Abstract
Congenital human cytomegalovirus (HCMV) infection is associated with neurodevelopmental disabilities. To dissect the earliest events of infection in the developing human brain, we studied HCMV infection during controlled differentiation of human embryonic stem cells (hESC) into neural precursors. We traced a transition from viral restriction in hESC, mediated by a block in viral binding, toward HCMV susceptibility in early hESC-derived neural precursors. We further revealed the role of platelet-derived growth factor receptor alpha (PDGFRα) as a determinant of the developmentally acquired HCMV susceptibility.
TEXT
Human cytomegalovirus (HCMV) is a leading cause of congenital infection (1), associated with neurodevelopmental disabilities (2, 3). The ability of the virus to infect the developing fetal brain is a key factor in its neuropathogenesis (3–8). While considerable experimental data were obtained from newborn and embryonic mouse models (6, 9–15), the strict species specificity precludes animal models of HCMV. Recent studies in human neuronal progenitor cells (NPC) derived from fetal and neonatal brains have revealed productive HCMV infection of NPC, with resultant functional alterations (16–20). Notably, NPC do not represent early neural embryonic development but rather a more advanced stage along the neuronal differentiation route. Hence, the earliest events defining the susceptibility of the developing human nervous system to HCMV have remained largely unknown.
Human embryonic stem cells (hESC) provide an opportunity to study early human neural development (21–23). We have developed highly reproducible protocols for controlled induction of differentiation of hESC toward a neural lineage, giving rise to enriched populations of proliferating, developmentally multipotent early NPC (24, 25).
Here, we have employed experimental HCMV infection in a dynamic model of controlled differentiation of hESC into neural precursors, to gain insight into the molecular events mediating HCMV infection during early human neurodevelopment.
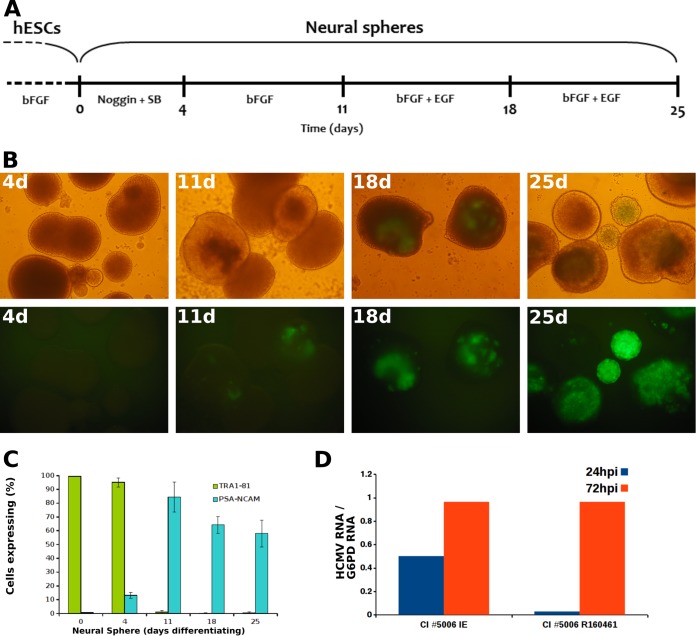
We first sought to track the earliest stage of neural differentiation at which the cells become susceptible to HCMV. To this end, differentiation of hESC was induced in floating cell clusters toward the formation of neural spheres (Fig. 1A) as detailed previously (24, 25). The emerging neural spheres were infected at sequential time points along the neural differentiation process with the broadly tropic HCMV TB40/E strain expressing green fluorescent protein (GFP) (26, 27) (Fig. 1B). In accordance with previous studies (25), immunostaining analysis for the pluripotent stem cell marker TRA 1-81 and the neural differentiation marker PSA-NCAM showed that the majority of the cells underwent early neural differentiation within 11 days (Fig. 1C). Importantly, we have identified a transition toward HCMV susceptibility occurring between 4 and 11 days post-differentiation induction (Fig. 1B). A similar susceptibility pattern was observed for low-passage-number, cell-associated, clinical strains isolated from urine samples of congenitally infected neonates, with viral gene expression first detected following infection of 11-day-old hESC-derived NPC (Fig. 1D). Our findings trace the switch toward HCMV susceptibility to early neural precursors, representing the primitive neuroepithelial cells (24, 25, 28), which form the neural plate and neural tube as early as 4 weeks of gestation—later giving rise to the progenitors of neural stem cells (28–32). Viral targeting of these primary predecessors of the developing nervous system could underlie the progressive neurodevelopmental disabilities associated with congenital HCMV infection.
FIG 1.
Transition toward HCMV susceptibility in early hESC-derived NPC. (A) Schematic presentation of induction of hESC differentiation into NPC. Undifferentiated hESC were maintained in culture medium supplemented with basic fibroblast growth factor 2 (bFGF). Upon initiation of neural differentiation (days 0 to 4), bFGF was withdrawn from the culture medium. The medium was supplemented during days 0 to 4 with two inhibitors of the SMAD signaling pathway (Noggin and SB-431542). From day 5, the SMAD signaling inhibitors were removed, and the medium was supplemented with bFGF. From day 12, the medium was further supplemented with epidermal growth factor (EGF) (25, 28). (B) Neurospheres formed at the indicated time points post-induction of differentiation were infected with HCMV strain TB40/E expressing the late gene pp150 fused to GFP (multiplicity of infection of 1 PFU/cell) and visualized at 7 days postinfection by inverted-phase (top row) and fluorescence (bottom row) microscopy. Similar findings were obtained using HCMV strain TB40/E expressing immediate-early GFP (not shown). (C) Expression of cell differentiation markers by neurospheres formed at the indicated times post-induction of differentiation, as analyzed by immunostaining and flow cytometry. (D) Neurospheres at day 11 post-induction of differentiation were infected with the low-passage-number clinical strain CI5006, recovered from the urine of a congenitally infected newborn. Shown are the viral IE-1 and late RNA R160461 mRNA levels, determined as described previously (27) at the indicated times postinfection and normalized by the housekeeping gene for glucose-6-phosphate dehydrogenase (G6PD) (similar results were obtained with three additional low-passage-number clinical isolates).
In line with the initial restriction to HCMV infection in hESC-derived NPC, we found that undifferentiated hESC (lines hES1 and HAD-C 102 [22, 33]) are resistant to infection by broadly tropic HCMV laboratory-derived and low-passage-number clinical strains. In contrast, hESC were susceptible to herpes simplex virus 1 (HSV-1) and adenovirus (data not shown). These findings, along with reports of their susceptibility to HSV-1, pseudorabies virus (PrV), coxsackie virus, and varicella-zoster virus (VZV), when grown in suspension (34, 35), suggest a virus-specific restriction mechanism(s).
We next studied the nature of the impediment to HCMV infection in hESC. Interestingly, we identified a block at the initial stage of viral binding, demonstrated by (i) failure of the major viral tegument protein pp65 to transport into the nucleus or cytoplasm (Fig. 2A and B), reflecting absence of viral internalization, and (ii) lack of HCMV DNA accumulation during viral incubation with hESC (under conditions favoring HCMV and HSV-1 binding onto susceptible human foreskin fibroblasts [HFF] and hESC, respectively) (Fig. 2C and D), compatible with HCMV-specific binding restriction in hESC. While we show a dominant viral entry block, we cannot rule out additional intracellular constraints, targeting downstream steps in the virus life cycle, including the recently demonstrated suppression of HCMV major immediate-early promoter (MIEP) in hESC lines Wisconsin H1 and H9, different from the ones used here (36, 37).
FIG 2.
HCMV binding and entry are blocked in hESC. (A and B) hESC (panels I and II) and control human foreskin fibroblasts (panels III) were mock infected (panels I) or infected with HCMV strain TB40/E expressing pp150-fused GFP or strain TB40/E expressing pp65-fused GFP (panels II and III, respectively) at a multiplicity of infection of 1 PFU/cell. At 24 h postinfection, the cells were fixed, immunostained for HCMV pp65, and visualized by confocal microscopy (A) or directly visualized by fluorescent-phase microscopy (B). Large photos are at ×40 magnification (bar, 50 μm); small photos are at ×20 magnification (bar, 100 μm). A similar lack of pp65 internalization in hESC was observed at sequential time points between 2 and 48 h postinfection (viral incubation times equaled inspection times for these experiments; data not shown). (C and D) Quantitative viral DNA measurements, performed as described previously (27) in the indicated cell lysates, normalized by RNase P (RnP) gene DNA copies. (C) Viral entry assay. Cells were inoculated with HCMV strain TB40/E (multiplicity of infection of 0.5 PFU/cell) in the presence or absence of heparin, incubated at 37°C for 2 h, and treated with trypsin-EDTA to remove cell-bound noninternalized virus, and viral DNA quantity was analyzed by quantitative real-time PCR (27). (D) Viral binding assay. Cells were incubated with HCMV strain TB40/E (multiplicity of infection of 1 PFU/cell) at 4°C for the indicated times, in the presence or absence of heparin, collected by gentle scraping, and analyzed for viral DNA quantity as described above. Panel II shows the viral DNA accumulation kinetics over the 2 h of incubation. The minimal viral DNA accumulation in hESC, detected in panels C and D, is attributed to low-level viral infection which was limited to the small fraction of spontaneously differentiating cells (data not shown) that are commonly present in hESC cultures (22, 50). The asterisks indicate undetectable levels.
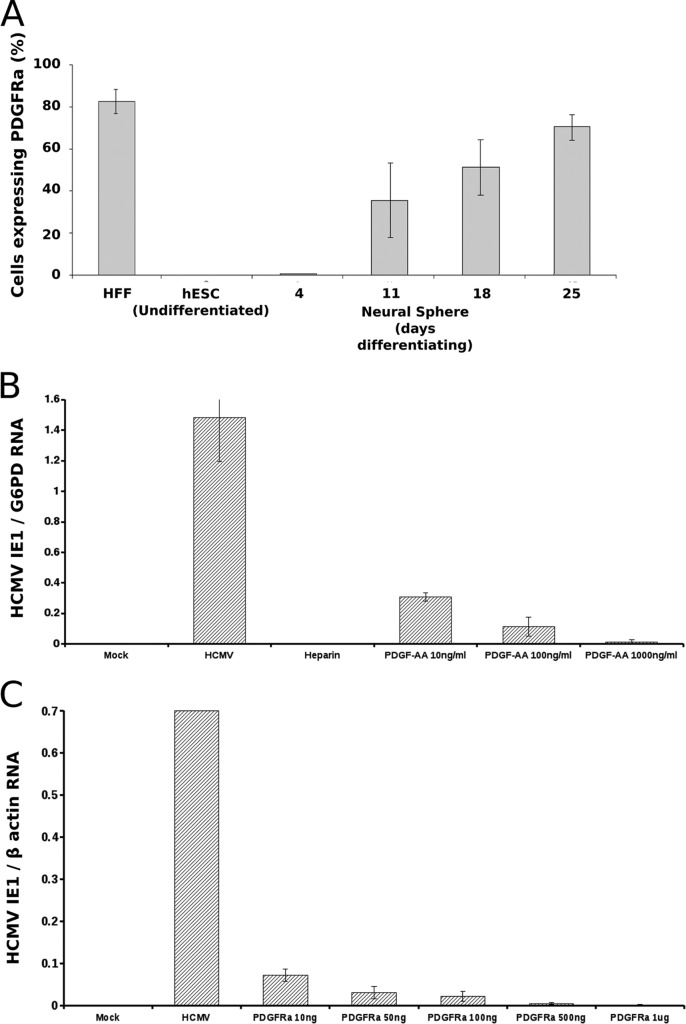
Having revealed the restriction at the stage of viral binding, we proceeded to identify the candidate cellular factor which mediates the transition toward viral susceptibility upon neural differentiation from hESC. Since platelet-derived growth factor receptor alpha (PDGFRα) has been shown to mediate HCMV entry in a cell-type-dependent manner (38, 39), we hypothesized that it plays a role in viral entry into NPC; we found a close correlation between PDGFRα expression along the neural differentiation route and the HCMV susceptibility pattern (Fig. 3A). Furthermore, we have shown a clear dose-dependent competitive inhibition of HCMV infection following pretreatment of early hESC-derived NPC with PDGFRα ligand PDGF-AA (Fig. 3B). An independent experimental approach consistently revealed that pretreatment of HCMV with increasing amounts of soluble PDGFRα resulted in remarkable dose-dependent inhibition of HCMV infection in hESC-derived NPC (Fig. 3C). These combined findings directly support the role of PDGFRα as a determinant of the developmentally acquired HCMV susceptibility. The mechanism by which PDGFRα regulates HCMV infection in hESC-derived NPC remains to be determined. In view of the documented functions of PDGFRα during neurodevelopment (30, 40–47), it is tempting to speculate that its engagement by HCMV and its virus-induced downregulation (48) could be a key factor in the neuropathogenesis of congenital HCMV. Our findings do not exclude a role for other potential cofactors (including additional surface receptors) in modulating the increased viral susceptibility during differentiation, which could be further analyzed by global gene expression studies. In fact, we observed a gradual increase in viral susceptibility along subsequent differentiation time points (Fig. 1), suggesting a dynamic process with temporal release of additional restriction checkpoints. This finding is in agreement with a recent report demonstrating multiple viral restriction levels in hESC-derived neural differentiating cells, which were gradually alleviated during differentiation progression (49).
FIG 3.
PDGFRα mediates HCMV susceptibility in early hESC-derived NPC. (A) Expression of PDGFRα, as analyzed by immunostaining and flow cytometry, is shown in hESC, in neural spheres formed at the indicated times post-induction of differentiation, and in control HFF. (B) Pretreatment of cells with PDGF-AA inhibits HCMV infection in hESC-derived NPC. Neural spheres at 18 days postinduction were infected with HCMV strain TB40/E following pretreatment with increasing concentrations of PDGF-AA. Control cultures were mock infected or infected in the presence of heparin. (C) Pretreatment of HCMV with soluble PDGFRα inhibits HCMV infection in hESC-derived NPC. HCMV was preincubated with increasing amounts of soluble PDGFRα prior to infection of neural spheres. Control experiments revealed no inhibition of infection following preincubation of the virus with similar amounts of bovine serum albumin (not shown). (B and C) Viral IE-1 mRNA levels in the cell lysates at 24 h postinfection, normalized by the housekeeping gene for glucose-6-phosphate dehydrogenase (G6PD) or β-actin.
The novel mechanism that we have discovered here, which restricts viral infection in hESC and confers a developmental window of susceptibility, unveils a potential survival strategy by which the virus avoids perturbing embryogenesis and instead targets early lineage-unrestricted neuroepithelial precursors. Future studies that will examine the effect of HCMV infection on the fate and function of the primitive neuroepithelial cells could pave the way to new directions for prevention and therapy of congenital HCMV.
ACKNOWLEDGMENTS
This work was supported by grants from the Israel Science Foundation, the Israeli Ministry of Health, and the European Union Seventh Framework Programme FP7/2012-2016 under grant agreement no. 316655 and by a generous gift from Julie Swartz and from Judy and Sidney Swartz.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Bracha Rager and Tamir Ben-Hur for ideas and helpful discussions.
REFERENCES
- 1.Cannon MJ. 2009. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol 46(Suppl 4):S6–S10. doi: 10.1016/j.jcv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Demmler GJ. 1996. Congenital cytomegalovirus infection and disease. Adv Pediatr Infect Dis 11:135–162. [PubMed] [Google Scholar]
- 3.Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, Veren DA, Page F, Alford CA. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904–1908. [PubMed] [Google Scholar]
- 4.Perlman JM, Argyle C. 1992. Lethal cytomegalovirus infection in preterm infants: clinical, radiological, and neuropathological findings. Ann Neurol 31:64–68. doi: 10.1002/ana.410310112. [DOI] [PubMed] [Google Scholar]
- 5.Gabrielli L, Bonasoni MP, Lazzarotto T, Lega S, Santini D, Foschini MP, Guerra B, Baccolini F, Piccirilli G, Chiereghin A, Petrisli E, Gardini G, Lanari M, Landini MP. 2009. Histological findings in foetuses congenitally infected by cytomegalovirus. J Clin Virol 46(Suppl 4):S16–S21. doi: 10.1016/j.jcv.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Cheeran MC, Lokensgard JR, Schleiss MR. 2009. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev 22:99–126. doi: 10.1128/CMR.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries LS, Gunardi H, Barth PG, Bok LA, Verboon-Maciolek MA, Groenendaal F. 2004. The spectrum of cranial ultrasound and magnetic resonance imaging abnormalities in congenital cytomegalovirus infection. Neuropediatrics 35:113–119. doi: 10.1055/s-2004-815833. [DOI] [PubMed] [Google Scholar]
- 8.Bale JF Jr, Bray PF, Bell WE. 1985. Neuroradiographic abnormalities in congenital cytomegalovirus infection. Pediatr Neurol 1:42–47. doi: 10.1016/0887-8994(85)90008-6. [DOI] [PubMed] [Google Scholar]
- 9.Koontz T, Bralic M, Tomac J, Pernjak-Pugel E, Bantug G, Jonjic S, Britt WJ. 2008. Altered development of the brain after focal herpesvirus infection of the central nervous system. J Exp Med 205:423–435. doi: 10.1084/jem.20071489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosugi I, Shinmura Y, Kawasaki H, Arai Y, Li RY, Baba S, Tsutsui Y. 2000. Cytomegalovirus infection of the central nervous system stem cells from mouse embryo: a model for developmental brain disorders induced by cytomegalovirus. Lab Invest 80:1373–1383. doi: 10.1038/labinvest.3780145. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Patel R, Ren C, Taggart MG, Firpo MA, Schleiss MR, Park AH. 2013. A comparison of different murine models for cytomegalovirus-induced sensorineural hearing loss. Laryngoscope 123:2801–2806. doi: 10.1002/lary.24090. [DOI] [PubMed] [Google Scholar]
- 12.Kashiwai A, Kawamura N, Kadota C, Tsutsui Y. 1992. Susceptibility of mouse embryo to murine cytomegalovirus infection in early and mid-gestation stages. Arch Virol 127:37–48. doi: 10.1007/BF01309573. [DOI] [PubMed] [Google Scholar]
- 13.Tebourbi L, Testart J, Cerutti I, Moussu JP, Loeuillet A, Courtot AM. 2002. Failure to infect embryos after virus injection in mouse zygotes. Hum Reprod 17:760–764. doi: 10.1093/humrep/17.3.760. [DOI] [PubMed] [Google Scholar]
- 14.Matsukage S, Kosugi I, Kawasaski H, Miura K, Kitani H, Tsutsui Y. 2006. Mouse embryonic stem cells are not susceptible to cytomegalovirus but acquire susceptibility during differentiation. Birth Defects Res A Clin Mol Teratol 76:115–125. doi: 10.1002/bdra.20233. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki H, Kosugi I, Arai Y, Iwashita T, Tsutsui Y. 2011. Mouse embryonic stem cells inhibit murine cytomegalovirus infection through a multi-step process. PLoS One 6:e17492. doi: 10.1371/journal.pone.0017492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheeran MC, Hu S, Ni HT, Sheng W, Palmquist JM, Peterson PK, Lokensgard JR. 2005. Neural precursor cell susceptibility to human cytomegalovirus diverges along glial or neuronal differentiation pathways. J Neurosci Res 82:839–850. doi: 10.1002/jnr.20682. [DOI] [PubMed] [Google Scholar]
- 17.Odeberg J, Wolmer N, Falci S, Westgren M, Seiger A, Soderberg-Naucler C. 2006. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J Virol 80:8929–8939. doi: 10.1128/JVI.00676-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odeberg J, Wolmer N, Falci S, Westgren M, Sundtrom E, Seiger A, Soderberg-Naucler C. 2007. Late human cytomegalovirus (HCMV) proteins inhibit differentiation of human neural precursor cells into astrocytes. J Neurosci Res 85:583–593. doi: 10.1002/jnr.21144. [DOI] [PubMed] [Google Scholar]
- 19.Luo MH, Schwartz PH, Fortunato EA. 2008. Neonatal neural progenitor cells and their neuronal and glial cell derivatives are fully permissive for human cytomegalovirus infection. J Virol 82:9994–10007. doi: 10.1128/JVI.00943-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo MH, Hannemann H, Kulkarni AS, Schwartz PH, O'Dowd JM, Fortunato EA. 2010. Human cytomegalovirus infection causes premature and abnormal differentiation of human neural progenitor cells. J Virol 84:3528–3541. doi: 10.1128/JVI.02161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 22.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. 2000. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 23.Petros TJ, Tyson JA, Anderson SA. 2011. Pluripotent stem cells for the study of CNS development. Front Mol Neurosci 4:30. doi: 10.3389/fnmol.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen MA, Itsykson P, Reubinoff BE. 2007. Neural differentiation of human ES cells. Curr Protoc Cell Biol Chapter 23:Unit 23.7. doi: 10.1002/0471143030.cb2307s36. [DOI] [PubMed] [Google Scholar]
- 25.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. 2009. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinzger C, Hahn G, Digel M, Katona R, Sampaio KL, Messerle M, Hengel H, Koszinowski U, Brune W, Adler B. 2008. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J Gen Virol 89:359–368. doi: 10.1099/vir.0.83286-0. [DOI] [PubMed] [Google Scholar]
- 27.Weisblum Y, Panet A, Zakay-Rones Z, Haimov-Kochman R, Goldman-Wohl D, Ariel I, Falk H, Natanson-Yaron S, Goldberg MD, Gilad R, Lurain NS, Greenfield C, Yagel S, Wolf DG. 2011. Modeling of human cytomegalovirus maternal-fetal transmission in a novel decidual organ culture. J Virol 85:13204–13213. doi: 10.1128/JVI.05749-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. 2001. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Zhang SC. 2011. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell Mol Life Sci 68:3995–4008. doi: 10.1007/s00018-011-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson EL, Alvarez-Buylla A. 2008. Characterization of adult neural stem cells and their relation to brain tumors. Cells Tissues Organs 188:212–224. doi: 10.1159/000114541. [DOI] [PubMed] [Google Scholar]
- 31.Merkle FT, Alvarez-Buylla A. 2006. Neural stem cells in mammalian development. Curr Opin Cell Biol 18:704–709. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Xia X, Zhang SC. 2009. Differentiation of neuroepithelia from human embryonic stem cells. Methods Mol Biol 549:51–58. doi: 10.1007/978-1-60327-931-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tannenbaum SE, Turetsky TT, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, Levy O, Arbell D, Simon A, Ben-Meir A, Shufaro Y, Laufer N, Reubinoff BE. 2012. Derivation of xeno-free and GMP-grade human embryonic stem cells—platforms for future clinical applications. PLoS One 7:e35325. doi: 10.1371/journal.pone.0035325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scassa ME, Jaquenod de Giusti C, Questa M, Pretre G, Richardson GA, Bluguermann C, Romorini L, Ferrer MF, Sevlever GE, Miriuka SG, Gomez RM. 2011. Human embryonic stem cells and derived contractile embryoid bodies are susceptible to Coxsackievirus B infection and respond to interferon Ibeta treatment. Stem Cell Res 6:13–22. doi: 10.1016/j.scr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Dukhovny A, Sloutskin A, Markus A, Yee MB, Kinchington PR, Goldstein RS. 2012. Varicella-zoster virus infects human embryonic stem cell-derived neurons and neurospheres but not pluripotent embryonic stem cells or early progenitors. J Virol 86:3211–3218. doi: 10.1128/JVI.06810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia X, Zhang Y, Zieth CR, Zhang SC. 2007. Transgenes delivered by lentiviral vector are suppressed in human embryonic stem cells in a promoter-dependent manner. Stem Cells Dev 16:167–176. doi: 10.1089/scd.2006.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penkert RR, Kalejta RF. 2013. Human embryonic stem cell lines model experimental human cytomegalovirus latency. mBio 4(3):e00298-13. doi: 10.1128/mBio.00298-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soroceanu L, Akhavan A, Cobbs CS. 2008. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 455:391–395. doi: 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- 39.Vanarsdall AL, Wisner TW, Lei H, Kazlauskas A, Johnson DC. 2012. PDGF receptor-alpha does not promote HCMV entry into epithelial and endothelial cells but increased quantities stimulate entry by an abnormal pathway. PLoS Pathog 8:e1002905. doi: 10.1371/journal.ppat.1002905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrae J, Hansson I, Afink GB, Nister M. 2001. Platelet-derived growth factor receptor-alpha in ventricular zone cells and in developing neurons. Mol Cell Neurosci 17:1001–1013. doi: 10.1006/mcne.2001.0989. [DOI] [PubMed] [Google Scholar]
- 41.Park JK, Williams BP, Alberta JA, Stiles CD. 1999. Bipotent cortical progenitor cells process conflicting cues for neurons and glia in a hierarchical manner. J Neurosci 19:10383–10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betsholtz C, Richardson WD. 1999. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development 126:457–467. [DOI] [PubMed] [Google Scholar]
- 43.Soriano P. 1997. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 124:2691–2700. [DOI] [PubMed] [Google Scholar]
- 44.Finzsch M, Stolt CC, Lommes P, Wegner M. 2008. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development 135:637–646. doi: 10.1242/dev.010454. [DOI] [PubMed] [Google Scholar]
- 45.Forsberg-Nilsson K, Behar TN, Afrakhte M, Barker JL, McKay RD. 1998. Platelet-derived growth factor induces chemotaxis of neuroepithelial stem cells. J Neurosci Res 53:521–530. [DOI] [PubMed] [Google Scholar]
- 46.Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. 1998. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron 20:869–882. doi: 10.1016/S0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 47.Hall A, Giese NA, Richardson WD. 1996. Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development 122:4085–4094. [DOI] [PubMed] [Google Scholar]
- 48.Gredmark S, Straat K, Homman-Loudiyi M, Kannisto K, Soderberg-Naucler C. 2007. Human cytomegalovirus downregulates expression of receptors for platelet-derived growth factor by smooth muscle cells. J Virol 81:5112–5120. doi: 10.1128/JVI.02197-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belzile JP, Stark TJ, Yeo GW, Spector DH. 2014. Human cytomegalovirus infection of human embryonic stem cell-derived primitive neural stem cells is restricted at several steps but leads to the persistence of viral DNA. J Virol 88:4021–4039. doi: 10.1128/JVI.03492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amit M, Itskovitz-Eldor J. 2002. Derivation and spontaneous differentiation of human embryonic stem cells. J Anat 200:225–232. doi: 10.1046/j.1469-7580.2002.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]