ABSTRACT
T cell memory is usually studied in the context of infection with a single pathogen in naive mice, but how memory develops during a coinfection with two pathogens, as frequently occurs in nature or after vaccination, is far less studied. Here, we questioned how the competition between immune responses to two viruses in the same naive host would influence the development of CD8 T cell memory and subsequent disease outcome upon challenge. Using two different models of coinfection, including the well-studied lymphocytic choriomeningitis (LCMV) and Pichinde (PICV) viruses, several differences were observed within the CD8 T cell responses to either virus. Compared to single-virus infection, coinfection resulted in substantial variation among mice in the size of epitope-specific T cell responses to each virus. Some mice had an overall reduced number of virus-specific cells to either one of the viruses, and other mice developed an immunodominant response to a normally subdominant, cross-reactive epitope (nucleoprotein residues 205 to 212, or NP205). These changes led to decreased protective immunity and enhanced pathology in some mice upon challenge with either of the original coinfecting viruses. In mice with PICV-dominant responses, during a high-dose challenge with LCMV clone 13, increased immunopathology was associated with a reduced number of LCMV-specific effector memory CD8 T cells. In mice with dominant cross-reactive memory responses, during challenge with PICV increased immunopathology was directly associated with these cross-reactive NP205-specific CD8 memory cells. In conclusion, the inherent competition between two simultaneous immune responses results in significant alterations in T cell immunity and subsequent disease outcome upon reexposure.
IMPORTANCE Combination vaccines and simultaneous administration of vaccines are necessary to accommodate required immunizations and maintain vaccination rates. Antibody responses generally correlate with protection and vaccine efficacy. However, live attenuated vaccines also induce strong CD8 T cell responses, and the impact of these cells on subsequent immunity, whether beneficial or detrimental, has seldom been studied, in part due to the lack of known T cell epitopes to vaccine viruses. We questioned if the inherent increased competition and stochasticity between two immune responses during a simultaneous coinfection would significantly alter CD8 T cell memory in a mouse model where CD8 T cell epitopes are clearly defined. We show that some of the coinfected mice have sufficiently altered memory T cell responses that they have decreased protection and enhanced immunopathology when reexposed to one of the two viruses. These data suggest that a better understanding of human T cell responses to vaccines is needed to optimize immunization strategies.
INTRODUCTION
Antiviral immunity is predominately studied in the context of infection with a single pathogen although simultaneous infection with two or more microorganisms is a common occurrence in nature. Simultaneous coinfections occur when pathogens share the same route of transmission, such as insect vectors or contaminated blood products. Multiple insect bites from virally infected insect vectors (e.g., mosquitoes) can cause coinfection, and mosquitoes can be coinfected and transmit multiple viruses (1, 2). These coinfections are commonly associated with enhanced disease severity. During a 2006 dengue virus outbreak in India, 19% of patients were coinfected with multiple serotypes of dengue virus. A higher percentage of these patients with coinfection developed the severe symptoms associated with dengue hemorrhagic fever (3). In another study, 13% of patients admitted to hospital during the 2009 H1N1 influenza A virus (IAV) pandemic were coinfected with at least one other respiratory virus (4). The patients coinfected with IAV and coronavirus or respiratory syncytial virus had enhanced disease severity compared to that of patients infected with only IAV (4, 5). Used hypodermic needles and contaminated blood products may also trigger coinfections as they harbor frequently more than one virus. Of intravenous drug users infected with human immunodeficiency virus (HIV), 90 to 95% are also infected with hepatitis C virus (HCV) (6), making these patients reservoirs for coinfecting other individuals. HIV/HCV coinfection is associated with faster progression to HCV-mediated liver disease than infection with only HCV and increased risk of cirrhosis in these patients (7). Simultaneous coinfection with hepatitis B and D viruses, which is more common in intravenous drug users, is also more frequently associated with fulminant hepatitis than sequential infection (8).
Multiple vaccines given simultaneously or as combination formulations are similar to a coinfection due to exposure to antigens from a number of different pathogens at the same time. Generally, physicians and parents are comfortable with a child receiving up to three vaccines simultaneously (9, 10). However, CDC protocols allow for children to receive up to nine vaccine injections containing 13 different vaccines at their 12- to 15-month doctor's visit if the child is behind in the vaccination schedule (11). Vaccine interference, where one vaccine dampens the antibody response to another during administration of multiple vaccines, has been reported (12, 13). For example, in Nigerian children the simultaneous administration of the measles vaccine with the smallpox, yellow fever, and the combination diphtheria, pertussis, and tetanus vaccines resulted in an 89% to 70% decrease in measles seroconversion rates (13). Vaccine interference would suggest that during a coinfection or during the concurrent development of two or more primary immune responses to multiple pathogens simultaneously, factors such as competition and stochasticity might influence the developing immune responses. Due to the paucity of identified human T cell epitopes in the majority of vaccines, it is unclear how concurrent administration of vaccines or simultaneous infection with viruses affects CD8 T cell immunity.
Here, we questioned if CD8 T cell responses were able to develop relatively independently during a simultaneous coinfection or if they would be altered by the inherent competition present in a coinfection environment. Furthermore, we questioned if these changes would have detrimental effects on disease outcome upon subsequent reexposure or challenge with either virus. In order to specifically study the effect of coinfection-induced competition on CD8 T cell memory, we focused on viral mouse models that have well-defined CD8 T cell responses and are dependent on CD8 T cell immunity for viral clearance. Mice were simultaneously coinfected with two different combinations of viruses: lymphocytic choriomeningitis (LCMV)/Pichinde viruses (PICV) or LCMV/vaccinia virus (VACV). Our group has previously extensively studied these viruses in both single-virus infection of naive hosts and sequential infections. During a sequential infection viruses are given singly, and memory is allowed to develop over 6 weeks before challenge with the second virus, which is analogous to more natural or seasonal infections. We have previously observed protective heterologous immunity during sequential infection of LCMV-immune mice with PICV or VACV that was mediated by the reactivation of clearly defined cross-reactive LCMV-specific CD8 memory T cells (14–20). These previous studies in LCMV-immune mice infected with PICV show that alteration in disease outcome is dependent on a CD8 T cell epitope that is cross-reactive between these two distantly related viruses that have 54% sequence homology. This cross-reactive subdominant epitope, nucleoprotein residues 205 to 212 (NP205–212, or NP205), is not identical between the two viruses and differs in 2 of 8 amino acids. Every naive mouse infected with LCMV or PICV generates a low frequency of memory CD8 T cells specific to the subdominant epitope NP205. When either LCMV- or PICV-immune mice are subsequently infected with the alternate virus, the NP205 memory response is reactivated and becomes immunodominant, and there is more rapid viral clearance than in naive mice. For instance, during PICV infection of LCMV-immune mice, the LCMV-NP205-specific memory T cells that recognize PICV-NP205 antigen are reactivated, resulting in an altered PICV-specific immunodominance hierarchy (14, 15, 17). Similar events occur in LCMV-immune mice challenged with VACV (15, 19, 21). LCMV-immune mice challenged with VACV have several well-defined cross-reactive memory responses, including LCMV-NP205, LCMV glycoprotein (GP) residues 34 to 41 (LCMV-GP34), and LCMV GP residues 118 to 125 (LCMV-GP118), that can expand and mediate faster viral clearance (18, 22). However, some of these mice also develop immunopathology in the form of increased weight loss and panniculitis presenting as acute fatty necrosis of abdominal fat (15, 21–23). The disease outcome, protective immunity and/or panniculitis, is dependent on which cross-reactive epitope-specific population expands (21), which is linked to the private specificity of the T cell receptor (TCR) repertoire of each individual host. Private specificity of the TCR repertoire develops due to the stochastic nature of thymic selection resulting in different TCR repertoires even in genetically identical mice, and during viral infection the stochastic nature of T cell selection further enhances TCR private specificity to a particular antigen (24). Thus, each LCMV-immune mouse would have a different cross-reactive T cell population of different avidities and functionalities. These characteristics of the cross-reactive T cell response play a major role in how quickly virus is cleared from peripheral organs, such as the abdominal fat pads. If the T cell response is highly efficient at clearing virus, minimal immunopathology develops; however, if virus lingers in tissues, increased numbers of effector cells migrate into these areas and induce enhanced collateral damage in the process of clearing virus or immunopathology.
All of our previous studies using two viruses have been in sequential infection models where we examined how prior immunity to an unrelated pathogen would influence disease outcome. We have never examined the effect on the primary immune response in a naive host when mice are infected with two viruses simultaneously. Our systems designed to study heterologous immunity can easily be utilized to address this important question also. In this study, we determined, for the first time to our knowledge, whether the competition during infection with two viruses at the same time would alter the qualitative and quantitative characteristics of CD8 T cell memory formation and thus immune protection and immunopathology upon reexposure. We would predict that the increased competition for resources within the coinfection environment would further enhance stochasticity of the TCR repertoire selection and lead to less predictable immune correlates of protection than single-virus infection.
(The data from the manuscript have been presented in part at the Co-infections Meeting, Bolton Landing, NY, 15 to 17 April 2012, and at the Keystone Symposia: Immunological Memory, Persisting Microbes, and Chronic Disease, Banff, Alberta, Canada, 22 March 2011).
MATERIALS AND METHODS
Mice.
Male C57BL/6J (B6, H-2b) mice, 5 to 6 weeks old, were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific-pathogen-free conditions at the Department of Animal Medicine, University of Massachusetts Medical School. This study was done in compliance within the guidelines of our Institutional Animal Care and Use Committee.
Viruses.
LCMV (Armstrong, clone 13 strain [CL-13], and rL212A variant) and PICV (AN3739 strain) stocks were propagated in BHK-21 cells (25, 26). The rL212A variant was generated using reverse genetics as previously described (16). Briefly, BHK-21 cells were transfected with polymerase II (Pol II)-based expression plasmids containing T7 RNA polymerase and viral trans-acting factors. The T7 RNA polymerase expressed full-length S and L genome RNA species of the LCMV Armstrong strain. The viral trans-acting factors (L and NP) were required for virus RNA replication and gene transcription. After 60 h, supernatants from cultures were collected and used to infect new BHK-21 cells. Supernatant was harvested at 48 h postinfection. The rL212A variant harbors a leucine-to-alanine mutation at position 8 of the NP205 epitope, one of the primary major histocompatibility complex (MHC) class I binding residues. This point mutation abrogates the CD8 T cell response to NP205 while the rest of the immune response is similar to that of wild-type LCMV (16). Vaccinia virus (WR strain) was propagated on NCTC-L929 cells (27).
Six- to eight-week-old mice were infected intraperitoneally (i.p.) with 5 × 104 PFU of LCMV Armstrong, 2 × 107 PFU of PICV, and/or 1 × 105 PFU of VACV, doses which induce the optimum CD8 T cell response in single-virus infections. Immune mice were challenged with either 2 × 106 PFU of LCMV CL-13 intravenously (i.v.) or 2 × 107 PFU of PICV i.p. To control for cell culture contaminants, supernatant from PICV-infected BHK-21 cells was purified through a sucrose gradient and diluted in Hanks balanced salt solution (HBSS), and LCMV was diluted >40-fold in HBSS.
Virus titers.
LCMV and PICV titers were determined by plaque assay with serial dilutions of 10% tissue homogenate or serum on American Type Culture Collection Vero cells (15). To determine LCMV viral load in coinfected mice, a plaque assay was stained with iodonitrotetrazolium chloride (INT), which stains uninfected and PICV-infected cells, thereby allowing for specific identification and enumeration of LCMV plaques (28, 29).
ALT assay.
An alanine transferase (ALT) assay was purchased from D-Tek LLC (Bensalem, PA). Kit directions were followed in this assay except that only 15 μl of serum was mixed with 150 μl of substrate.
Synthetic peptides.
The following peptides were purchased from 21st Century Biochemicals (Marlboro, MA) at 90% purity: synthetic LCMV peptides NP396–404 (FQPQNGQFI; NP396), GP33–41 (KAVYNFATC; GP33), GP276–286 (SGVENPGGYCL; GP276), NP205–212 (YTVKYPNL), and GP118–125 (ISHNFCNL); PICV peptides NP38–45 (SALDFHKV; NP38), NP122–132 (VYEGNLTNTQL; NP122), and NP205–212 (YTVKFPNM); and VACV peptides B8R20–27 (TSYKFESV; B8R), A47L138–146 (AAFEFINSL; A47L), E7R130–137 (STLNFNNL; E7R), and A11R198–205 (AIVNYANL; A11R).
Intracellular cytokine staining (ICS).
Single-cell suspensions from peripheral blood or spleens were treated with 0.84% NH4Cl to lyse red blood cells, stimulated with 1 μM peptide, and incubated with Golgi Plug (BD Bioscience) and recombinant human interleukin-2 (IL-2) for 4.5 h at 37°C. Samples were treated with Fc receptor-blocking antibody and stained for CD8 and gamma interferon (IFN-γ) (clone XMG1.2; BD Bioscience). Samples were collected on an LSRII instrument (BD Bioscience) and analyzed with FlowJo software (Tree Star, Inc.). The total number of LCMV-, PICV-, or VACV-specific CD8 T cells was calculated by summing the total number of cells producing IFN-γ to multiple epitopes specific to each virus. For each virus, the response to immunodominant epitope(s) was included along with at least one subdominant epitope, depending on the virus. For LCMV the total response was the sum of the responses to NP396, GP33, GP276, and NP205. For PICV the total response was the sum of the responses to all of the known CD8 T cell epitopes: NP38, NP122, and NP205. For VACV the total response was the sum of the responses to B8R, A47L, E7R, and A11R.
Surface and tetramer staining.
Single-cell suspensions from peripheral blood or spleens were treated with Fc receptor-blocking antibody (clone 24.G2; BD Bioscience) and then stained with phycoerythrin (PE)-labeled tetramers (17). Surface stains including those for KLRG1 (clone 2F1; eBioscience), IL-7 receptor (IL-7R) (clone A7R34; eBioscience), CD62L (clone MEL-14; BD Bioscience), CD44 (clone IM7; BioLegend and eBioscience), and CD8 (clone 53-6.7; BD Bioscience) were then added.
Panniculitis scoring.
Levels 1 to 7 of panniculitis were scored visually based on the severity of necrosis as previously described: levels 1 to 2, mild disease with a few necrotic white spots on abdominal fat pads; levels 3 to 4, moderate disease with larger patches of necrosis that extends into the upper quadrant of the fat pad; levels 5 to 6, severe disease with extensive large patches of necrosis throughout; level 7, very severe disease such that the abdominal organs adhere to each other; and level 8, mice moribund with panniculitis and unlikely to survive (15, 21).
Fat pad histology scoring.
Abdominal fat pads were fixed in 10% neutral buffered formaldehyde and paraffin embedded. Tissue sections were stained with hematoxylin and eosin (H&E) and analyzed microscopically. Fat pad pathology was blindly graded by a pathologist based on the distribution and severity of disease from 0 to 6, as follows: 0, within normal limits; 1, mononuclear infiltrate; 2, mononuclear infiltrate with small areas of necrosis; 3, greater than 10 areas of necrosis that are less than 1/25 of the visual field; 4, areas of necrosis that are 1/10 of the visual field; 5, areas of necrosis that are 1/2 of the visual field; 6, areas of necrosis that are greater than 1/2 of the visual field.
Analysis of sequence similarity between viruses.
The sequence similarity between measles and mumps viruses and LCMV and PICV were determined using William Pearson's Lalign program (ch.EMBnet.org). Global alignments were done on L and S segments for LCMV (L segment, GenBank accession number NC_004291; S segment, GenBank accession number NC_004294) and PICV (L segment, GenBank accession number NC_006439; S segment, GenBank accession number JN378747). Whole-genome alignments were done for measles virus (GenBank accession number NC_001498) and mumps virus (GenBank accession number AB744048).
Statistics.
Descriptive statistics are expressed as means ± standard errors of the means. Statistical analysis was done using a two-tailed Student's t test in comparisons of results from two groups and using analysis of variance (ANOVA) with a Bonferroni posttest in comparisons of three or more. Linear regression was used to measure the correlation between two independent variables. Fischer's exact test was used to measure differences between categorical data sets. Statistical analysis was performed using Prism software (GraphPad Software, La Jolla, CA).
RESULTS
LCMV/PICV-coinfected mice showed reduced protective immunity and increased immunopathology compared to single-virus-immune mice after challenge.
We began these studies by first determining, based on a disease outcome of either protective immunity or immunopathology, if there is any evidence after secondary challenge consistent with our hypothesis that coinfection will alter CD8 memory T cell responses to either virus.
Naive mice were infected with the Armstrong strain of LCMV, with PICV, or with both simultaneously. LCMV and PICV both induce strong CD8 T cell responses that are required to clear the virus, with weak and no neutralizing antibody response, respectively (30, 31). To determine if coinfection alters the protective quality of immune memory, mice were challenged with either a high dose of the clone 13 (CL-13) strain of LCMV or the usual dose of PICV and compared with homologously challenged single-virus-infected mice (Fig. 1A). We used CL-13 because of its ability at a high i.v. dose to establish a persistent infection in naive mice that lasts 60 to 100 days and is characterized by viremia and detection of infectious virus in most organs (32). In naive mice the high dose of CL-13 used in this study induces transient T cell-mediated immunopathology during the first week of infection (33–35). We have previously shown that TCRβ knockout (KO) mice infected with this dose of CL-13 have no immunopathology as measured by weight loss, ALT levels, and lung and liver histology but have very high viral loads (33). Wild-type mice will eventually undergo complete clonal exhaustion of the T cell response, after which mice regain weight and at day 14 postinfection have minimal lung and liver pathology (33). Unfortunately, there was no such virulent high-dose model that could be used for PICV challenge. We could use only the same PICV dose that was used for the first infection.
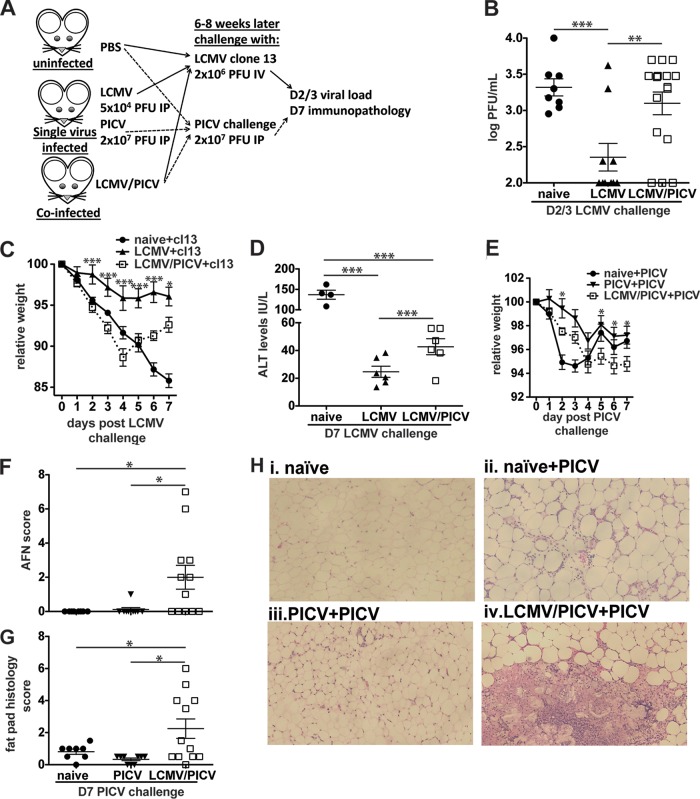
FIG 1.
LCMV/PICV-coinfected mice had significantly increased viral loads and immunopathology after challenge. (A) Naive mice were infected with LCMV (Armstrong strain at 5 × 104 PFU i.p.), PICV (2 × 107 PFU i.p.), or both simultaneously. After at least 6 weeks mice were considered immune and challenged with either CL-13 or PICV. Viral loads were determined on days 2 to 3 (D2/3) postchallenge, and immunopathology was assessed on day 7 (D7). (B to D) Naive, LCMV-infected, and LCMV/PICV-coinfected mice were challenged with 2 × 106 PFU of CL-13 i.v. (B) Viral loads were determined in serum at days 2 to 3 postchallenge. Data were pooled from three similar experiments. (C) Mice were weighed daily, and relative weight was calculated from day 0 (n = 3 to 8 mice/group). Data are representative of three similar experiments. Statistics compare LCMV-infected and LCMV/PICV-coinfected mice. (D) ALT levels were determined at day 7 post-CL-13 challenge in serum. Data were pooled from two similar experiments. (E to H) Naive, PICV-infected, and coinfected mice were challenged with 2 × 107 PFU of PICV i.p. (E) Mice were weighed daily, and relative weight was calculated from day 0 (n = 7 to 12 mice/group). Statistics show differences between PICV-infected and coinfected mice. Data were pooled from two similar experiments. The AFN score of abdominal/epididymal fat pad (F) and fat pad histopathology scores (G) were determined at day 7 post-PICV challenge. Data were pooled from two similar experiments. (H) Fat pad sections from naive, naive PICV-challenged (naive+PICV) mice, PICV-infected PICV-challenged (PICV+PICV) mice, and coinfected PICV-challenged (LCMV/PICV+PICV) mice were stained with H&E at day 7 of PICV-challenge. Naive mice showed normal fat pad structure, naive+PICV mice showed focal patches of mononuclear infiltrates, PICV+PICV mice showed mild mononuclear infiltrates around the periphery, and LCMV/PICV+PICV mice showed severe necrosis of up to 50% of the fat pad. *, P < 0.05; **, P < 0.01;***, P < 0.001.
As expected, LCMV-immune, but not naive, mice were protected against high-dose i.v. CL-13, as evidenced by the significant reduction in serum viral load at days 2 to 3 postchallenge (Fig. 1B). In contrast to LCMV-immune mice where 60% (6/10) of the mice had cleared virus, virus was cleared in only 23% (3/16) of the LCMV/PICV-coinfected mice (P < 0.05, by Fischer's exact test) (Fig. 1B). However, both LCMV-infected and coinfected mice were able to clear CL-13 from the serum by day 5 postchallenge (data not shown). Similarly, after PICV challenge 92% (11/12) of PICV-immune mice but only 20% (3/15) of LCMV/PICV-coinfected mice cleared PICV by day 3 postchallenge (P < 0.0004, by Fischer's exact test). By day 5 postchallenge virus could not be detected in either PICV-infected or coinfected mice (data not shown).
Weight loss was used as a surrogate to assess the degree of immunopathology in LCMV/PICV- or LCMV-immune mice following homologous challenge with CL-13 (Fig. 1C). Naive mice infected with a high dose of CL-13 will have some initial weight loss mediated by T cell responses that are unable to clear the virus but eventually undergo T cell clonal exhaustion and, therefore, do not develop long-lasting severe immunopathology (33–35). In this study, naive mice infected with CL-13 progressively lost up to 15% of their body weight by day 7, whereas LCMV-immune mice were largely protected from weight loss. Interestingly, after CL-13 challenge coinfected mice exhibited a weight loss similar to that observed in naive mice during the first 4 days of CL-13 challenge, consistent with the initial poor control of virus and enhanced T cell-mediated pathology, and then started to regain weight until day 7.
CL-13 infection has been associated with lung and liver pathology (33). To define immunopathology more specifically, liver damage was quantified by measuring alanine transaminase (ALT), an enzyme used to diagnose hepatocellular injury (36) (Fig. 1D). At day 7 post CL-13 challenge, naive mice had higher ALT levels than those of either of the immune groups. However, coinfected mice had higher ALT levels than LCMV-immune mice, consistent with a higher viral load driving T cell-mediated enhanced liver damage.
Coinfected mice also had enhanced immunopathology compared to PICV-infected mice following challenge with PICV (Fig. 1E). PICV-immune mice had the least relative weight loss following PICV challenge. Naive mice infected with PICV had a substantial drop in relative weight by day 2 postchallenge but by day 7 their weights were similar to those of PICV-immune mice (Fig. 1E). Coinfected mice had protracted weight loss, losing weight gradually until day 4, but unlike the naive group, coinfected mice did not regain weight by day 7 postchallenge.
In sequential heterologous challenge models, such as LCMV-immune mice challenged with VACV (LCMV+VACV) and mice sequentially infected with PICV then LCMV and rechallenged with PICV (PICV+LCMV+PICV), where cross-reactive T cell responses have been identified, mice can develop severe panniculitis (15, 16, 21), a necrosis of the abdominal fat known as acute fatty necrosis (AFN) during the last virus infection. The most common form of human panniculitis is erythema nodosum, which affects subcutaneous fat and can occur following certain acute viral infections or vaccinations (37, 38). As in these sequential series of unrelated infections that result in panniculitis, we found that coinfection followed by homologous challenge with PICV also caused fat pad immunopathology, something almost never seen in single-virus challenge (15, 16, 21–23) (Fig. 1F to H). In fact, naive mice did not develop AFN, and only 1 out of 9 PICV-immune mice had very mild AFN (AFN score of 1) (Fig. 1F). Coinfected mice had significantly greater panniculitis (mean AFN score of 2.0 ± 0.69 versus a PICV-immune mouse average AFN score of 0.11 ± 0.11), and individuals exhibited greater variability in AFN severity, ranging from AFN scores of 0 to 7, similar to previous findings of private specificity previously observed in sequential infections (16, 21). These data show that LCMV/PICV-coinfected mice had significantly enhanced immunopathology and slower viral clearance than single-virus-immune mice during either LCMV or PICV homologous challenges. These data are consistent with the coinfected mice having a less efficient memory T cell response that allows for prolonged viral replication in peripheral organs, leading to the recruitment of a greater number of effector cells and enhanced T cell-mediated immunopathology. These studies are particularly important in their implication that there are most likely changes in the primary immune responses to either virus following coinfection and that these changes will have significant clinical impact when an individual is reexposed to that virus subsequently.
Having observed differences in the immune protection and immunopathology after homologous challenge of coinfected mice, we designed our experiments to determine the mechanism behind these changes in disease outcome. Our primary hypothesis is that the competition for resources during coinfection alters CD8 memory responses to each virus, rendering it less efficient at clearing a homologous challenge. Specifically, we questioned if there was evidence of altered CD8 TCR repertoire selection, such as changes in immunodominance hierarchies, enhanced private specificity, such as increased variability between individual mice, and changes in the overall size of the immune response to each virus.
Coinfected mice had decreased numbers of LCMV-specific CD8 T cells after primary infection.
Previous studies suggest that the number of virus-specific T cells is positively correlated with protection against lethal viral infection (39–41). In LCMV/PICV coinfection we found decreased numbers of LCMV-specific CD8 T cells at both the peak of infection (day 8) and in memory (day 30) of primary infection (Fig. 2A and B). In contrast, there was no difference in the generally much smaller size of the PICV-specific responses between PICV-infected and coinfected mice at day 8 postinfection, but during the memory phase PICV-immune mice had more PICV-specific cells than coinfected mice (Fig. 2B).
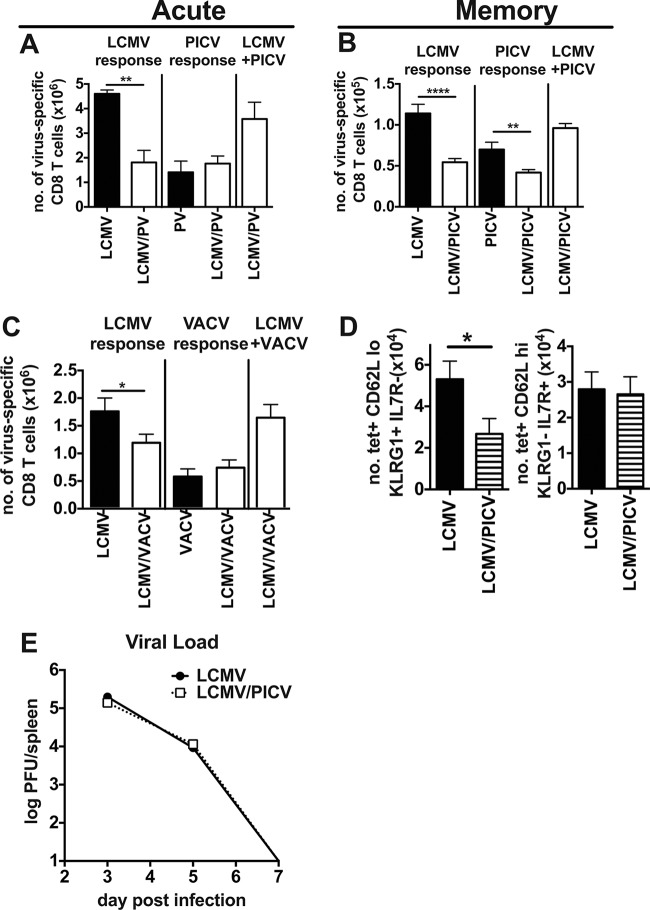
FIG 2.
Coinfected mice had smaller LCMV-specific CD8 T cell responses after primary infection. On day 8 (A) and 8 weeks postinfection (B) total numbers of virus-specific CD8 T cells were determined based on IFN-γ production in an ICS assay to multiple virus-specific epitopes in LCMV-, PICV-, and LCMV/PICV-infected mice. The total LCMV or PICV response represents summation of well-described LCMV or PICV epitope-specific responses for each virus. LCMV+PICV responses represent the summation of responses for the two viruses in coinfected mice. For the experiment shown in panel A, n = 3 to 4 mice/group; data are representative of three similar experiments. For the experiment shown in panel B, n = 10 mice/group; data were pooled from two similar experiments. (C) Day 7 postinfection total numbers of virus-specific CD8 T cells were determined for LCMV-, VACV-, and LCMV/VACV-infected mice (n = 5 to 9 mice/group). Data are from two similar experiments pooled. (D) The total number of LCMV-specific CD8 T cells was calculated for CD62Llo KLRG1+ IL7R− cells and CD62Lhi KLRG1− IL7R+ cells at 8 weeks postinfection in LCMV/PICV-coinfected mice (n = 10 mice/group). Data are pooled from two similar experiments. tet, tetramer. (E) Splenic viral load was determined by INT-stained plaque assay in LCMV-infected and LCMV/PICV-coinfected mice after primary infection (n = 4 to 5 mice/group/time point). Data are representative of two similar experiments. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
In a second coinfection model where mice were simultaneously infected with two completely unrelated viruses, LCMV and VACV, there was also a 33% reduction in the LCMV-specific response compared with that in LCMV-infected mice (Fig. 2C). These data show that during coinfections with different combinations of viruses, there can be a dramatic reduction in the total number of cells specific to one of the viruses.
In the LCMV/PICV coinfection model we also examined the phenotype of the memory CD8 T cells, specifically analyzing effector (expressing low levels of CD62L [CD62Llo] and killer cell lectin-like receptor subfamily G member 1 [KLRG1+] and negative for IL-7 receptor [IL-7R−]) and central (expressing high levels of CD62L [CD62Lhi] and KLRG1− IL-7R+) memory-like cell populations. LCMV-specific CD62Lhi CD8 T cells, which are associated with central memory, have previously been shown to mediate protection in LCMV-immune mice during high-dose CL-13 challenge (42). We questioned if the reduced LCMV-specific memory pool found in LCMV/PICV-coinfected mice (Fig. 2B) would contain fewer central memory-like cells. Interestingly, we found that there was no difference in the number of LCMV-specific central memory-like cells (CD62Lhi KLRG1− IL-7R+) between LCMV-infected and LCMV/PICV-coinfected mice at 8 weeks postinfection (Fig. 2D). However, there was a significant reduction in the number of effector memory-like cells (CD62Llo KLRG1+ IL7R−), suggesting that this population may play an important role in protection when there are an equal number of central memory cells, as has been previously observed in a parabiosis model of VACV infection (43).
Antigen load can alter the differentiation and proliferation of T cells (44–48). With such a dramatic difference in numbers of LCMV-specific cells between LCMV-infected and LCMV/PICV-coinfected mice, we examined whether LCMV antigen load or the kinetics of viral clearance during the primary infection were altered between these two groups. However, there was no difference in LCMV viral load in the spleen between LCMV-infected and coinfected mice on day 3, 5, or 7 after primary infection (Fig. 2E).
Coinfected mice had variability in which virus-specific responses dominated.
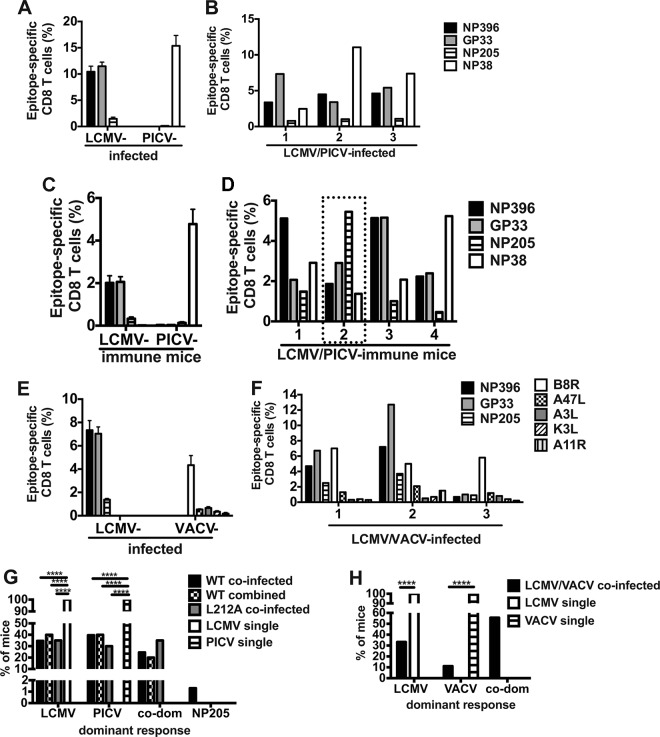
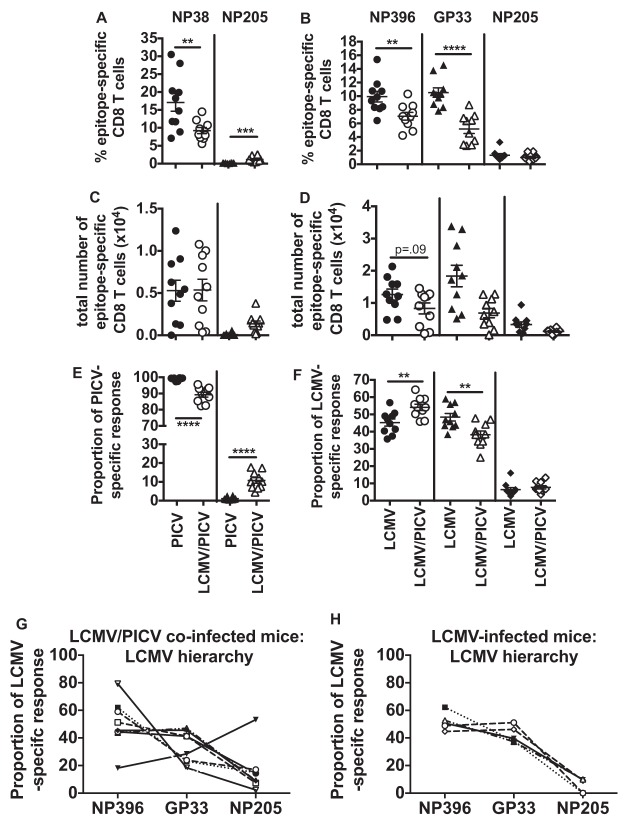
We questioned if the decrease in the size of the LCMV-specific CD8 T cell response after coinfection would be explained by changes in the epitope-specific responses to either virus. We analyzed the response to several epitopes from each of the viruses and found that in coinfected mice there was variability in which of the virus-specific responses dominated, also known as intervirus variability of the immune response (Fig. 3). After a single-virus infection the dominant response was always to the one infecting virus as there was no other virus-specific response competing with it (Fig. 3A, C, E, G, and H). LCMV/PICV-coinfected mice, however, could be subcategorized by which virus-specific response dominated, as follows: (i)the LCMV-specific response was dominant (Fig. 3B, mouse 1, D, mouse 1 and 3, and G), (ii) the PICV-specific response was dominant (Fig. 3B, mouse 2,D, mouse 4, and G), (iii) the LCMV and PICV responses were codominant (Fig. 3B, mouse 3, and G), or (iv) the cross-reactive, subdominant NP205-specific response was dominant (Fig. 3D, mouse 2, dotted box, and G). These virus-specific response-dominant groups were also observed at memory time points (Fig. 3D) and if the viruses were combined within the same needle prior to injection (Fig. 3G). The variability in which virus-specific response dominates was also reproducible in a second coinfection model using LCMV/VACV (Fig. 3F and H). In mice infected with a single virus, either LCMV or PICV, the normally subdominant, cross-reactive NP205-specific response was absolutely never dominant in thousands of mice infected over the last 20 years (14, 16, 17). To our surprise, after coinfection in a small proportion (1.8%, or 3 out of 159) of these mice this response actually dominated (Fig. 3D, mouse 2, dotted box, and G).
FIG 3.
Coinfected mice have variability in which virus-specific or cross-reactive responses dominated the CD8 T cell response. CD8 T cell immunodominance hierarchies were determined on either day 7 to 8 (A, B, E, and F) or day 42 (B and C) postinfection on lymphocytes isolated from spleen or peripheral blood by ICS assay for IFN-γ production. (A) LCMV- or PICV-infected mice (n = 25 mice/group from five similar experiments). (B) Three individual LCMV/PICV-coinfected mice representative of immunodominance patterns observed in four similar experiments. (C) Memory frequencies of epitope-specific CD8 T cells in LCMV- or PICV-immune mice (n = 25 mice/group from five similar experiments). (D) Four individual LCMV/PICV-coinfected mice representative of immunodominance patterns observed 8 weeks postinfection in 15 similar experiments. (E) LCMV- or VACV-infected mice (n = 5 mice/group from two similar experiments). (F) Three individual LCMV/VACV-coinfected mice representative of immunodominance patterns observed in two similar experiments. (G) The frequency of mice that had a dominant LCMV-specific, PICV-specific, codominant LCMV- and PICV-specific (co-dom), or cross-reactive NP205-specific CD8 T cell response. Wild-type (WT)-coinfected mice, n = 164 from 15 similar experiments; wild-type combined infection (wild-type viruses were combined in the same injection) n = 15 from 2 similar experiments; L212A-coinfected mice, n = 20 from 4 similar experiments; LCMV single and PICV single infections, n = 20 (each) from 4 similar experiments. (H) The frequency of mice that had a dominant LCMV-specific, VACV-specific, or codominant (co-dom) LCMV- and VACV-specific CD8 T cell response. LCMV/VACV-coinfected mice, n = 5 to 9 from two similar experiments; LCMV single and VACV single infections, n = 20 (each) from 4 similar experiments.
When the total number of LCMV-specific CD8 T cells was determined for these subgroups of coinfected mice, the LCMV response-dominant mice had a significantly greater number of LCMV-specific CD8 T cells than mice that had a codominant or PICV-dominant response (Fig. 4A). Similarly, there were an increased number of LCMV-specific CD8 T cells in LCMV response-dominant LCMV/VACV-coinfected mice (Fig. 4B) and a higher number of PICV-specific cells in PICV response-dominant LCMV/PICV-coinfected mice (Fig. 4C). Collectively, these data show that there is a high degree of variability in which a virus-specific response dominates during a simultaneous coinfection, and this correlates with the decreased virus-specific CD8 T cell response.
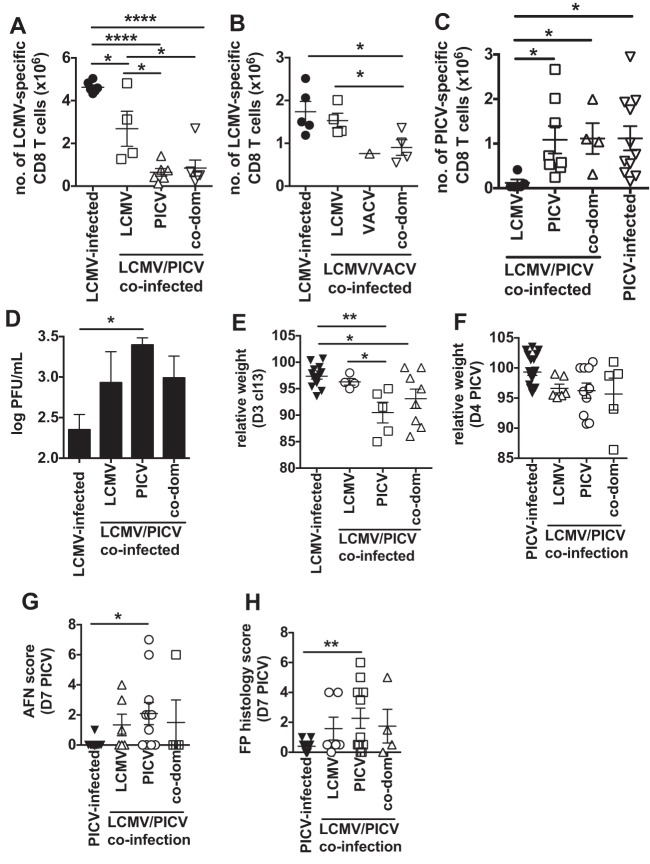
FIG 4.
In coinfected mice the dominating virus-specific response correlates with the total number of virus-specific CD8 T cells and immunopathology after CL-13 challenge. (A) The total number of LCMV-specific CD8 T cells for LCMV/PICV-coinfected mice that were categorized by the dominance of their CD8 T cell response into LCMV-, PICV-, or codominant (co-dom) and compared with responses of LCMV-infected mice (n = 4 to 7 mice/group from 2 similar experiments). (B) The total number of LCMV-specific CD8 T cells for LCMV/VACV-coinfected mice that were categorized by the dominance of their CD8 T cell response into LCMV-, VACV-, or codominant compared with responses of LCMV-infected mice (n = 1 to 5 mice/group from 2 similar experiments). (C) The total number of PICV-specific CD8 T cells for LCMV/PICV-coinfected mice that were categorized by their dominating CD8 T cell response into LCMV-, PICV-, or codominant compared with responses of PICV-infected mice (n = 4 to 10 mice/group from 3 similar experiments). (D and E) Serum viral load on day 2 to 3 post-CL-13 challenge (D) and weight loss on day 3 postchallenge (E) of LCMV/PICV-coinfected mice that were LCMV-, PICV-, or codominant compared with responses of LCMV-infected mice during CL-13 challenge (n = 4 to 12 mice/group from 3 similar experiments). (F to H) Immunopathology of LCMV/PICV-coinfected mice that were LCMV-, PICV-, or codominant compared with responses of PICV-infected mice during PICV challenge. (F) Weight loss. (G) AFN score. (H) Fat pad (FP) histology score (n = 5 to 18 mice/group from 4 similar experiments). *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
Since the number of virus-specific cells has previously been shown to correlate with protection against lethal viral infection (39–41), we divided the coinfected mice based upon their dominant virus-specific responses. We found that the coinfected mice with the lowest number of LCMV-specific memory cells (i.e., those that were PICV-specific response dominant) had significantly higher viral load and greater weight loss than LCMV-immune mice (Fig. 4D and E). Furthermore, the LCMV response-dominant coinfected mice showed weight loss and viral load similar to those of LCMV-only immune mice (Fig. 4D and E). These data suggest that the reduction in the number of LCMV-specific memory cells in some coinfected mice led to slower clearance of virus, increasing the time to amplify memory T cell-mediated immunopathology upon CL-13 challenge. Furthermore, the interviral variability that occurs between individual coinfected mice results in a lack of predictability of the disease outcome upon reexposure to the pathogen.
In the less virulent PICV challenge, there was no association between relative weight loss during PICV challenge and interviral response dominance in coinfected mice (Fig. 4F). However, interestingly, there was an increase in fat pad pathology in coinfected mice that had a PICV-dominant response (Fig. 4G and H). These data suggest that the presence of a greater number of PICV-specific CD8 T cells was contributing to enhanced fat pad immunopathology in some coinfected mice after PICV challenge. Thus, coinfection may cause some other qualitative changes in the immune response to PICV that leads to increased immunopathology upon PICV challenge.
Coinfected mice had overall altered, but predictable, intravirus-specific immunodominance hierarchies.
We therefore next questioned whether there were significant changes within the immunodominance hierarchies to each virus resulting from the enhanced competition and stochasticity during coinfection that might account for the increased immunopathology upon PICV challenge. Previously, immunopathology has been correlated with cross-reactive epitope-specific responses in models of heterologous immunity where mice are sequentially infected with pathogens (16, 49). Furthermore, by ablation of these responses using peptide tolerization techniques or mutant viruses, which lack these epitopes, immunopathology can be reduced. Therefore, we analyzed the data more closely and compared the immunodominance hierarchies of the epitopes from each virus (Fig. 5 and 6). The different epitope-specific T cell populations can be arranged into a hierarchy based on the size of the response, also known as an immunodominance hierarchy. In LCMV/PICV-coinfected mice a larger fraction of the PICV-specific response was directed at the cross-reactive subdominant NP205 epitope instead of at the normally very dominant NP38 than in PICV-immune mice (Fig. 5A, C, and E). There was a tremendous variation in the size of the NP205-specific response between individual mice. Both the frequency and proportional NP205-specific response in coinfected mice had a greater mean (7.44%) and range (4.42 to 17.68%) than the NP205-specific response in PICV-infected mice (mean, 0.76%; range, 0.37 to 2.68%). In order to normalize the data we used proportional epitope-specific responses to collapse the effect of private specificity in the size of the response in individual mice. Thus, we could assess whether the ratio of the NP205 to NP38 response played a role in mediating immunopathology.
FIG 5.
LCMV/PICV-coinfected mice had increased private specificity with new predictable intravirus-specific immunodominance hierarchies. Peripheral blood lymphocytes were isolated from LCMV-infected, PICV-infected, and LCMV/PICV-coinfected mice at least 6 weeks post-primary infection, and immunodominance was determined by IFN-γ production. (A and B) Epitope-specific CD8 T cell responses for PICV (A) and LCMV (B) directly comparing responses in single-virus-infected mice with those in coinfected mice. (C and D) The total number of virus-specific CD8 T cells was determined for PICV (C) and LCMV (D). (E and F) The epitope-specific proportional responses were determined for PICV and LCMV (n = 9 to 10 mice/group from two similar experiments pooled). (G and H) The LCMV-specific proportional response for a representative experiment using LCMV/PICV-coinfected and LCMV-infected mice illustrates the intraviral variability. **, P < 0.01; ****, P < 0.0001.
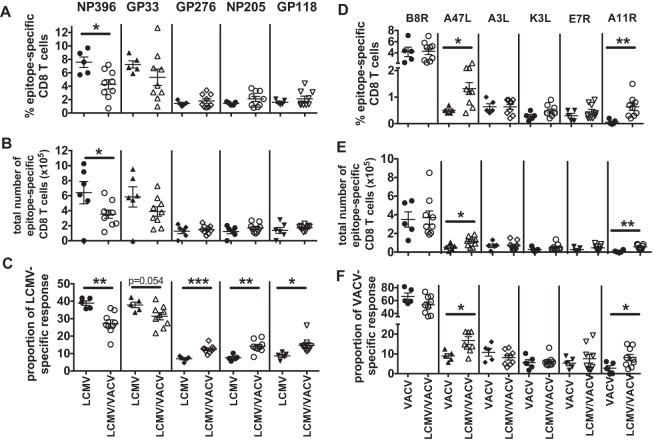
FIG 6.
LCMV/VACV-coinfected mice have altered immunodominance hierarchies and overall increased frequencies of cross-reactive CD8 T cells. Splenocytes were isolated from LCMV-infected, VACV-infected, and LCMV/VACV-coinfected mice, and immunodominance hierarchies were determined by IFN-γ production on day 7 postinfection. (A and D) Epitope-specific CD8 T cell responses for LCMV (A) and VACV (D) directly comparing single-virus-infected mice with coinfected mice. (B and E) Total numbers of epitope-specific CD8 T cell responses for LCMV (B) and VACV (E). (C and F) The proportional response for each epitope was determined for LCMV (C) and VACV (F). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (n = 5 to 9 mice/group from two similar experiments pooled).
LCMV/PICV-coinfected mice presented with a new LCMV-specific immunodominance hierarchy (Fig. 5 B, D, and F). Coinfected mice had a higher proportion of NP396-specific T cells and a lower proportion of GP33/34-specific T cells than LCMV-immune mice. In contrast to the PICV-specific response, no difference was found in the NP205-specific responses between coinfected and LCMV-immune mice. This is consistent with the very small size (less than 1% of the CD8 T cell response) of the PICV-NP205 response having minimal additive effect on the size of the NP205 response in the LCMV-specific immunodominance hierarchy of coinfected mice. But, within the PICV-specific immunodominance hierarchy, the addition of the larger LCMV-NP205 response (up to 4% of the CD8 T cell response) could be responsible for the significant increase in the NP205 response in coinfected mice. It is important to note that whereas NP396- and GP33/34-specific responses are codominant in LCMV-immune mice, the proportion of NP396-specific cells is significantly (P < 0.003) greater than the proportion of GP33/34-specific cells among coinfected mice (Fig. 5F).
These data show that there are new patterns of epitope dominance for each infected virus after coinfection; however, during analysis we also noticed individual intravirus-specific variability. The LCMV-specific immunodominance hierarchies of individual LCMV/PICV-coinfected mice are highly variable (Fig. 5 G and H). In mice infected with LCMV only, there is only one pattern of immunodominance (NP396 = GP33 > NP205). However, in coinfected mice there are three distinct patterns: (i) NP396 = GP33 > NP205, (ii) (NP396 > GP33 ≥ NP205, and (iii)NP205 > GP33 > NP396. These data show that even though after coinfection there was an overall new pattern of immunodominance, there was significant individual intravirus-specific variability that may play a role in the private specificity of the immunopathology found after challenge.
Skewing of the immunodominance hierarchies was also observed in the LCMV/VACV-coinfected mice. Within the LCMV-specific immunodominance hierarchy, there was a reduction in the NP396-specific T cells in LCMV/VACV-coinfected mice (Fig. 6A to C). Within the VACV-specific immunodominance hierarchy, there is a significant increase in the known cross-reactive A11R response and also in the A47L-specific response, an epitope that has not been examined for cross-reactivity between LCMV and VACV (Fig. 6D to F). Collectively, these data show that coinfection leads to differences within virus-specific immunodominance hierarchies to both viruses compared to those in single-virus-infected mice.
Increased NP205-specific memory T cells mediated enhanced immunopathology in LCMV/PICV-coinfected mice during PICV challenge.
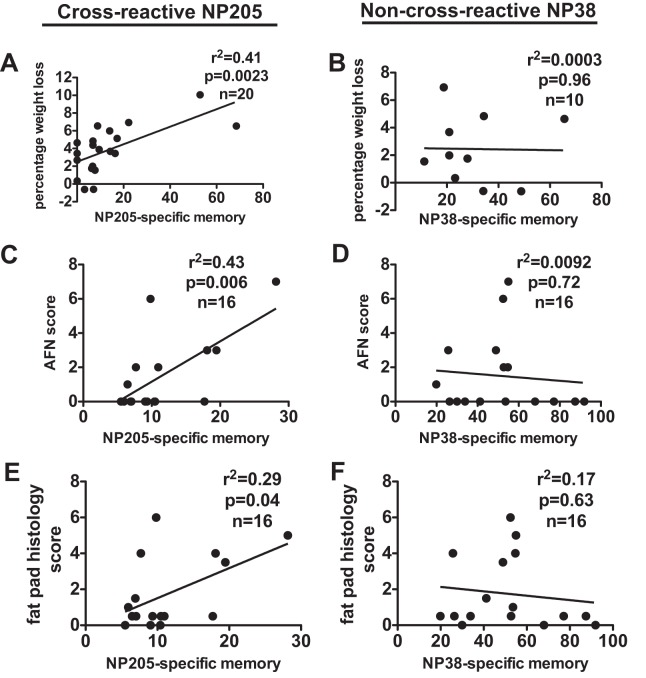
In previous studies using sequential infection with LCMV and PICV, enhanced fat pad pathology was mediated by the reactivation of the cross-reactive NP205-specific memory cells (16). NP205 was the dominant response in some LCMV/PICV-coinfected mice (Fig. 3D, dotted box, and G and 5G), which is never seen in mice infected with LCMV or PICV alone. Furthermore, within the PICV-specific memory response in LCMV/PICV-coinfected mice, there was an increase in both the frequency and proportion of NP205-specific cells compared to that in mice infected with only PICV (Fig. 5A and E). Therefore, we questioned if the cross-reactive NP205-specific memory population in LCMV/PICV-coinfected mice was playing a role in the enhanced immunopathology during PICV challenge. Weight loss (Fig. 7A) and severity of panniculitis (Fig. 7C and E) after PICV challenge directly correlated with the proportion of memory NP205-specific CD8 T cells in the peripheral blood of LCMV/PICV-coinfected mice prior to challenge with PICV. By comparison, there was no correlation between the proportion of non-cross-reactive NP38-specific memory cells and any measure of PICV-induced immunopathology (Fig. 7B, D, and F). Furthermore, there was no correlation of the cross-reactive NP205-specific population with immunopathology in coinfected mice after CL-13 challenge (data not shown).
FIG 7.
NP205-specific memory proportions prior to PICV challenge directly correlated with severity of immunopathology in LCMV/PICV-coinfected mice after PICV challenge. Linear regression analyses comparing the proportion of memory cells specific to the cross-reactive NP205 or the non-cross-reactive NP38 epitope to the severity of disease as indicated by percentage of weight loss (A and B), AFN score (C and D), or fat pad histopathology score (E and F) in LCMV/PICV-coinfected mice.
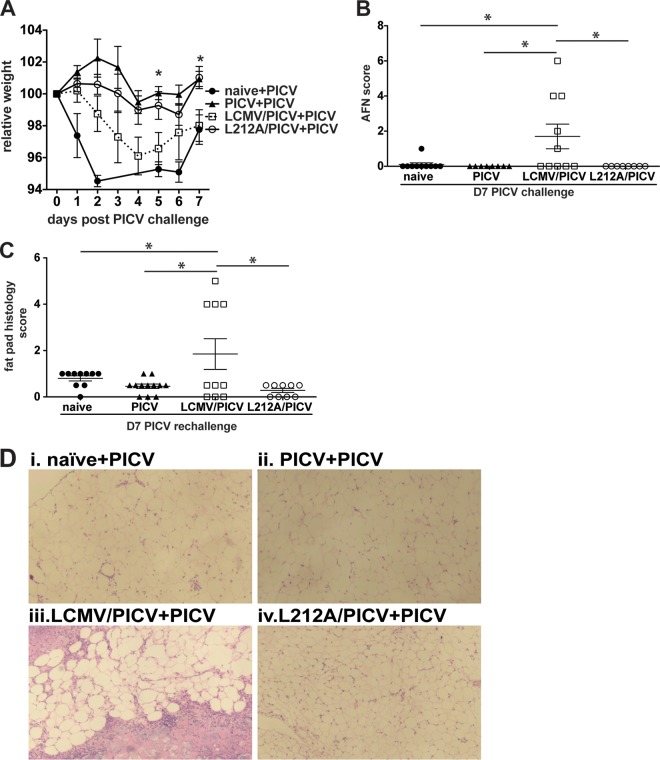
To directly test whether the NP205-specific response was involved in induction of immunopathology, we utilized a recombinant LCMV (rL212A) that does not induce an LCMV-NP205-specific response due to a leucine-to-alanine mutation at position 8, one of the MHC class I binding residues, of this epitope, thus preventing presentation (16). These mice will develop only a PICV-NP205-specific response but not an LCMV-NP205-specific response, thus negating the potential cross-reactive response during PICV challenge. Coinfection with the rL212A variant and PICV (rL212A/PICV) resulted in a variation in the interviral immunodominance hierarchy similar to that observed with LCMV/PICV coinfection, suggesting that the presence of a cross-reactive response did not control the variability in the interviral immunodominance hierarchy between individuals (Fig. 3G). After PICV challenge, mice coinfected with rL212A/PICV experienced significantly less weight loss than the mice coinfected with LCMV/PICV (Fig. 8A). Of note, weight loss in rL212A/PICV-coinfected mice subsequently challenged with PICV mirrored that of PICV-immune mice challenged with PICV. rL212A/PICV coinfection prevented panniculitis and widespread necrosis of fat pads associated with PICV challenge of LCMV/PICV-coinfected mice (Fig. 8B to D). These results indicate that the enhanced responses to the cross-reactive NP205-specific response mediated the increased immunopathology in LCMV/PICV-coinfected mice upon PICV challenge.
FIG 8.
NP205 memory response mediates immunopathology in LCMV/PICV-coinfected mice during PICV challenge. Naive, PICV-, wild-type LCMV/PICV-, and L212A/PICV-immune mice were challenged with 2 × 107 PFU PICV i.p. (A) Mice were weighed daily, and relative weight was calculated from day 0 (n = 7 to 10 mice/group). Data are from two similar experiments pooled. Statistics show differences between wild-type- and L212A-coinfected mice. AFN score of fat pads (B) and fat pad histopathology scores (C) were determined at day 7 post-PICV challenge. Data are from two similar experiments pooled. (D) Fat pad sections were stained with H&E. Results after PICV challenge were as follows: naive mice (naive+PICV) showed focal patches of mononuclear infiltrate, PICV-immune mice challenged with PICV (PICV+PICV) showed very mild mononuclear infiltrate, wild-type LCMV/PICV-immune mice challenged with PICV (LCMV/PICV+PICV) showed severe necrosis, and L212A/PICV-immune mice challenged with PICV (L212A/PICV+PICV) displayed mild mononuclear infiltration. *, P < 0.05.
DISCUSSION
This study demonstrates that the inherent stochasticity and increased competition present during a simultaneous coinfection with two viruses led to altered immune responses to the two individual viruses compared to the responses when the viruses were administered alone. These changes resulted in reduced immune protection and enhanced immunopathology upon homologous challenge with either of the immunizing viruses. We identified enhanced variability in the interviral response, in which virus-specific responses dominated, as well as predictable alterations in the intravirus-specific immunodominance hierarchies reproducible in two different coinfection models, LCMV/PICV and LCMV/VACV. Consistent with previous observations that the number of memory T cells is important in protection from lethal infection (39–41), LCMV/PICV-coinfected mice with a dominant PICV-specific response had fewer numbers of LCMV-specific cells, which was associated with higher viral loads and more severe immunopathology after CL-13 challenge. Furthermore, coinfected mice had an overall enhancement of the cross-reactive NP205-specific response that directly correlated with the severity of disease during PICV challenge and was ablated by using an LCMV mutant that does not induce an NP205-specific response. These mice developed only a PICV-NP205-specific response negating the cross-reactive response. In both PICV and CL-13 challenge models, these changes in CD8 T cell responses led to a less effective memory T cell response in LCMV/PICV-coinfected mice. This may have led to the more protracted viral clearance with enhanced and prolonged accumulation of secondary effector T cells in the tissues increasing the risk of enhanced immune pathology, as has been previously demonstrated in our infection models of heterologous immunity and extensively summarized in our recent review (50). Thus, the magnitude and character of CD8 T cell responses generated in response to simultaneous coinfections differed substantially from those induced by infection with a single virus.
During coinfection there are two different immune responses being generated within the same host, leading to a more competitive environment with enhanced stochasticity for naive T cells to become activated. During coinfection, where viral peptides from two viruses are presented, there is a reduced number of antigen-presenting cells (APCs) presenting antigens from each virus. Decreased numbers of APCs would drive increased competition between T cells specific to different antigens for access to APC binding sites, but there would also be increased competition between T cells specific to the same antigen, with higher-affinity clones out-competing lower affinity clones (51). This high level of competition may further impact the inherent stochasticity of T cell activation and play a role in the high level of variability in the T cell responses between individual coinfected mice.
Since genetically identical mice have unique TCR repertoires, under the highly competitive conditions of a simultaneous coinfection, the unique variability in the TCR repertoire of individual mice led to enhanced private specificity in both intra- and interviral immunodominance hierarchies. The variability in immunodominance hierarchy resulted in a significant amount of private specificity in terms of which of the infecting virus-specific CD8 T cell responses dominated. Coinfected mice could be subcategorized by the dominating response. PICV response-dominant LCMV/PICV-coinfected mice had fewer LCMV-specific CD8 T cells, and during a CL-13 challenge these mice also had significantly higher viral loads and greater weight loss than their LCMV-dominant counterparts and LCMV-only-infected mice. A previous study using three different doses of CL-13 found that the disease outcome or level of immunopathology was determined by the balance of the efficiency of the T cell response and viral load (33). For example, at a low dose the virus is quickly cleared from the host by the T cell response without the development of lung or liver immunopathology, but if the virus is given at a medium dose, the T cell response becomes partially clonally exhausted and cannot efficiently control the quickly replicating virus, leading to T cell-mediated death in up to 60% of mice. At a high dose of CL-13, the T cell response is completely clonally exhausted before it becomes lethal. Since LCMV CL-13 is a fast-replicating virus, the number of LCMV-specific CD8 T cells has a large impact in the disease outcome of individual LCMV/PICV-coinfected mice. The memory cells, specifically the effector memory cells, may need to be present at high levels to quickly respond to infection and protect the mouse from collateral damage and immunopathology that can develop if virus is not cleared quickly (33). PICV, in comparison, is a slow-replicating, innocuous challenge virus. In this study, we found that the number of PICV-specific CD8 T cells in LCMV/PICV-coinfected mice had no impact on weight loss after PICV challenge. This may be because the slower replication rate of PICV does not put as much pressure on the memory cells to clear the infection quickly. Even though in the memory phase LCMV/PICV-coinfected mice had fewer PICV-specific CD8 T cells, the slow replication of the virus may have allowed memory cells to expand and have little to no impact on disease outcome.
However, the increased disease severity of LCMV/PICV-coinfected mice during PICV challenge was found to be mediated by the cross-reactive NP205-specific memory response. Cross-reactive responses have also been shown to mediate the severity of immunopathology in both humans (52, 53) and mice during heterologous viral infections (49). In the current study, we found a direct correlation between the severity of pathology and the size of the NP205-specific memory response prior to challenge. Mice coinfected with a mutant LCMV virus (rL212A), which lacks the cross-reactive NP205 epitope, did not develop panniculitis when challenged with PICV. In previous studies, NP205 cross-reactivity was found to be beneficial in LCMV-immune mice challenged with PICV and in PICV-immune mice challenged with LCMV (14, 15, 17). However, this cross-reactive response can also lead to detrimental effects. Some mice that are infected sequentially, first with PICV and then 6 weeks later with LCMV, and subsequently rechallenged with PICV at 12 weeks post-primary PICV infection (PICV+LCMV+PICV) can develop severe panniculitis dependent on the private specificity of the cross-reactive response (16). In a previous study, when PICV-immune mice were sequentially challenged with LCMV (PICV+LCMV), a narrow cross-reactive portion of the memory NP205-specific pool was selected to expand by the LCMV-NP205 epitope. When these mice were reexposed to PICV, the LCMV-centric NP205-specific response was no longer beneficial and could contribute to disease (PICV+LCMV+PICV) (16). During an LCMV/PICV coinfection, where mice were challenged with the two viruses simultaneously, some mice developed an enhanced NP205-specific response that was detrimental during a PICV challenge, causing panniculitis similar to that observed in some mice infected with PICV plus LCMV plus PICV. The mechanism for the development of panniculitis in the sequential (PICV+LCMV+PICV) model is the repeated expansion of the LCMV-centric NP205-specific cross-reactive TCR repertoire that increases the probability for a detrimental clone to dominate the secondary response to PICV. In the current study of simultaneous LCMV/PICV coinfection, there are no prior sequential infections with LCMV and PICV that increased or altered the NP205-specific memory response, further highlighting the high degree of variability that can occur between individual coinfected mice during a primary infection.
It may be that the enhanced, even immunodominant, cross-reactive response during a coinfection results from enhanced exposure to a similar antigen present in both viruses, doubling the opportunity for exposure. During simultaneous coinfection with LCMV and PICV, NP205 epitopes from both viruses were most likely presented. NP205-specific cells are very subdominant after PICV infection, usually making up less than 1% of the acute CD8 T cell response. Following LCMV infection, however, although the NP205-specific response is also subdominant, it can be up to 5% of the acute response. This may explain why there is no difference in the NP205-specific response between LCMV/PICV-coinfected and LCMV-infected mice. The addition of the PICV-NP205-specific cells is trivial and does not alter the proportion of NP205-specific cells in coinfected mice. However, the addition of the larger population of LCMV-NP205-specific cells within the PICV-specific immunodominance hierarchies of coinfected mice may have resulted in a significantly higher proportion of cross-reactive CD8 T cells that became reactivated during the subsequent PICV challenge.
The inherent ability of the TCR to recognize multiple antigens would suggest that T cells that are capable of recognizing antigens from two different, unrelated pathogens are relatively common. However, the implications of cross-reactive T cells influencing disease outcome during infection, autoimmunity, and cancer in human patients are further complicated by genetic diversity, including HLA type, and sequence/history of infection for each individual. Do these issues make the influence of cross-reactivity and heterologous immunity too complicated to address? Recent scientific advances would suggest that this is not the case as even with these complexities several studies have already shown that, in both humans and mice, disease can be associated with cross-reactive responses (14–19, 21–23, 27, 49, 52–68). In mice and in some human infections, where CD8 T cell epitopes are well defined for several viruses, clear networks of cross-reactivity have been determined (14–19, 21–23, 27, 49, 64–68). These types of networks are the basis for understanding the implications of cross-reactivity in human disease and vaccines.
In this study, as a proof-of-principal study using viruses that have been extensively studied in both single and sequential infection models, we have uncovered several alterations in immune responses which would not be predicted from previous studies with individual virus infections. The results presented in this study underscore that we need to have a better understanding of human T cell responses during coinfection by two or more viruses which could lead to optimizing strategies for currently used vaccines. The measles, mumps, and rubella (MMR) vaccine and the yearly influenza vaccine (FluMist and Fluenza) (69) are both important vaccines that save numerous lives and drastically reduce hospitalization. Protection from these viruses correlates with antibody responses (70), but since these are live attenuated viruses, these vaccines will also induce T cell responses. It is unknown if the combined administration of these vaccines alters epitope-specific T cell responses compared to responses to vaccines that contain only one virus. Measles and mumps viruses are both members of the Paramyxoviridae family and have 48% sequence similarity, suggesting a high likelihood that there are cross-reactive epitopes shared between these two viruses. Coinfection with LCMV and PICV, with 54% sequence similarity, resulted in enhanced disease severity in some mice upon challenge with PICV, which was mediated by a cross-reactive CD8 T cell response. This study shows that simultaneous coinfection can cause unpredictable and highly variable alterations in T cell responses that are not identified in single-virus studies and that these alterations can result in loss of immune protection and enhanced disease upon reexposure to that pathogen.
ACKNOWLEDGMENTS
We thank Keith Daniels for tetramers, Stephen Waggoner, Megan Enos, and Keith Daniels for i.v. injections, and Ray Welsh, Stephen Waggoner, Levi Watkin, Tara Strutt, and Kai McKinstry for critical reviews of the manuscript.
This study was funded by NIH grants AI-054455 (L.K.S.), AI-046578 (L.K.S.), AI-046629 (L.K.S.), T32-007349 (L.L.K.), AI-047140 (J.C.D.L.T.), AI-077719 (J.C.D.L.T.), AI-079665 (J.C.D.L.T.), and DK-032520 and German Research Foundation fellowship CO310-2/1 (M.C.).
We have no conflicting financial interests.
The contents of this publication are solely the responsibility of the authors and do not represent the official view of the NIH.
REFERENCES
- 1.Lam KS, Marshall ID. 1968. Dual infections of Aedes aegypti with arboviruses. I. Arboviruses that have no apparent cytopathic effect in the mosquito. Am J Trop Med Hyg 17:625–636. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain RW, Sudia WD. 1957. Dual infections of eastern and western equine encephalitis viruses in Culex tarsalis. J Infect Dis 101:233–236. doi: 10.1093/infdis/101.3.233. [DOI] [PubMed] [Google Scholar]
- 3.Bharaj P, Chahar HS, Pandey A, Diddi K, Dar L, Guleria R, Kabra SK, Broor S. 2008. Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J 5:1. doi: 10.1186/1743-422X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esper FP, Spahlinger T, Zhou L. 2011. Rate and influence of respiratory virus co-infection on pandemic (H1N1) influenza disease. J Infect 63:260–266. doi: 10.1016/j.jinf.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palacios G, Hornig M, Cisterna D, Savji N, Bussetti AV, Kapoor V, Hui J, Tokarz R, Briese T, Baumeister E, Lipkin WI. 2009. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One 4:e8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maier I, Wu GY. 2002. Hepatitis C and HIV co-infection: a review. World J Gastroenterol 8:577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singal A-K, Anand BS. 2009. Management of hepatitis C virus infection in HIV/HCV co-infected patients: clinical review. World J Gastroenterol 15:3713–3724. doi: 10.3748/wjg.15.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabowski J, Wedemeyer H. 2010. Hepatitis delta: immunopathogenesis and clinical challenges. Dig Dis 28:133–138. doi: 10.1159/000282076. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhoff A, Jacobs RJ, Greenberg DP, Yagoda B, Castles CG. 2003. Clinician satisfaction with vaccination visits and the role of multiple injections, results from the COVISE Study (Combination Vaccines Impact on Satisfaction and Epidemiology). Clin Pediatr 43:87–93. [DOI] [PubMed] [Google Scholar]
- 10.Woodin KA, Rodewald LE, Humiston SG, Carges MS, Schaffer SJ, Szilagyi PG. 1995. Physician and parent opinions. Are children becoming pincushions from immunizations? Arch Pediatr Adolesc Med 149:845–849. [DOI] [PubMed] [Google Scholar]
- 11.Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. 2011. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend Rep 60(RR-2):1–60. [PubMed] [Google Scholar]
- 12.Vidor E. 2007. The nature and consequences of intra- and inter-vaccine interference. J Comp Pathol 137(Suppl 1):S62–S66. doi: 10.1016/j.jcpa.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Ruben FL, Smith EA, Foster SO, Casey HL, Pifer JM, Wallace RB, Atta AI, Jones WL, Arnold RB, Teller BE, Shaikh ZQ, Lourie B, Eddins DL, Doko SM, Foege WH. 1973. Simultaneous administration of smallpox, measles, yellow fever, and diphtheria-pertussis-tetanus antigens to Nigerian children. Bull World Health Organ 48:175–181. [PMC free article] [PubMed] [Google Scholar]
- 14.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. 2002. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol 3:627–634. [DOI] [PubMed] [Google Scholar]
- 15.Selin LK, Varga SM, Wong IC, Welsh RM. 1998. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med 188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen AT, Cornberg M, Gras S, Guillonneau C, Rossjohn J, Trees A, Emonet S, la Torre de JC, Welsh RM, Selin LK. 2012. Loss of anti-viral immunity by infection with a virus encoding a cross-reactive pathogenic epitope. PLoS Pathog 8:e1002633. doi: 10.1371/journal.ppat.1002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornberg M, Chen AT, Wilkinson LA, Brehm MA, Kim S-K, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM, Selin LK. 2006. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest 116:1443–1456. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornberg M, Clute SC, Watkin LB, Saccoccio FM, Kim S-K, Naumov YN, Brehm MA, Aslan N, Welsh RM, Selin LK. 2010. CD8 T cell cross-reactivity networks mediate heterologous immunity in human EBV and murine vaccinia virus infections. J Immunol 184:2825–2838. doi: 10.4049/jimmunol.0902168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. 2001. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol 2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 20.Welsh RM, McNally JM, Brehm MA, Selin LK. 2000. Consequences of cross-reactive and bystander CTL responses during viral infections. Virology 270:4–8. doi: 10.1006/viro.2000.0278. [DOI] [PubMed] [Google Scholar]
- 21.Nie S, Lin S-J, Kim S-K, Welsh RM, Selin LK. 2010. Pathological features of heterologous immunity are regulated by the private specificities of the immune repertoire. Am J Pathol 176:2107–2112. doi: 10.2353/ajpath.2010.090656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S-K, Cornberg M, Wang XZ, Chen HD, Selin LK, Welsh RM. 2005. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J Exp Med 201:523–533. doi: 10.1084/jem.20041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nie S, Cornberg M, Selin LK. 2009. Resistance to vaccinia virus is less dependent on TNF under conditions of heterologous immunity. J Immunol 183:6554–6560. doi: 10.4049/jimmunol.0902156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cibotti R, Cabaniols JP, Pannetier C, Delarbre C, Vergnon I, Kanellopoulos JM, Kourilsky P. 1994. Public and private Vβ T cell receptor repertoires against hen egg white lysozyme (HEL) in nontransgenic versus HEL transgenic mice. J Exp Med 180:861–872. doi: 10.1084/jem.180.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsh RM, Lampert PW, Burner PA, Oldstone MB. 1976. Antibody-complement interactions with purified lymphocytic choriomeningitis virus. Virology 73:59–71. doi: 10.1016/0042-6822(76)90060-X. [DOI] [PubMed] [Google Scholar]
- 26.Welsh RM. 1978. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med 148:163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selin LK, Nahill SR, Welsh RM. 1994. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med 179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper PD. 1959. Tetrazolium salts as stains for animal virus plaque assays. Virology 7:469–470. doi: 10.1016/0042-6822(59)90077-7. [DOI] [PubMed] [Google Scholar]
- 29.Logan JC, Fox MP, Morgan JH, Makohon AM, Pfau CJ. 1975. Arenavirus inactivation on contact with N-substituted isatin beta-thiosemicarbazones and certain cations. J Gen Virol 28:271–283. doi: 10.1099/0022-1317-28-3-271. [DOI] [PubMed] [Google Scholar]
- 30.Walker CM, Rawls WE, Rosenthal KL. 1984. Generation of memory cell-mediated immune responses after secondary infection of mice with pichinde virus. J Immunol 132:469–474. [PubMed] [Google Scholar]
- 31.Cerny A, Sutter S, Bazin H, Hengartner H, Zinkernagel RM. 1988. Clearance of lymphocytic choriomeningitis virus in antibody- and B-cell-deprived mice. J Virol 62:1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med 160:521–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornberg M, Kenney LL, Chen AT, Waggoner SN, Kim S-K, Dienes HP, Welsh RM, Selin LK. 2013. Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front Immunol 4:475. doi: 10.3389/fimmu.2013.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamm A, Valentine L, Potts R, Premenko-Lanier M. 2012. An intermediate dose of LCMV clone 13 causes prolonged morbidity that is maintained by CD4+ T cells. Virology 425:122–132. doi: 10.1016/j.virol.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waggoner SN, Cornberg M, Selin LK, Welsh RM. 2012. Natural killer cells act as rheostats modulating antiviral T cells. Nature 481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheehan M, Haythorn P. 1979. Predictive values of various liver function tests with respect to the diagnosis of liver disease. Clin Biochem 12:262–263. doi: 10.1016/S0009-9120(79)80122-8. [DOI] [PubMed] [Google Scholar]
- 37.Smoller BR, Weishar M, Gray MH. 1990. An unusual cutaneous manifestation of Crohn's disease. Arch Pathol Lab Med 114:609–610. [PubMed] [Google Scholar]
- 38.Maggiore G, Grifeo S, Marzani MD. 1983. Erythema nodosum and hepatitis B virus (HBV) infection. J Am Acad Dermatol 9:602–603. doi: 10.1016/S0190-9622(83)80256-4. [DOI] [PubMed] [Google Scholar]
- 39.Cerwenka A, Morgan TM, Harmsen AG, Dutton RW. 1999. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J Exp Med 189:423–434. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamada H, Garcia-Hernandez M de LL Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. 2009. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinkernagel RM, Welsh RM. 1976. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol 117:1495–1502. [PubMed] [Google Scholar]
- 42.Nolz JC, Harty JT. 2011. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity 34:781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badovinac VP, Porter BB, Harty JT. 2002. Programmed contraction of CD8+ T cells after infection. Nat Immunol 3:619–626. doi: 10.1038/nrm880. [DOI] [PubMed] [Google Scholar]
- 45.Kaech SM, Ahmed R. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol 2:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prlic M, Hernandez-Hoyos G, Bevan MJ. 2006. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med 203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiemann M, Busch V, Linkemann K, Huster KM, Busch DH. 2003. Differences in maintenance of CD8+ and CD4+ bacteria-specific effector-memory T cell populations. Eur J Immunol 33:2875–2885. doi: 10.1002/eji.200324224. [DOI] [PubMed] [Google Scholar]
- 48.van Heijst JWJ, Gerlach C, Swart E, Sie D, Nunes-Alves C, Kerkhoven RM, Arens R, Correia-Neves M, Schepers K, Schumacher TNM. 2009. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient. Science 325:1265–1269. doi: 10.1126/science.1175455. [DOI] [PubMed] [Google Scholar]
- 49.Wlodarczyk MF, Kraft AR, Chen HD, Kenney LL, Selin LK. 2013. Anti-IFN-γ and peptide-tolerization therapies inhibit acute lung injury induced by cross-reactive influenza A-specific memory T cells. J Immunol 190:2736–2746. doi: 10.4049/jimmunol.1201936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selin LK, Wlodarczyk MF, Kraft AR, Nie S, Kenney LL, Puzone R, Celada F. 2011. Heterologous immunity: immunopathology, autoimmunity and protection during viral infections. Autoimmunity 44:328–347. doi: 10.3109/08916934.2011.523277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. 2000. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med 192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clute SC, Watkin LB, Cornberg M, Naumov YN, Sullivan JL, Luzuriaga K, Welsh RM, Selin LK. 2005. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J Clin Invest 115:3602–3612. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urbani S, Amadei B, Fisicaro P, Pilli M, Missale G, Bertoletti A, Ferrari C. 2005. Heterologous T cell immunity in severe hepatitis C virus infection. J Exp Med 201:675–680. doi: 10.1084/jem.20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welsh RM, Rothman AL. 2003. Dengue immune response: low affinity, high febrility. Nat Med 9:820–822. doi: 10.1038/nm0703-820. [DOI] [PubMed] [Google Scholar]
- 55.Antunes DA, Rigo MM, Silva JP, Cibulski SP, Sinigaglia M, Chies JAB, Vieira GF. 2011. Structural in silico analysis of cross-genotype-reactivity among naturally occurring HCV NS3-1073-variants in the context of HLA-A*02:01 allele. Mol Immunol 48:1461–1467. doi: 10.1016/j.molimm.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Sinigaglia M, Antunes DA, Rigo MM, Chies JAB, Vieira GF. 2013. CrossTope: a curate repository of 3D structures of immunogenic peptide: MHC complexes. Database (Oxford) 2013:bat002. doi: 10.1093/database/bat002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. 2013. Virus-specific CD4+ memory-phenotype T cells are abundant in unexposed adults. Immunity 38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Motozono C, Miles JJ, Hasan Z, Gatanaga H, Meribe SC, Price DA, Oka S, Sewell AK, Ueno T. 2013. CD8+ T cell cross-reactivity profiles and HIV-1 immune escape towards an HLA-B35-restricted immunodominant Nef epitope. PLoS One 8:e66152. doi: 10.1371/journal.pone.0066152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sewell AK. 2012. Why must T cells be cross-reactive? Nat Rev Immunol 12:669–677. doi: 10.1038/nri3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haanen JB, Wolkers MC, Kruisbeek AM, Schumacher TN. 1999. Selective expansion of cross-reactive CD8+ memory T cells by viral variants. J Exp Med 190:1319–1328. doi: 10.1084/jem.190.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maeda K, West K, Toyosaki-Maeda T, Rothman AL, Ennis FA, Terajima M. 2004. Identification and analysis for cross-reactivity among hantaviruses of H-2b-restricted cytotoxic T-lymphocyte epitopes in Sin Nombre virus nucleocapsid protein. J Gen Virol 85:1909–1919. doi: 10.1099/vir.0.79945-0. [DOI] [PubMed] [Google Scholar]
- 62.Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. 2001. Cross-reactivity between hepatitis C virus and Influenza A virus determinant-specific cytotoxic T cells. J Virol 75:11392–11400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savage PA, Boniface JJ, Davis MM. 1999. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity 10:485–492. doi: 10.1016/S1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 64.Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. 2003. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol 163:1341–1355. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim S-K, Clute SC, Welsh RM. 2006. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev 211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S-K, Brehm MA, Welsh RM, Selin LK. 2002. Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J Immunol 169:90–98. doi: 10.4049/jimmunol.169.1.90. [DOI] [PubMed] [Google Scholar]
- 67.Kraft ARM, Wlodarczyk MF, Kenney LL, Selin LK. 2013. PC61 (anti-CD25) treatment inhibits influenza A virus-expanded regulatory T cells and severe lung pathology during a subsequent heterologous lymphocytic choriomeningitis virus infection. J Virol 87:12636–12647. doi: 10.1128/JVI.00936-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cornberg M, Sheridan BS, Saccoccio FM, Brehm MA, Selin LK. 2007. Protection against vaccinia virus challenge by CD8 memory T cells resolved by molecular mimicry. J Virol 81:934–944. doi: 10.1128/JVI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scott LJ, Carter NJ, Curran MP. 2012. Live attenuated influenza vaccine (Fluenz™): a guide to its use in the prevention of seasonal influenza in children in the EU. Paediatr Drugs 14:271–279. doi: 10.2165/11207080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 70.Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]