated by native PAGE. As previously reported (19), KatB, whose expression is directly repressed by OxyR, was the only CAT visible in the staining analysis (Fig. 5A). As expected, production of KatB was substantially increased in samples treated with H2O2 or in an oxyR mutant. H2S, although less effective, was also able to induce KatB production. Noticeable induction was observed with H2S at 0.25 mM, and at higher concentrations (0.5 to 2 mM), induction was more robust.
FIG 5.
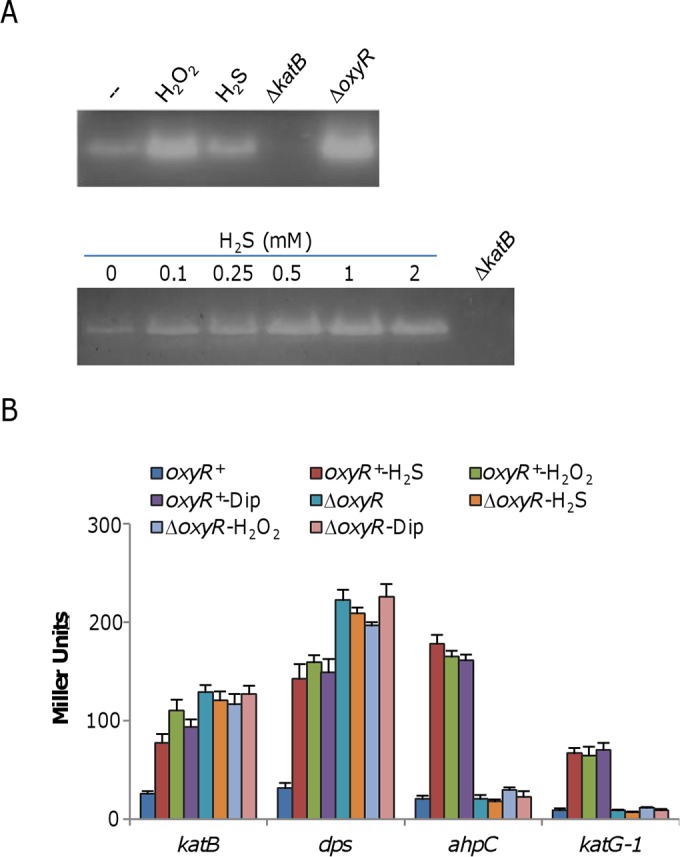
H2S induces the OxyR-mediated stress response. (A) CAT staining analysis. Cells were harvested just prior to (−) and 30 min after the addition of 0.25 mM H2S or 0.1 mM H2O2 (top). Protein samples of ∼10 μg from the cell lysates indicated were separated by native PAGE and stained for CAT activity. The ΔkatB and ΔoxyR mutant strains (constitutive high-level expression) were used as negative and positive controls. In the analysis shown at the bottom, various concentrations of H2S were examined for the ability to induce expression of the katB gene. (B) Impact of H2S on the expression of four members of the OxyR regulon. β-Galactosidase assays were carried out with lacZ reporter vectors. Cells grown to mid-log phase were treated with the chemicals indicated for 30 min and then harvested for the assays. Concentrations: H2S, 0.25 mM; H2O2, 0.2 mM; dipyridyl (Dip), 0.25 mM. β-Galactosidase activities are reported as the mean ± SD (n = 4). Similar results were obtained by qRT-PCR assay (see Fig. S3 in the supplemental material).
The observation that H2S alone induces enhanced production of KatB, similar to that from treatment with H2O2, supports the idea that the OxyR system mediates the cytoprotection of H2S. To confirm this possibility, we examined the expression of three other genes (ahpC, dps, and katG1) of the OxyR regulon, as well as the katB gene, in samples treated with H2S by using an integrative lacZ reporter system. While the ahpC and katG1 genes (encode alkylhydroperoxide reductase and CAT KatG1, respectively) are positively regulated by OxyR, the dps gene, which encodes an iron storage protein, is repressed by OxyR, as is the katB gene (19). As shown in Fig. 5B, in the presence of H2S or H2O2, the β-galactosidase activities driven by promoters of the test genes were upregulated in the oxyR+ background but no longer responsive to either compound in the absence of OxyR. A similar trend was observed by qRT-PCR (see Fig. S3 in the supplemental material). In summary, these data indicate that H2S, when added after 10 min, enhanced resistance to H2O2 by activating OxyR.
Iron sequestration likely triggers activation of OxyR.
Because iron is both essential and potentially harmful (42), bacteria have evolved various mechanisms to ensure adequate supplies and at the same time tight restriction of free-iron levels to protect against iron-induced toxicity. As H2S is an iron-sequestering agent, we first tested whether this feature is associated with the activation of OxyR. The intracellular levels of total and unincorporated iron after the addition of H2S were measured (Fig. 6A). Surprisingly, the addition of H2S had a minor effect on the concentrations of unincorporated iron but substantially increased the amounts of total iron. This result implies that H2S may cause an iron influx, presumably triggered by the decreased intracellular levels of free iron immediately after its addition. If this were the case, then the addition of dipyridyl should produce a similar result. Indeed, total intracellular iron levels were elevated in the presence of 0.5 mM dipyridyl, although unincorporated iron was diminished to some extent, implying that dipyridyl may be able to activate OxyR, although it differs from H2S in its inability to promote H2O2 killing when added simultaneously with H2O2, as shown in Fig. 4. To test this, we measured KatB activity by in-gel staining and found that it was increased after the addition of dipyridyl (Fig. 6B). Furthermore, the expression pattern of four OxyR-regulated genes in cells treated with dipyridyl resembled that found after H2S treatment (Fig. 6B). These data, collectively, suggest that iron sequestration is the likely mechanism by which H2S activates the OxyR-mediated cellular response to oxidative stress.
FIG 6.
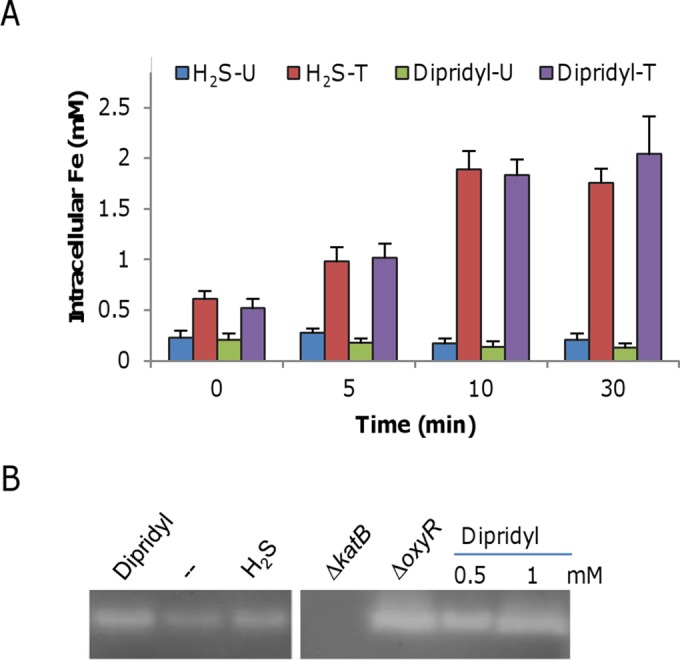
H2S induces the OxyR-mediated stress response. (A) Intracellular iron levels induced by H2S. One-liter cultures grown to mid-log phase (OD600 of ∼0.2) were harvested just before (0 min) and 5, 10, and 30 min after the addition of 0.25 mM H2S or 0.2 mM dipyridyl, and the unincorporated (U) and total (T) intracellular iron concentrations were measured. The experiments were performed at least three times. Error bars show standard deviations. (B) CAT staining analysis. Cells were harvested 30 min after the addition of 0.25 mM H2S or 0.2 mM dipyridyl along with the untreated control (--). Ten-microgram samples of protein from the cell lysates indicated were separated by native PAGE and stained for CAT activity. The ΔkatB and ΔoxyR mutant strains (constitutive high-level expression) were used as negative and positive controls. In the right panel, two higher concentrations of dipyridyl were tested for enhanced induction of the katB gene. The data are representative of three independent experiments.
DISCUSSION
Previous studies have demonstrated that H2S may be either detrimental or beneficial to bacterial cells (3, 7, 8). Growth inhibition is attributed to the abilities of H2S to damage DNA and denature proteins. In contrast, a beneficial role played by H2S is its ability to combat oxidative stress. However, there are reports that, in some organisms, H2S stimulates rather than inhibits ROS production (4, 5, 43). The basis of these differences has not been resolved. One possible explanation is that species inhabiting various niches may evolve different mechanisms for responding to H2S, given the unparalleled diversity of all physiological aspects of living organisms. But the presence of a general strategy is equally possible. Here, we present evidence that H2S is indeed able to perform these contrasting functions, depending upon the time of addition.
In this study, endogenous H2S was found to be dispensable for protecting S. oneidensis cells from oxidative stress, in contrast to findings recently reported (3). Conceivably, the production of H2S from metabolic processes is continuous and steady but unable to reach levels sufficiently high to trigger a cellular response. This possibility is supported by the finding that mutation of cysK, resulting in substantially enhanced production of H2S, sensitizes cells to H2O2. Using exogenous H2S, we demonstrated that it promotes H2O2 killing by specifically damaging heme-containing proteins, especially the CAT KatB, even though it is able to induce the protective OxyR regulon, as described in other bacterial species (3). Such an inhibitory effect on heme-containing proteins such as Cco (mitochondrial aa3 type), myoglobin, and hemoglobin by H2S is well documented in eukaryotes (44–46). In general, the reaction between these proteins and H2S induced modification of the heme component, reversibly inhibiting activity (46, 47). Sulfheme species, resulting from the binding of H2S, are reduced because of this interaction (48). Intriguingly, although the inhibitory effect of H2S on mitochondrial Cco was first reported more than 80 years ago, the cellular targets of H2S are still largely restricted to proteins implicated in oxygen transport and consumption (46). Results presented here clearly show that CATs, at least those from S. oneidensis and bovine liver, and cytochrome cbb3 oxidase are inactivated by H2S, expanding the repertoire of H2S targets. It is very likely that there are more H2S targets, based on the very large pool of heme-containing proteins. Nevertheless, with respect to H2O2 killing, CAT is the most crucial target of H2S because its loss largely disables the cell's ability to degrade H2O2. Furthermore, it should be noted that the effect of H2S on different heme-containing proteins may vary considerably, given that amino acid residues near the heme play an essential role in sulfheme formation (46).
In contrast to the inhibitory effect, activation of OxyR by H2S appears to rely on its iron-sequestering capability, as a similar result was obtained with the iron-chelating agent dipyridyl. Iron- and heme-containing proteins, mononuclear iron proteins in particular, are primary targets of H2O2 (49, 50). Some bacteria, such as E. coli, are able to replace the iron atom of these enzymes with manganese to maintain activity in the presence of H2O2 (51, 52). However, in S. oneidensis, this protective mechanism is likely to be heavily compromised because of the extremely low Mn/Fe ratio (19, 53). More importantly, S. oneidensis lacks an H2O2-inducible manganese transporter, preventing the accelerated import of Mn for protection during oxidative stress. As a result, free iron is abundant for the Fenton reaction when challenged by exogenous H2O2. To effectively prevent the formation of lethal hydroxyl radicals, S. oneidensis activates OxyR, resulting in the rapid removal of H2O2 and reduction of intracellular unincorporated iron concentrations. For the former, the strategy is to produce multiple scavenging enzymes, with KatB dominating. For the latter, the situation is rather complicated, however, because there are a large number of iron- and heme-containing proteins in S. oneidensis (such as up to 42 c-type cytochromes, while E. coli has 5 to 7), thus requiring relatively high levels of iron for its normal physiological activities (54, 55).
To control intracellular free-iron levels under stress conditions, the iron-sequestering ferritin Dps is extensively induced upon the activation of OxyR (19). In addition, S. oneidensis limits its iron uptake rate to prevent a sudden increase, as evidenced by its accumulation of 20 times less iron than by E. coli in a 24-h period (53). It is therefore conceivable that although addition of H2S, as well as dipyridyl, reduces levels of free iron to some extent and thus transiently impedes growth, the process overall triggers an iron influx, which likely serves as a signal for activation of OxyR. This hypothesis gains support from the finding that the total iron concentration is significantly elevated but the amounts of free iron are rather constant. Presumably, most of the imported free iron is bound to Dps under oxidative stress conditions. This protective effect of H2S as an iron-sequestering agent seems to coincide with that of superoxide (56). While superoxide can lead to cellular damage and death, its accumulation induces the protective SoxRS and MarRAB regulons (57). Thus, pretreatment with low-to-moderate concentrations of superoxide provides cells a greater ability to cope with subsequent more severe stress and, more importantly, prevents cells from entering programmed cell death (56).
Shewanella bacteria thrive in redox-stratified environments where ROS are likely to form (9) and microbes with high resistance to ROS are presumably favored. However, S. oneidensis is very susceptible to ROS, in comparison to E. coli (19, 53, 58). We do not yet know the reason for this difference, but it may be associated with novel properties of this species. We have previously shown that S. oneidensis prefers insoluble to soluble electron acceptors for respiration, such as Fe (III) oxide versus oxygen, thus preventing endogenous generation of ROS from autoxidation of components of the respiratory chain (35, 59). In addition, unlike other organisms containing cytochrome caa3-type and cbb3-type oxidases, where the low-affinity (caa3-type) oxidase plays a dominant role under high-oxygen conditions and the cbb3 oxidase is induced only at low O2 concentrations, S. oneidensis utilizes the cbb3-type oxidase under both high- and low-oxygen conditions and does not express the caa3 oxidase (39, 40). As respiration of oxygen by the cbb3 oxidase is relatively slow, less ROS would be generated. Moreover, H2S that is produced endogenously may play a beneficial role. The elevated sensitivity to H2O2 is observed only in the H2S-hyperproducing (ΔcysK mutant) strain, implying that H2S generated in the wild type is not sufficient to elicit a detrimental response. Hence, H2S may simply act as an agent to help maintain a relatively reduced environment for cells, either inside or outside in proximity.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (31270097, 41476105), the Major State Basic Research Development Program (973 Program: 2010CB833803), and the Doctoral Fund of the Ministry of Education of China (20130101110142).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00603-15.
REFERENCES
- 1.Wang R. 2012. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 2.Kimura H. 2014. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal 20:783–793. doi: 10.1089/ars.2013.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shatalin K, Shatalina E, Mironov A, Nudler E. 2011. H2S: a universal defense against antibiotics in bacteria. Science 334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 4.Eghbal MA, Pennefather PS, O'Brien PJ. 2004. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology 203:69–76. doi: 10.1016/j.tox.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Lalucat J, Bennasar A, Bosch R, García-Valdés E, Palleroni NJ. 2006. Biology of Pseudomonas stutzeri. Microbiol Mol Biol Rev 70:510–547. doi: 10.1128/MMBR.00047-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caro A, Thompson S, Tackett J. 2011. Increased oxidative stress and cytotoxicity by hydrogen sulfide in HepG2 cells overexpressing cytochrome P450 2E1. Cell Biol Toxicol 27:439–453. doi: 10.1007/s10565-011-9198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirzoyan N, Schreier H. 2014. Effect of sulfide on growth of marine bacteria. Arch Microbiol 196:279–287. doi: 10.1007/s00203-014-0968-0. [DOI] [PubMed] [Google Scholar]
- 8.Fu LH, Hu KD, Hu LY, Li YH, Hu LB, Yan H, Liu YS, Zhang H. 2014. An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum. PLoS One 9:e104206. doi: 10.1371/journal.pone.0104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JLM, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM. 2008. Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6:592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- 10.Lovley DR. 2012. Electromicrobiology. Annu Rev Microbiol 66:391–409. doi: 10.1146/annurev-micro-092611-150104. [DOI] [PubMed] [Google Scholar]
- 11.Janda JM, Abbott SL. 2014. The genus Shewanella: from the briny depths below to human pathogen. Crit Rev Microbiol 40:293–312. doi: 10.3109/1040841X.2012.726209. [DOI] [PubMed] [Google Scholar]
- 12.Myers CR, Nealson KH. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- 13.Burns JL, DiChristina TJ. 2009. Anaerobic respiration of elemental sulfur and thiosulfate by Shewanella oneidensis MR-1 requires psrA, a homolog of the phsA gene of Salmonella enterica serovar Typhimurium LT2. Appl Environ Microbiol 75:5209–5217. doi: 10.1128/AEM.00888-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirodkar S, Reed S, Romine M, Saffarini D. 2011. The octahaem SirA catalyses dissimilatory sulfite reduction in Shewanella oneidensis MR-1. Environ Microbiol 13:108–115. doi: 10.1111/j.1462-2920.2010.02313.x. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Li N, Mao Y, Zhou G, Gao H. 2015. Endogenous generation of hydrogen sulfide and its regulation in Shewanella oneidensis. Front Microbiol 6:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinzinger NK, Fujimoto SY, Clark MA, Moreno MS, Barrett EL. 1995. Sequence analysis of the phs operon in Salmonella typhimurium and the contribution of thiosulfate reduction to anaerobic energy metabolism. J Bacteriol 177:2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato D, Nozaki T. 2009. Methionine gamma-lyase: the unique reaction mechanism, physiological roles, and therapeutic applications against infectious diseases and cancers. IUBMB Life 61:1019–1028. doi: 10.1002/iub.255. [DOI] [PubMed] [Google Scholar]
- 18.Flynn TM, O'Loughlin EJ, Mishra B, DiChristina TJ, Kemner KM. 2014. Sulfur-mediated electron shuttling during bacterial iron reduction. Science 344:1039–1042. doi: 10.1126/science.1252066. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Dong Y, Luo Q, Li N, Wu G, Gao H. 2014. Protection from oxidative stress relies mainly on derepression of OxyR-dependent KatB and Dps in Shewanella oneidensis. J Bacteriol 196:445–458. doi: 10.1128/JB.01077-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Luo Q, Jiang Y, Wu G, Gao H. 2014. Managing oxidative stresses in Shewanella oneidensis: intertwined roles of the OxyR and OhrR regulons. Environ Microbiol 16:1821–1834. doi: 10.1111/1462-2920.12418. [DOI] [PubMed] [Google Scholar]
- 21.Shi M, Wan F, Mao Y, Gao H. 2015. Unraveling the mechanism for the viability deficiency of Shewanella oneidensis oxyR null mutant. J Bacteriol 197:2179–2189. doi: 10.1128/JB.00154-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel LM. 1965. A direct microdetermination for sulfide. Anal Biochem 11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- 23.Jin M, Jiang Y, Sun L, Yin J, Fu H, Wu G, Gao H. 2013. Unique organizational and functional features of the cytochrome c maturation system in Shewanella oneidensis. PLoS One 8:e75610. doi: 10.1371/journal.pone.0075610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L, Wang J, Tang P, Chen H, Gao H. 2011. Genetic and molecular characterization of flagellar assembly in Shewanella oneidensis. PLoS One 6:e21479. doi: 10.1371/journal.pone.0021479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao H, Wang X, Yang ZK, Chen J, Liang Y, Chen H, Palzkill T, Zhou J. 2010. Physiological roles of ArcA, Crp, and EtrA and their interactive control on aerobic and anaerobic respiration in Shewanella oneidensis. PLoS One 5:e15295. doi: 10.1371/journal.pone.0015295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seaver LC, Imlay JA. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol 183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan J, Wei B, Shi M, Gao H. 2011. Functional assessment of EnvZ/OmpR two-component system in Shewanella oneidensis. PLoS One 6:e23701. doi: 10.1371/journal.pone.0023701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macomber L, Rensing C, Imlay JA. 2007. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol 189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maehly AC. 2006. The assay of catalases and peroxidases, p 357–424. In Suelter CH. (ed), Methods of biochemical analysis. John Wiley & Sons, Inc, New York, NY. [DOI] [PubMed] [Google Scholar]
- 30.Marklund S, Marklund G. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. [DOI] [PubMed] [Google Scholar]
- 31.Yin J, Jin M, Zhang H, Ju L, Zhang L, Gao H. 2015. Regulation of nitrite resistance of the cytochrome cbb3 oxidase by cytochrome c ScyA in Shewanella oneidensis. Microbiologyopen 4:84–99. doi: 10.1002/mbo3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu H, Jin M, Ju L, Mao Y, Gao H. 2014. Evidence for function overlapping of CymA and the cytochrome bc1 complex in the Shewanella oneidensis nitrate and nitrite respiration. Environ Microbiol 16:3181–3195. doi: 10.1111/1462-2920.12457. [DOI] [PubMed] [Google Scholar]
- 33.Martin JE, Waters LS, Storz G, Imlay JA. 2015. The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet 11:e1004977. doi: 10.1371/journal.pgen.1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deb S, Johnson EE, Robalinho-Teixeira RL, Wessling-Resnick M. 2009. Modulation of intracellular iron levels by oxidative stress implicates a novel role for iron in signal transduction. Biometals 22:855–862. doi: 10.1007/s10534-009-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S, Imlay JA. 2003. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J Bacteriol 185:1942–1950. doi: 10.1128/JB.185.6.1942-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu H, Jin M, Wan F, Gao H. 2015. Shewanella oneidensis cytochrome c maturation component CcmI is essential for heme attachment at the non-canonical motif of nitrite reductase NrfA. Mol Microbiol 95:410–425. doi: 10.1111/mmi.12865. [DOI] [PubMed] [Google Scholar]
- 38.Mueller S, Riedel H-D, Stremmel W. 1997. Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal Biochem 245:55–60. doi: 10.1006/abio.1996.9939. [DOI] [PubMed] [Google Scholar]
- 39.Fu H, Chen H, Wang J, Zhou G, Zhang H, Gao H. 2013. Crp-dependent cytochrome bd oxidase confers nitrite resistance to Shewanella oneidensis. Environ Microbiol 15:2198–2212. doi: 10.1111/1462-2920.12091. [DOI] [PubMed] [Google Scholar]
- 40.Zhou G, Yin J, Chen H, Hua Y, Sun L, Gao H. 2013. Combined effect of loss of the caa3 oxidase and Crp regulation drives Shewanella to thrive in redox-stratified environments. ISME J 7:1752–1763. doi: 10.1038/ismej.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gusarov I, Nudler E. 2005. NO-mediated cytoprotection: instant adaptation to oxidative stress in bacteria. Proc Natl Acad Sci U S A 102:13855–13860. doi: 10.1073/pnas.0504307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrews SC, Robinson AK, Rodríguez-Quiñones F. 2003. Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 43.Borkowska A, Sielicka-Dudzin A, Herman-Antosiewicz A, Wozniak M, Fedeli D, Falcioni G, Antosiewicz J. 2012. Diallyl trisulfide-induced prostate cancer cell death is associated with Akt/PKB dephosphorylation mediated by P-p66shc. Eur J Nutr 51:817–825. doi: 10.1007/s00394-011-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collman JP, Ghosh S, Dey A, Decréau RA. 2009. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc Natl Acad Sci U S A 106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kabil O, Banerjee R. 2010. Redox biochemistry of hydrogen sulfide. J Biol Chem 285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietri R, Roman-Morales E, Lopez-Garriga J. 2011. Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid Redox Signal 15:393–404. doi: 10.1089/ars.2010.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper CE, Brown GC. 2008. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Rose P, Moore PK. 2011. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 49.Sobota JM, Imlay JA. 2011. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci U S A 108:5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anjem A, Imlay JA. 2012. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem 287:15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anjem A, Varghese S, Imlay JA. 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol 72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imlay JA. 2014. The mismetallation of enzymes during oxidative stress. J Biol Chem 289:28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D. 2004. Accumulation of MnII in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 54.Meyer TE, Tsapin AI, Vandenberghe I, De Smet L, Frishman D, Nealson KH, Cusanovich MA, Van Beeumen JJ. 2004. Identification of 42 possible cytochrome c genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes. OMICS 8:57–77. doi: 10.1089/153623104773547499. [DOI] [PubMed] [Google Scholar]
- 55.Gao H, Barua S, Liang Y, Wu L, Dong Y, Reed S, Chen J, Culley D, Kennedy D, Yang Y, He Z, Nealson KH, Fredrickson JK, Tiedje JM, Romine M, Zhou J. 2010. Impacts of Shewanella oneidensis c-type cytochromes on aerobic and anaerobic respiration. Microb Biotechnol 3:455–466. doi: 10.1111/j.1751-7915.2010.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dorsey-Oresto A, Lu T, Mosel M, Wang X, Salz T, Drlica K, Zhao X. 2013. YihE kinase is a central regulator of programmed cell death in bacteria. Cell Rep 3:528–537. doi: 10.1016/j.celrep.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y, Vulić M, Keren I, Lewis K. 2012. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother 56:4922–4926. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin J, Gao H. 2011. Stress responses of Shewanella. Int J Microbiol 2011:863623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan J, Chen Y, Zhou G, Chen H, Gao H. 2013. Investigation of roles of divalent cations in Shewanella oneidensis pellicle formation reveals unique impacts of insoluble iron. Biochim Biophys Acta 1830:5248–5257. doi: 10.1016/j.bbagen.2013.07.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.