ABSTRACT
Mature dendritic cells (mDCs) are known as the most potent antigen-presenting cells (APCs) since they are also able to prime/induce naive T cells. Thus, mDCs play a pivotal role during the induction of antiviral immune responses. Remarkably, the cell surface molecule CD83, which was shown to have costimulatory properties, is targeted by herpes simplex virus 1 (HSV-1) for viral immune escape. Infection of mDCs with HSV-1 results in downmodulation of CD83, resulting in reduced T cell stimulation. In this study, we report that not only infected mDCs but also uninfected bystander cells in an infected culture show a significant CD83 reduction. We demonstrate that this effect is independent of phagocytosis and transmissible from infected to uninfected mDCs. The presence of specific viral proteins found in these uninfected bystander cells led to the hypothesis that viral proteins are transferred from infected to uninfected cells via L particles. These L particles are generated during lytic replication in parallel with full virions, called H particles. L particles contain viral proteins but lack the viral capsid and DNA. Therefore, these particles are not infectious but are able to transfer several viral proteins. Incubation of mDCs with L particles indeed reduced CD83 expression on uninfected bystander DCs, providing for the first time evidence that functional viral proteins are transmitted via L particles from infected mDCs to uninfected bystander cells, thereby inducing CD83 downmodulation.
IMPORTANCE HSV-1 has evolved a number of strategies to evade the host's immune system. Among others, HSV-1 infection of mDCs results in an inhibited T cell activation caused by degradation of CD83. Interestingly, CD83 is lost not only from HSV-1-infected mDCs but also from uninfected bystander cells. The release of so-called L particles, which contain several viral proteins but lack capsid and DNA, during infection is a common phenomenon observed among several viruses, such as human cytomegalovirus (HCMV), Epstein-Barr virus, and HSV-1. However, the detailed function of these particles is poorly understood. Here, we provide for the first time evidence that functional viral proteins can be transferred to uninfected bystander mDCs via L particles, revealing important biological functions of these particles during lytic replication. Therefore, the transfer of viral proteins by L particles to modulate uninfected bystander cells may represent an additional strategy for viral immune escape.
INTRODUCTION
Dendritic cells (DCs) are known as the most potent antigen-presenting cells (APCs) due to their unique ability to prime naive T cells. Thus, they are vital to induce effective antiviral immune responses. In their immature state, DCs reside as sentinels of the immune system in almost all peripheral tissues until they encounter and take up antigens, resulting in maturation of DCs. As a consequence, expression of major histocompatibility complex (MHC) classes I and II as well as of costimulatory molecules, such as CD40, CD80, CD86, and also CD83, is strongly induced (1–3). As CD83 is not expressed on immature, tolerogenic DCs but is highly upregulated during DC maturation, this protein has become one of the best surface markers for mature DCs (3–5). Nevertheless, CD83 is also expressed on subsets of activated T cells, B cells, granulocyte precursor cells, myelocytes, neutrophils, and thymus epithelial cells as well as on regulatory T cells (5–10).
In addition to this membrane-bound CD83 molecule (mCD83), a soluble form of CD83 (sCD83), consisting of the extracellular Ig domain of mCD83, also has been described previously (11, 12). This soluble form, which was shown to possess potent immunosuppressive properties, is released from activated DCs as well as from B cells and can be detected at low levels in sera of healthy individuals (11) and at highly elevated concentrations in patients suffering from malignant disorders (12). Using animal models, it could be demonstrated that a recombinant expressed sCD83 molecule inhibits disease-associated symptoms in experimental autoimmune encephalomyelitis as well as in an inflammatory bowel disease model. In addition, sCD83 was shown to prevent graft rejection in different transplantation models (13–17).
In contrast, mCD83 expressed on human mature DCs (mDCs) has been suggested to have costimulatory properties, since in vitro knockdown of mCD83, using small interfering RNA (siRNA) technology, resulted in a significantly reduced stimulatory capacity of these DCs (18, 19). Furthermore, several viruses, especially herpesviruses, including human cytomegalovirus (HCMV), varicella-zoster virus, and also herpes simplex virus 1 (HSV-1), modulate the surface expression of mCD83, resulting in inhibition of T cell proliferation and thus in reduced antiviral immune responses (20–22).
HSV-1 is the prototype of the alphaherpesvirus subgroup and is characterized by low species specificity and, in addition, by an extremely fast lytic replication cycle. During replication, not only are complete virions, called H particles (heavy particles), formed, but also so-called L particles (light particles) are released. L particles resemble H particles, but they lack the capsid and viral DNA. Interestingly, it has been shown previously that these L particles contain many cellular factors as well as viral proteins, including infected cell protein 0 (ICP0) and ICP4 (23–25), which could be transferred to other cells. However, until now a transfer of functional proteins by L particles has not been reported.
HSV-1 establishes latency in sensory neurons and ganglia after primary infection (26, 27). The infected cell protein 0 (ICP0) stimulates the initiation of lytic replication and is responsible for viral reactivation, which makes ICP0 one of the most important immediate early (IE) proteins and a key regulator of lytic and latent infection (28–30). Furthermore, ICP0 was shown previously to mediate proteasomal degradation of several cellular proteins, including the promyelocytic leukemia (PML) protein (31, 32) and the Sp100 protein (33), which results in disruption of ND10 domains (34, 35). These observations led to the hypothesis that ND10 domains might repress viral replication and that disruption of these structures by viral proteins might represent a strategy to overcome this repression (36). We showed earlier that during HSV-1 infection of mDCs, ICP0 induces the degradation of CD83 in a proteasome-dependent but ubiquitin-independent manner (20, 37, 38).
In addition, we observed that not only HSV-1-infected mDCs but also HSV-1-negative (uninfected) bystander mDCs, present within an infected culture, show a significant reduction of CD83 on their cell surface compared to separately cultured mock-infected cells. Hence, the aim of the present study was to investigate how CD83 downregulation in HSV-1-negative bystander DCs is induced. Here, we provide for the first time evidence that L particles are released into the supernatant of HSV-1-infected mDCs and that these L particles are able to transmit functional viral proteins to uninfected bystander DCs and to modulate these cells.
MATERIALS AND METHODS
Virus strains, virus preparation, and virus titration.
In the present study, HSV-1/17+/CMV-EGFP/UL43 (HSV-1), which is derived from the HSV-1 strain 17+, was used. This HSV-1 variant expresses the enhanced green fluorescent protein (EGFP) marker gene driven by the CMV promoter which has been inserted into the UL43 gene. As this gene has been described as nonessential (39, 40) and has previously been demonstrated not to affect the kinetics of HSV-1 reactivation and latency (41), this virus was used as wild-type (WT) virus. Preparation of virus stocks and determination of virus titers were carried out as described previously (42).
Generation of mature dendritic cells (mDCs).
Peripheral blood mononuclear cells (PBMCs) were isolated from different healthy donors using a Lymphoprep gradient (Nycomed Pharma AS, Oslo, Norway) as described previously (37).
Infection procedure.
Mature DCs (2 × 106) were infected with HSV-1 in a total volume of 300 μl RPMI 1640 medium supplemented with 20 mM HEPES at a multiplicity of infection (MOI) of 0.6. Infection was performed for 1 h at 37°C at 300 rpm in a shaking heating block (Eppendorf, Hamburg, Germany). Subsequently, cells were transferred to RPMI 1640 medium containing 1% autologous serum, 10 mM HEPES, 2 mM l-glutamine, 100 U penicillin ml−1, 100 μg streptomycin ml−1, 40 U granulocyte-macrophage colony-stimulating factor (GM-CSF) ml−1, and 250 U interleukin-4 (IL-4) ml−1 at a concentration of 0.5 × 106 ml−1. Where indicated, cells were treated with 2 μM cytochalasin D (Enzo Life Sciences, Lörrach, Germany) 1 h postinfection.
Flow cytometry and FACS.
Changes in cell surface expression of CD83 were analyzed by fluorescence-activated cell sorting (FACS) using a CD83-specific antibody (clone HB15e; BD Biosciences, Heidelberg, Germany). To ensure analysis of living cells, we additionally stained the cells using the LIVE/DEAD Fixable Violet dead cell stain kit (Life Technologies, Carlsbad, CA, USA). Cells were sorted for their GFP expression using a BD Aria FACS cell sorter (BD Biosciences, Heidelberg, Germany).
Cell lysis and immunoblotting.
Mature DCs (1 × 106) were harvested and resuspended in 50 μl lysis buffer (10% glycerol, 2 mM EDTA [pH 8], 137 mM NaCl, 10 mM sodium phosphate [pH 7.2], 1% NP-40, 2 mM phenylmethanesulfonyl fluoride, 20 mM sodium fluoride). After 20 min on ice, lysates were cleared by centrifugation at 4°C and full speed for 20 min. Free virus as well as L and H particle preparations was directly resuspended in SDS loading buffer and incubated for 10 min at 95°C. Protein samples were separated using SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred onto a nitrocellulose membrane. After blocking with 5% (wt/vol) dry milk, the membrane was incubated with the primary antibody at 4°C overnight. After incubation with the appropriate secondary horseradish peroxidase (HRP)-labeled antibody, detection was performed using Amersham ECL Prime Western blotting detection reagent (GE Healthcare, Solingen, Germany). The following primary antibodies were used for Western blot analyses: anti-CD83 (clone F-5; Santa Cruz Biotech Inc., Heidelberg, Germany; 1:1,000), anti-β-actin (clone AC-74; Sigma-Aldrich, St. Louis, MO, USA; 1:2,000), anti-ICP0 (clone 11060; Santa Cruz; 1:1,000), anti-GFP (clone B-2; Santa Cruz; 1:1,000), anti-ICP4 (clone 10F1; Santa Cruz; 1:1,000), anti-gB (clone 10B7; Santa Cruz; 1:1,000), and anti-ICP5 (major capsid protein [MCP]; clone 3B6; Santa Cruz; 1:1,000).
RNA isolation and reverse transcription.
Mature DCs were harvested and washed once with phosphate-buffered saline (PBS). Total RNA was isolated using the QIAshredder kit and the RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Genomic DNA was removed by on-column DNase digestion with the RNase-free DNase set (Qiagen). Afterwards, cDNA was synthesized using the QuantiTect reverse transcription kit (Qiagen) according to the manufacturer's recommendations.
qRT-PCR.
The following primers were used for quantitative reverse transcription-PCR (qRT-PCR): ICP0 sense (5′-CCC CCC TTT TTT CCC CTT AGC C-3′), ICP0 antisense (5′-AAC AGT TCC GTG TCC GTG CTG TCC-3′), S14 sense (5′-GGC AGA CCG AGA TGA ATC CTC A-3′), S14 antisense (5′-CAG GTC CAG GGG TCT TGG TCC-3′), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense (5′-ATC CCA TCA CCA TCT TCC AGG-3′), and GAPDH antisense (5′-CAT GGT TCA CAC CCA TGA CG-3′). The cycling profile for qRT-PCR (45 cycles) was 15 s at 95°C, 15 s at 55°C, and 20 s at 72°C. After finishing the cycling process, a melting-curve analysis was performed by subjecting the samples to a temperature ramp (from 65°C to 95°C at 0.1°C/s). qRT-PCR was performed using the Touch Thermal Cycler CFX96 real-time system (Bio-Rad, Munich, Germany). Analysis was carried out with CFX Manager 3.0 software (Bio-Rad).
Coculture experiments.
For coculture experiments, 4 × 106 mDCs were infected with HSV-1 as described earlier at an MOI of 1, and at 6 h postinfection, cells were sorted for HSV-1/GFP-positive cells. Afterward, 1 × 105 GFP-positive cells were cocultured with an equal amount of uninfected mDCs in 96-well round-bottom plates for 16 h. Finally, cells were analyzed for CD83 surface expression using flow cytometry.
Isolation of H and L particles.
Preparation of H and L particles was performed as described previously (43). In brief, to isolate H and L particles, virus was propagated in 15 T-175 cell culture flasks of BHK-21 cells. Infection was carried out at an MOI of 0.01, and after 4 days of incubation, particles were harvested by centrifugation for 2 h at 23,000 × g at 4°C. Virus particles were resuspended in Dulbecco modified Eagle medium (DMEM) without phenol red (Sigma-Aldrich, Deisenhofen, Germany). A gradient of 5 to 20% Ficoll 400 (Sigma-Aldrich) diluted in DMEM without phenol red in 5% steps was prepared, and the particle solution was carefully layered on top of this gradient. After centrifugation for 2.5 h at 26,000 × g and 4°C, particles were well separated in two distinct bands and collected by side punctures. Finally, particles were pelleted by centrifugation for 1.5 h at 80,000 × g at 4°C, resuspended in 100 μl DMEM without phenol red, and used immediately for further experiments. To inactivate contaminating H particles, L particles were treated 6 times with 0.12 J/cm2 UV light in a Vilber Luormat (Biometra, Göttingen, Germany). To isolate L particles generated from HSV-1-infected mDCs, 8 × 106 mDCs were infected with HSV-1 at an MOI of 1. After 24 h of incubation, viral particles were harvested, and L particles were isolated as described above.
Electron microscopy.
Isolated H and L particles were directly dialyzed against 20 mM HEPES overnight at 4°C using SnakeSkin pleated dialysis tubing with a 10,000-molecular-weight cutoff (MWCO) (Thermo Scientific, Rockford, IL, USA). The next day, 20 μl of particle solution was made to adhere on carbon-coated 400-square-mesh copper grids (Electron Microscopy Sciences, Hatfield, PA, USA) for 20 min at room temperature. After fixation with 2% glutaraldehyde, particles were stained using 1% uranyl acetate diluted in 50% ethanol for 10 min and lead citrate for 5 min. Samples were analyzed using a transmission electron microscope (Leo 912; Zeiss, Oberkochen, Germany).
Treatment of mDCs with L particles.
mDCs (2.5 × 105) were harvested, washed with PBS, and resuspended in 25 μl (BHK-21-derived) or 100 μl (mDC-derived) UV-irradiated L particles. After addition of 25 μl RPMI 1640 and 20 mM HEPES, cells were incubated for 1 h at 37°C. Subsequently, 200 μl DC medium supplemented with GM-CSF (final concentration, 40 U/ml) and IL-4 (final concentration, 250 U/ml) was added, and cells were incubated overnight.
Statistical analyses.
Results are displayed as means ± standard deviations (SD). For multiple comparisons, data were analyzed using one-way analysis of variance (ANOVA) and Bonferroni's multiple-comparison post hoc test. For comparison of two data sets, P values were determined via Student's t test. Significance was accepted if P was <0.05.
Approvals and legal requirements.
For the generation of monocyte-derived DCs from leukapheresis products of healthy donors, a positive vote from the local ethics committee has been obtained (reference number 4261).
RESULTS
GFP-negative bystander DCs lose CD83 after HSV-1 infection.
It has previously been described that HSV-1 infection of mDCs leads to the specific degradation of CD83 whereas other costimulatory molecules such as CD80 and CD86 are not influenced. Additionally, it was reported that the HSV-1 immediate early protein ICP0 plays an important role in the induction of CD83 downmodulation and that this CD83 reduction can be prevented by addition of the proteasome inhibitor MG-132 (20, 38). Interestingly, we observed that also in uninfected DCs, present within HSV-1-infected DC cultures, CD83 expression was strongly reduced. To analyze this phenomenon in more detail, we performed the following investigations.
The use of a GFP-expressing HSV-1 variant allows direct analysis of HSV-1/GFP-positive (infected) or HSV-1/GFP-negative (uninfected) cells within an infected cell culture. Thus, mDCs were mock infected or HSV-1 infected at an MOI of 0.6, resulting in an infection efficiency of approximately 50%. After 16 h of incubation, cells were harvested and stained for CD83 surface expression (Fig. 1a). To ensure that only living cells were analyzed, we additionally stained the cells using LIVE/DEAD Fixable Violet in all subsequent experiments. GFP-expressing HSV-1-positive cells as well as HSV-1-negative cells were directly gated to analyze CD83 surface expression. Flow cytometric analysis revealed that in infected cultures not only HSV-1-positive mDCs (GFP+) but also HSV-1-negative mDCs (GFP−) significantly lost their CD83 expression from the cell surface (P < 0.001). Western blot analysis of HSV-1-infected mDCs sorted into GFP+ and GFP− cells confirmed that HSV-1-negative mDCs, within the infected cultures, also showed reduced amounts of CD83 expression in comparison to mock-infected cells (Fig. 1b, upper panel). The membrane was reprobed with a specific GFP antibody to control the purity of the sorting procedure (Fig. 1b, middle panel) and with a β-actin-specific antibody to verify equal loading (Fig. 1b, lower panel).
FIG 1.
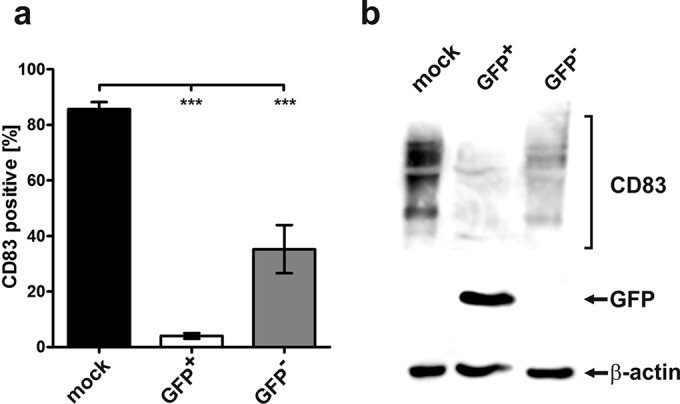
GFP-negative bystander DCs reveal reduced CD83 expression levels after HSV-1 infection. (a) Mature DCs were mock infected or HSV-1 infected. At 16 h postinfection, CD83 surface expression on mock-infected (black bar), GFP-positive (GFP+, white bar), and GFP-negative (GFP−, gray bar) mDCs was analyzed by flow cytometry. The experiments were performed at least five times with cells from different donors. Significant changes (P < 0.001) are indicated by asterisks. (b) Mature DCs were mock infected or HSV-1 infected. After 16 h, cells were sorted into GFP+ and GFP− mDCs, and CD83 expression was analyzed using Western blot analysis (upper panel). The membrane was reprobed with an anti-GFP-specific antibody (middle panel) and an anti-β-actin antibody (bottom panel) to verify equal loading. The experiments were performed three times with mDCs from different healthy donors, and representative data are shown.
Loss of CD83 in bystander DCs occurs in a time-delayed fashion.
In order to examine more closely the course of CD83 reduction in GFP-negative bystander mDCs compared to GFP-positive mDCs, a time course assay was performed. Mature DCs were mock infected or HSV-1 infected at an MOI of 0.6. After 4, 6, 8, and 16 h of incubation, mDCs were harvested and cells were stained for CD83 surface expression and analyzed using flow cytometry as described above (Fig. 2). In the GFP-positive population (GFP+, squares), a significant (P < 0.001) CD83 downmodulation occurred already at 6 h postinfection, compared to mock-infected control cells (filled circles). In contrast, CD83 loss in GFP-negative (GFP−, triangles) cells became significant no earlier than 16 h postinfection. These data clearly demonstrate that CD83 downmodulation in HSV-1-negative mDCs occurs at a later time than in HSV-1-positive cells. Thus, we hypothesized that viral factors which are responsible for the downmodulation of CD83 could be transmitted from infected to uninfected bystander DCs.
FIG 2.
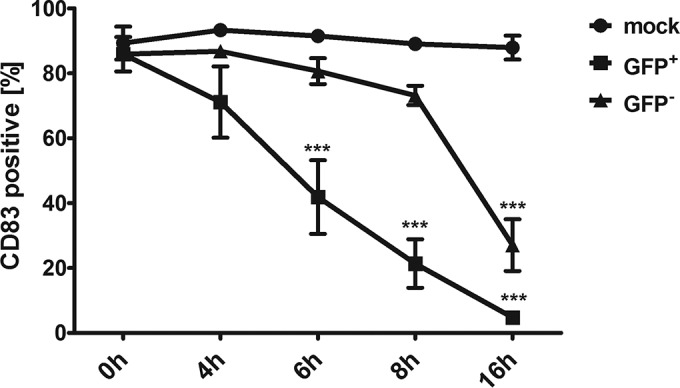
CD83 reduction in bystander DCs is time delayed. Mature DCs were mock infected or HSV-1 infected. At indicated time points, cells were harvested and CD83 surface expression of GFP-positive (GFP+, squares) and GFP-negative (GFP−, triangles) mDCs was analyzed by flow cytometry. Mock-infected cells (circles) were used as a control. Experiments were performed three times with cells from different healthy donors. Significant changes (P < 0.001) are indicated by asterisks.
Bystander DCs are not secondarily infected.
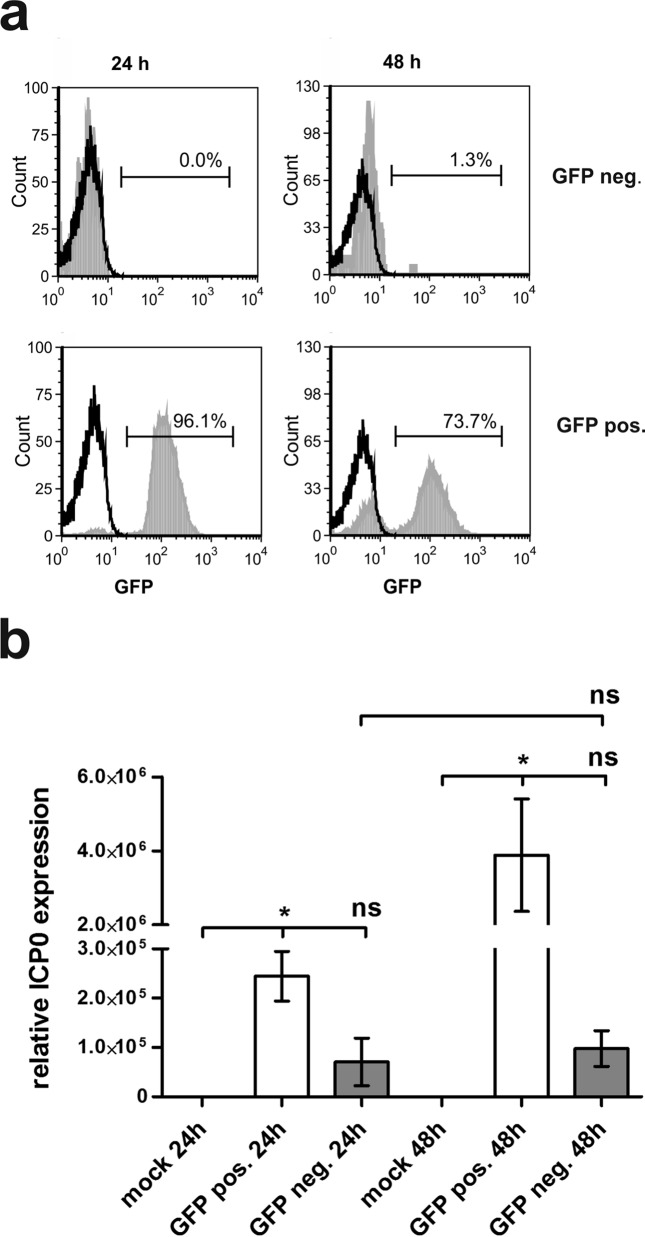
In order to rule out the possibility that GFP-negative bystander DCs have been infected in a second round of infection and hence GFP expression would not yet be detectable, we infected mDCs with HSV-1 and sorted these cells 24 h postinfection into GFP-negative and GFP-positive cells. The purity of the sorting process was controlled using flow cytometry (Fig. 3a, 24 h). An aliquot of these cells was cultured for an additional 24 h and subsequently analyzed for GFP expression (Fig. 3a, 48 h). Mock controls are depicted as black histograms, and GFP-negative cells (Fig. 3a, upper panel) and GFP-positive cells (Fig. 3a, lower panel) are depicted as filled light gray histograms. Figure 3a clearly shows that even after 48 h of incubation, only 1.3% GFP-positive cells are detectable in the GFP-negative sorted fraction. These results strongly indicate that these cells are not secondarily infected.
FIG 3.
GFP-negative bystander DCs are not secondarily infected. Mature DCs were either mock or HSV-1 infected. At 16 h postinfection, cells were sorted into GFP+ and GFP− cells and analyzed directly or after an additional 24 h of incubation. (a) Cells were analyzed for their GFP expression using flow cytometry. Mock controls are depicted as black histograms; GFP− (upper panel) and GFP+ (lower panel) cells are depicted as filled light gray histograms. The percentage of GFP+ cells is depicted in each histogram. (b) qRT-PCR analyses were performed to assess relative ICP0 expression levels. S14 and GAPDH served as controls. The experiment was carried out three times with cells from different healthy donors. Significant changes (P < 0.05) are indicated by asterisks. Nonsignificant changes (P > 0.05) are depicted as “ns.” neg., negative; pos., positive.
Moreover, it is well known that GFP cassettes inserted into viruses can be lost during lytic replication (44). Thus, in a subsequent step we wanted to exclude the possibility that GFP-negative cells were infected but lost their GFP expression only during replication. To address this question, qRT-PCR analyses for ICP0 transcripts were performed. ICP0 is an immediate early protein of the virus and is readily detected in HSV-1-infected mDCs (28). For this purpose, mDCs were mock infected or HSV-1 infected. After 24 h of incubation, cells were sorted as described above and an aliquot of these sorted cells was incubated for an additional 24 h. RNA was isolated, and after cDNA synthesis, qRT-PCR analyses were carried out. In Fig. 3b, relative ICP0 expression levels are depicted. As reference transcripts, GAPDH and S14 were used, and mock infection served as the negative control. Compared to mock-infected cells, relative ICP0 expression in GFP-positive cells is 2.5 × 105-fold higher and approximately 4 × 106-fold higher, 24 h and 48 h postinfection, respectively. ICP0 expression in GFP-negative cells is only marginally upregulated; however, these changes are not significant (P > 0.05), do not increase from 24 h to 48 h of incubation, and thus are most probably results of cross-contamination of GFP-positive cells in GFP-negative samples, occurring during the cell sorting procedure. These results support the fact that GFP-negative cells are indeed not productively infected.
Coculture of mDCs with HSV-1-infected mDCs induces CD83 downmodulation on uninfected bystander cells.
In order to investigate if the loss of CD83, observed on uninfected bystander DCs, is transmissible from HSV-1-infected cells to mock-infected cells, coculture experiments were performed. Therefore, mDCs were mock infected or HSV-1 infected, and after 6 h, GFP-positive cells were sorted and then cocultured with an equal amount of mock-infected cells. After an additional 16 h, mDCs were harvested and CD83 expression on GFP-negative mDCs was determined using flow cytometry. Figure 4 illustrates that GFP-negative cells (gray bar) have a significantly (P < 0.01) reduced CD83 expression on their cell surface compared to mock-infected cells (black bar). At the same time, we asked if responsible factors are possibly released into the supernatant of HSV-1-infected cells. Therefore, mDCs were inoculated with supernatant of HSV-1-infected mDCs and cultured for at least an additional 16 h. Afterward, cells were screened for their CD83 surface expression as described above. As displayed in Fig. 4 these mDCs (white bar) showed a significantly reduced CD83 expression (P < 0.05) compared to mock-infected cells. These observations suggest that specific factors, which mediate the induction of CD83 downmodulation in uninfected bystander DCs, are released into the supernatant of infected mDCs.
FIG 4.
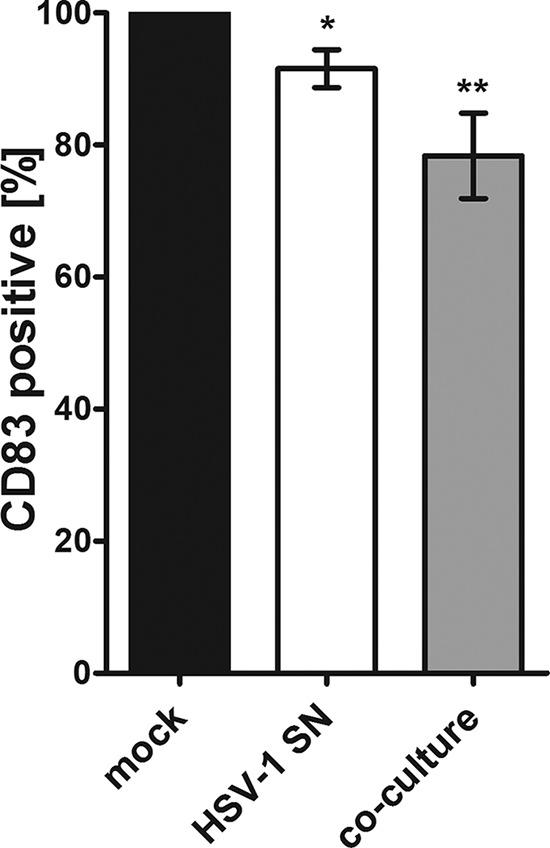
CD83 reduction in HSV-1-negative bystander DCs is transmissible from infected to uninfected cells. Mature DCs were either incubated with supernatant of HSV-1-infected mDCs or cocultured directly with HSV-1-infected mDCs. After 16 h of incubation, HSV-1-negative mDCs were analyzed for their CD83 surface expression using flow cytometry. The white bar shows GFP-negative cells cultured together with supernatant (SN) derived from HSV-1-infected mDCs, and the gray bar represents GFP-negative cells from coculture experiments. Mock controls (black bar) were set to 100%, and relative surface expression is shown. Significant changes (P < 0.05) are indicated by asterisks.
Reduction of CD83 on HSV-1-negative bystander DCs is independent of phagocytosis.
If CD83 degradation in uninfected bystander DCs is a consequence of HSV-1 infection, it was reasonable to assume that viral proteins might be detectable inside these bystander cells. Thus, mDCs were mock infected or HSV-1 infected as described above, and after sorting GFP-positive and GFP-negative mDCs, cells were lysed and Western blot analyses were performed (Fig. 5a). In HSV-1-positive mDCs, the IE proteins ICP0 and ICP4, the envelope protein gB, and the major capsid protein (MCP) could be detected. GFP-negative mDCs revealed reduced but detectable signals for ICP0, ICP4, and gB but not for MCP.
FIG 5.
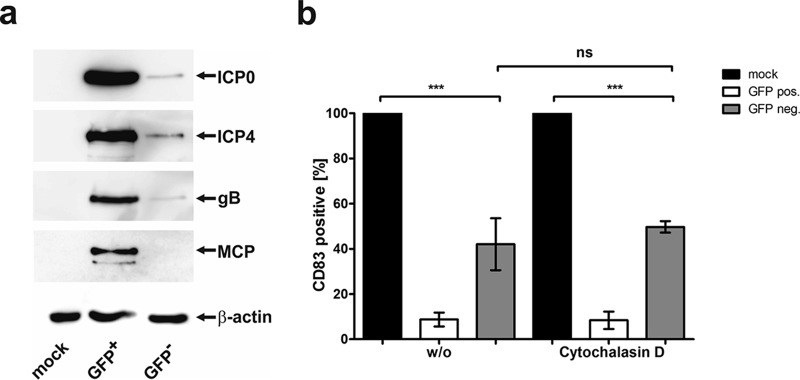
GFP-negative, uninfected bystander DCs contain several viral proteins, and CD83 downmodulation is independent of phagocytosis. (a) Mature DCs were mock infected or HSV-1 infected. At 16 h postinfection, cells were sorted into GFP+ and GFP− mDCs, and expression of viral proteins (ICP0, ICP4, gB, and MCP) was analyzed by immunoblotting. The membrane was reprobed with an anti-β-actin antibody as a loading control. Experiments were performed three times with mDCs from different donors, and representative data are shown. (b) Mature DCs were mock or HSV-1 infected and treated with 2 μM cytochalasin D 1 h postinfection or were left untreated (w/o). CD83 surface expression on mDCs was analyzed by flow cytometry 16 h postinfection. Mock controls (black bars) were set to 100%, and relative CD83 surface expression on GFP-positive (pos., white bars) and GFP-negative (neg., gray bars) cells is shown. Experiments were performed four times with cells from different healthy donors. Significant changes (P < 0.001) are indicated by asterisks, and nonsignificant changes (P > 0.05) are shown as “ns.”.
In a subsequent step, we wanted to rule out the possibility that uninfected bystander DCs engulfed these viral proteins via phagocytosis of infected cells or viral debris. Therefore, mDCs were mock or HSV-1 infected and treated with 2 μM cytochalasin D, 1 h postinfection, or were left untreated. After 16 h of incubation, CD83 surface expression was analyzed by flow cytometry (Fig. 5b). Even though phagocytosis was inhibited by cytochalasin D, HSV-1-negative bystander DCs showed reduced CD83 surface expression levels (Fig. 5b, right), comparable to those of the untreated samples (Fig. 5b, left).
Thus, these data strongly suggest that specific viral proteins are transferred into uninfected, GFP-negative bystander DCs, without the involvement of phagocytosis. The presence of the viral proteins ICP0, ICP4, and gB within these bystander DCs as well as the lack of capsid proteins led to the hypothesis that L particles might be responsible for transmission of viral proteins (45, 46) and consequently for the induced CD83 downmodulation.
CD83 loss on bystander DCs is transmitted by L particles.
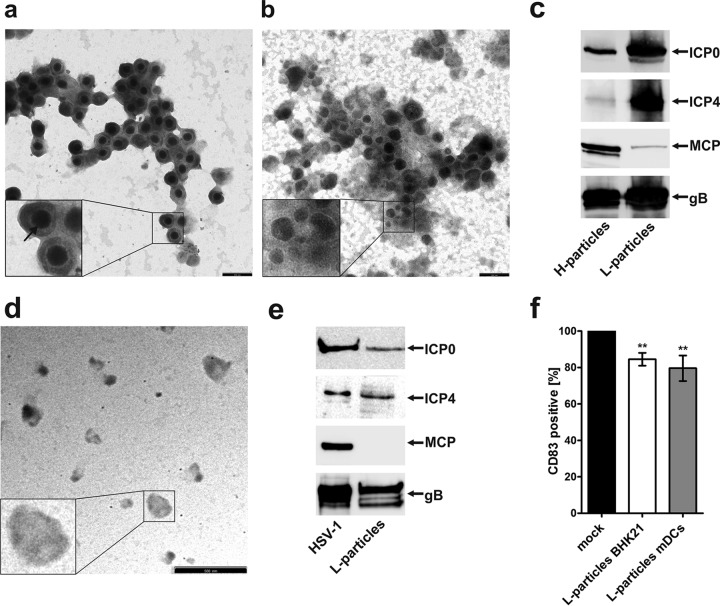
In order to prove the abovementioned hypothesis, H and L particles were isolated from the supernatant of HSV-1-infected BHK-21 cells using a Ficoll gradient. To test the purity of the different particle preparations, electron microscopy was used, whereby Fig. 6a illustrates H particles and Fig. 6b illustrates L particles. H particles contain a nucleocapsid surrounded by the envelope, whereas L particles are not as regular in size as are full virions and lack the capsid. The preparations showed a very high purity with small amounts of contaminating H particles within the L particle preparation and were thus subsequently used to further characterize and detect viral proteins by Western blotting (Fig. 6c). These preparations were directly taken up in SDS loading buffer, and after boiling for 5 min at 95°C, samples were loaded onto a 10% SDS gel, and subsequently, Western blot analyses were performed. Both H particles and L particles showed a clear signal for ICP0; however, L particles contained a larger amount of ICP0 in comparison to full virions (i.e., H particles). ICP4 was abundantly present in L particles but only to a minor degree in H particles. This observation is in agreement with results previously published by Szilagyi and Cunningham (46) reporting ICP4 as an L particle-specific protein. In contrast, MCP is readily detectable in H particles but only in very small amounts in L particles. This slight band could possibly be explained by a 1 to 5% (47) cross-contamination of H particles within the L particle preparation. As expected, the envelope protein gB is expressed in the two samples at comparable levels. These data clearly demonstrate that H and L particles have been successfully separated and that the preparations are of high purity. L particle preparations were then used for further experiments.
FIG 6.
CD83 downmodulation in HSV-1-negative bystander DCs is mediated by L particles. H and L particle preparations were analyzed by electron microscopy and further characterized using Western blot analysis. Viral particles were isolated using a Ficoll gradient and dialyzed against 20 mM HEPES buffer. (a and b) For electron microscopy, H particles (a) as well as L particles (b) were made to adhere to carbon-coated grids and stained using 1% uranyl acetate and lead citrate. Two independent preparations were analyzed by electron microscopy, and representative data are shown. The insets show particles that have been enlarged 2.5-fold. The arrow indicates the viral capsid. Bars (a and b), 250 nm. (c) H and L particles were analyzed by Western blotting for the presence of ICP0, ICP4, MCP, and gB. Three independent preparations were analyzed, and representative data are shown. (d) Electron microscopy analyses of L particles isolated from HSV-1-infected DC supernatant are shown. Bar, 500 nm. (e) L particles derived from mDCs were analyzed by Western blotting for the presence of viral proteins. As a positive control, BHK-21-derived virus was used. (f) Mature DCs were left untreated (black bar) or treated with UV-inactivated L particles derived from BHK-21 cells (white bar) or from DCs (gray bar). After 20 h, cells were harvested, and CD83 surface expression was analyzed by flow cytometry. Untreated controls were set to 100%, and relative surface expression is shown. The experiment was performed at least five times with cells from different healthy donors. Significant changes are indicated by asterisks (P < 0.01).
To assess whether L particles mediate the observed reduced CD83 expression in uninfected bystander DCs, L particles were isolated, UV irradiated to inactivate possible contaminating virions, and directly used to inoculate mDCs. Mature DCs were cocultured with L particles for at least 16 h and subsequently analyzed by flow cytometry for their CD83 surface expression (Fig. 6f). Interestingly, cells incubated with BHK-21 L particles (white bar) showed a significant (P < 0.01) reduction of CD83 surface expression, compared with mock-treated control cells (black bar). To further strengthen our hypothesis that L particles induce the reduction of CD83 surface expression in uninfected mDCs, we isolated L particles from the supernatant of HSV-1-infected mDCs. Interestingly, in contrast to HSV-1-infected BHK-21 supernatants, the supernatant of HSV-1-infected mDCs contained only L particles but no H particles. These mDC-derived L particles were controlled for their purity using electron microscopy (Fig. 6d) as well as Western blotting (Fig. 6e), as described above. Also, these particles contained ICP0, ICP4, and gB but no MCP. Mature DCs were then treated with the purified L particles and analyzed for their CD83 surface expression using flow cytometry (Fig. 6f). Interestingly, the treatment with mDC-derived L particles also resulted in a significant reduction (P < 0.01) of CD83 surface expression (gray bar), supporting the hypothesis that L particles are indeed responsible for CD83 downmodulation in uninfected bystander DCs.
Taken together, the above reported data clearly demonstrate that CD83 is downmodulated in uninfected bystander DCs and that this reduction occurs in a time-delayed fashion compared to infected mDCs. This CD83 downmodulation is not due to a secondary infection and is independent of phagocytosis. Finally, we were able to show that CD83 reduction in HSV-1-negative bystander DCs is mediated by L particles, which transmit viral proteins, including ICP0, which has previously been reported to be involved in CD83 degradation in HSV-1-infected mDCs (37, 38). Therefore, here we provide for the first time evidence that during lytic replication, L particles are able to transfer important biological functions to uninfected bystander cells.
DISCUSSION
In the present study, we report that not only HSV-1-positive mDCs but also HSV-1-negative bystander mDCs within an infected cell culture significantly downmodulate CD83 expression. Western blotting as well as flow cytometric analyses revealed that CD83 reduction in uninfected bystander DCs occurs to a significant extent. In comparison to infected DCs, CD83 downmodulation in uninfected bystander DCs occurred in a time-delayed fashion, suggesting that potential effector proteins, responsible for CD83 degradation, must be first synthesized and then transferred from infected to uninfected cells. This hypothesis was supported by the finding that uninfected bystander DCs contain viral proteins, including ICP0, ICP4, and gB, but no viral transcripts. As ICP0 was shown previously to be involved in CD83 degradation (37, 38), we hypothesized that, among others, ICP0 may be transmitted from infected to uninfected cells to induce CD83 degradation. Considering the detected viral proteins in uninfected DCs and the lack of capsid proteins, L particles came into focus as possible carriers of viral proteins (46).
Formation of full virions, so-called H particles, and that of noninfectious L particles have been shown to occur in close proximity, leading to similar envelope structures of these particles (48). Interestingly, we observed only L particles but no H particles in the supernatant of HSV-1-infected mDCs, which is in agreement with earlier studies demonstrating that HSV-1-infected mDCs do not release progeny virus into the supernatant (20, 41). Furthermore, virus transmission from infected mDCs to permissive Vero cells as well as to keratinocytes occurs only via cell-cell-contact (49). Moreover, similar observations were made for HCMV-infected DCs, which were shown to transfer the infection primarily via cell-cell-contact-dependent mechanisms (50). In contrast, in the present study, we were able to show that also uninfected bystander DCs are modulated by viral proteins. These proteins are transferred via L particles to uninfected bystander DCs, which have previously been released into the supernatant by HSV-1-infected DCs. In fact, incubation of mDCs with L particles derived from HSV-1-infected BHK-21 cells as well as with those derived from HSV-1-infected mDCs resulted in significantly reduced CD83 cell surface expression. Thus, our data suggest that L particles transfer functionally active viral proteins, including ICP0, to HSV-1-negative mDCs and thereby induce CD83 degradation.
Several studies have reported that microvesicles, such as exosomes and secreted vesicles, as well as the alphaherpesvirus-specific L particles have the potential to deliver a wide range of active molecules and agents (45, 51, 52). These molecules include mRNAs, proteins, microRNAs (miRNAs), noncoding RNAs, and DNA, which all can be transferred by microvesicles between cells (43, 53–55). Vesicle release from mammalian cells as a result of infection with bacteria and viruses was shown to account for their pathogenesis. Exosome release by an intracellular pathogen is suggested to serve as a general mechanism for effector molecule delivery from a pathogen to the cytosol of a host cell (52). There are several viruses, including HSV-1, HIV, and HCMV as well as hepatitis C virus (HCV), which induce the release of exosomes and other microvesicles. These vesicles contribute to communications between cells and also to a modulation of immune responses in the host (56, 57). HIV-infected cells, for example, were shown to release exosomes containing the virally encoded accessory protein negative factor (Nef), which is known to induce apoptosis in CD4+ T cells (58). In addition, Nef induces exosome release from infected cells, and these Nef-containing microvesicles then induce cell death in uninfected bystander cells (58). In contrast, Epstein-Barr virus-infected B cells secrete exosomes containing virally encoded miRNAs. These exosomes were able to deliver the functional miRNAs to DCs in vitro as well as to uninfected bystander B cells (59).
Since it was demonstrated previously that L particles also contain the virion host shutoff protein (vhs) and that this protein is transferred in a functional state (23, 51), one has to consider that CD83 downmodulation could also result from reduced transcription. vhs is responsible for rapid degradation of host mRNAs, thus supporting viral protein expression (60, 61). However, in an earlier publication we could show that CD83 transcription is not influenced by HSV-1 (38), and therefore, it is quite unlikely that CD83 downmodulation in bystander DCs is mediated via a vhs-dependent mechanism.
Under our experimental conditions, the observed effect of L particles, in respect to CD83 downmodulation, was rather modest. This could be explained by the fact that in our experiments only a restricted amount of particles is present. In a productively infected culture, however, L particles could be built continuously, and therefore, the expected effect would be increased.
We cannot definitively exclude the possibility that, besides L particles, other, not-yet-identified soluble factors released from HSV-1-infected DCs could interfere with CD83 expression and/or modulate DC maturation. However, this is rather unlikely and has to be investigated in future studies.
The data presented here clearly show for the first time that functional viral proteins are transferred via L particles from HSV-1-infected mDCs to uninfected bystander DCs, where they induce CD83 downmodulation. This may represent an additional, yet-unknown viral escape mechanism, since CD83-depleted mDCs are less potent immune stimulators. Thus, in summary, these data demonstrate that L particles are relevant virus-induced factors able to functionally modulate uninfected bystander cells.
ACKNOWLEDGMENTS
We thank the Core Unit “Cell Sorting and Immunomonitoring” Erlangen for cell sorting. We thank Kerstin Prechtel for critical proofreading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) via the SFB796 (project B2) and the Graduate Training Program 1071.
REFERENCES
- 1.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Zhou LJ, Schwarting R, Smith HM, Tedder TF. 1992. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol 149:735–742. [PubMed] [Google Scholar]
- 3.Zhou LJ, Tedder TF. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol 154:3821–3835. [PubMed] [Google Scholar]
- 4.Berchtold S, Jones T, Muhl-Zurbes P, Sheer D, Schuler G, Steinkasserer A. 1999. The human dendritic cell marker CD83 maps to chromosome 6p23. Ann Hum Genet 63:181–183. doi: 10.1046/j.1469-1809.1999.6320181.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhou LJ, Tedder TF. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U S A 93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimoto Y, Tu L, Miller AS, Bock C, Fujimoto M, Doyle C, Steeber DA, Tedder TF. 2002. CD83 expression influences CD4+ T cell development in the thymus. Cell 108:755–767. doi: 10.1016/S0092-8674(02)00673-6. [DOI] [PubMed] [Google Scholar]
- 7.Iking-Konert C, Wagner C, Denefleh B, Hug F, Schneider M, Andrassy K, Hansch GM. 2002. Up-regulation of the dendritic cell marker CD83 on polymorphonuclear neutrophils (PMN): divergent expression in acute bacterial infections and chronic inflammatory disease. Clin Exp Immunol 130:501–508. doi: 10.1046/j.1365-2249.2002.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozlow EJ, Wilson GL, Fox CH, Kehrl JH. 1993. Subtractive cDNA cloning of a novel member of the Ig gene superfamily expressed at high levels in activated B lymphocytes. Blood 81:454–461. [PubMed] [Google Scholar]
- 9.Oehler L, Majdic O, Pickl WF, Stockl J, Riedl E, Drach J, Rappersberger K, Geissler K, Knapp W. 1998. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J Exp Med 187:1019–1028. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolenski M, Cramer SO, Ehrlich S, Steeg C, Fleischer B, Avon B. 2003. Enhanced activation of CD83-positive T cells. Scand J Immunol 58:306–311. doi: 10.1046/j.1365-3083.2003.01303.x. [DOI] [PubMed] [Google Scholar]
- 11.Hock BD, Kato M, McKenzie JL, Hart DN. 2001. A soluble form of CD83 is released from activated dendritic cells and B lymphocytes, and is detectable in normal human sera. Int Immunol 13:959–967. doi: 10.1093/intimm/13.7.959. [DOI] [PubMed] [Google Scholar]
- 12.Hock BD, Haring LF, Steinkasserer A, Taylor KG, Patton WN, McKenzie JL. 2004. The soluble form of CD83 is present at elevated levels in a number of hematological malignancies. Leuk Res 28:237–241. doi: 10.1016/S0145-2126(03)00255-8. [DOI] [PubMed] [Google Scholar]
- 13.Bock F, Rossner S, Onderka J, Lechmann M, Pallotta MT, Fallarino F, Boon L, Nicolette C, Debenedette MA, Tcherepanova IY, Grohmann U, Steinkasserer A, Cursiefen C, Zinser E. 2013. Topical application of soluble CD83 induces IDO-mediated immune modulation, increases Foxp3+ T cells, and prolongs allogeneic corneal graft survival. J Immunol 191:1965–1975. doi: 10.4049/jimmunol.1201531. [DOI] [PubMed] [Google Scholar]
- 14.Eckhardt J, Kreiser S, Dobbeler M, Nicolette C, Debenedette MA, Tcherepanova IY, Ostalecki C, Pommer AJ, Becker C, Gunther C, Zinser E, Mak TW, Steinkasserer A, Lechmann M. 2014. Soluble CD83 ameliorates experimental colitis in mice. Mucosal Immunol 7:1006–1018. doi: 10.1038/mi.2013.119. [DOI] [PubMed] [Google Scholar]
- 15.Ge W, Arp J, Lian D, Liu W, Baroja ML, Jiang J, Ramcharran S, Eldeen FZ, Zinser E, Steinkasserer A, Chou P, Brand S, Nicolette C, Garcia B, Wang H. 2010. Immunosuppression involving soluble CD83 induces tolerogenic dendritic cells that prevent cardiac allograft rejection. Transplantation 90:1145–1156. doi: 10.1097/TP.0b013e3181f95718. [DOI] [PubMed] [Google Scholar]
- 16.Lan Z, Lian D, Liu W, Arp J, Charlton B, Ge W, Brand S, Healey D, DeBenedette M, Nicolette C, Garcia B, Wang H. 2010. Prevention of chronic renal allograft rejection by soluble CD83. Transplantation 90:1278–1285. doi: 10.1097/TP.0b013e318200005c. [DOI] [PubMed] [Google Scholar]
- 17.Zinser E, Lechmann M, Golka A, Lutz MB, Steinkasserer A. 2004. Prevention and treatment of experimental autoimmune encephalomyelitis by soluble CD83. J Exp Med 200:345–351. doi: 10.1084/jem.20030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aerts-Toegaert C, Heirman C, Tuyaerts S, Corthals J, Aerts JL, Bonehill A, Thielemans K, Breckpot K. 2007. CD83 expression on dendritic cells and T cells: correlation with effective immune responses. Eur J Immunol 37:686–695. doi: 10.1002/eji.200636535. [DOI] [PubMed] [Google Scholar]
- 19.Prechtel AT, Turza NM, Theodoridis AA, Steinkasserer A. 2007. CD83 knockdown in monocyte-derived dendritic cells by small interfering RNA leads to a diminished T cell stimulation. J Immunol 178:5454–5464. doi: 10.4049/jimmunol.178.9.5454. [DOI] [PubMed] [Google Scholar]
- 20.Kruse M, Rosorius O, Kratzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol 74:7127–7136. doi: 10.1128/JVI.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow G, Slobedman B, Cunningham AL, Abendroth A. 2003. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J Virol 77:4950–4959. doi: 10.1128/JVI.77.8.4950-4959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senechal B, Boruchov AM, Reagan JL, Hart DN, Young JW. 2004. Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood 103:4207–4215. doi: 10.1182/blood-2003-12-4350. [DOI] [PubMed] [Google Scholar]
- 23.McLauchlan J, Addison C, Craigie MC, Rixon FJ. 1992. Noninfectious L-particles supply functions which can facilitate infection by HSV-1. Virology 190:682–688. doi: 10.1016/0042-6822(92)90906-6. [DOI] [PubMed] [Google Scholar]
- 24.Rixon FJ, Addison C, McLauchlan J. 1992. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J Gen Virol 73:277–284. doi: 10.1099/0022-1317-73-2-277. [DOI] [PubMed] [Google Scholar]
- 25.Sathananthan B, Rodahl E, Ekberg T, Langeland N, Haarr L. 1996. Two-dimensional gel analysis of [35S]methionine labelled and phosphorylated proteins present in virions and light particles of herpes simplex virus type 1, and detection of potentially new structural proteins. Virus Res 46:1–18. [DOI] [PubMed] [Google Scholar]
- 26.Whitley RJ. 1996. Herpesviruses, p 2–5. In Baron S. (ed), Medical microbiology, 4th ed, Chapter 68 University of Texas Medical Branch at Galveston, Galveston, TX. [PubMed] [Google Scholar]
- 27.Whitley RJ, Roizman B. 2001. Herpes simplex virus infections. Lancet 357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 28.Everett RD. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761–770. [DOI] [PubMed] [Google Scholar]
- 29.Everett RD, Boutell C, Orr A. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J Virol 78:1763–1774. doi: 10.1128/JVI.78.4.1763-1774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagglund R, Roizman B. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J Virol 78:2169–2178. doi: 10.1128/JVI.78.5.2169-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everett RD, Rechter S, Papior P, Tavalai N, Stamminger T, Orr A. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J Virol 80:7995–8005. doi: 10.1128/JVI.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maul GG, Everett RD. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J Gen Virol 75:1223–1233. doi: 10.1099/0022-1317-75-6-1223. [DOI] [PubMed] [Google Scholar]
- 33.Gu H, Roizman B. 2003. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc Natl Acad Sci U S A 100:8963–8968. doi: 10.1073/pnas.1533420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everett RD, Maul GG. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J 13:5062–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Everett RD, Freemont P, Saitoh H, Dasso M, Orr A, Kathoria M, Parkinson J. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J Virol 72:6581–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chelbi-Alix MK, de Thé H. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935–941. doi: 10.1038/sj.onc.1202366. [DOI] [PubMed] [Google Scholar]
- 37.Heilingloh CS, Muhl-Zurbes P, Steinkasserer A, Kummer M. 2014. Herpes simplex virus type 1 ICP0 induces CD83 degradation in mature dendritic cells independent of its E3 ubiquitin ligase function. J Gen Virol 95:1366–1375. doi: 10.1099/vir.0.062810-0. [DOI] [PubMed] [Google Scholar]
- 38.Kummer M, Turza NM, Muhl-Zurbes P, Lechmann M, Boutell C, Coffin RS, Everett RD, Steinkasserer A, Prechtel AT. 2007. Herpes simplex virus type 1 induces CD83 degradation in mature dendritic cells with immediate-early kinetics via the cellular proteasome. J Virol 81:6326–6338. doi: 10.1128/JVI.02327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coffin RS, MacLean AR, Latchman DS, Brown SM. 1996. Gene delivery to the central and peripheral nervous systems of mice using HSV1 ICP34.5 deletion mutant vectors. Gene Ther 3:886–891. [PubMed] [Google Scholar]
- 40.Samady L, Costigliola E, MacCormac L, McGrath Y, Cleverley S, Lilley CE, Smith J, Latchman DS, Chain B, Coffin RS. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs− HSV vectors for dendritic cell-mediated immunotherapy. J Virol 77:3768–3776. doi: 10.1128/JVI.77.6.3768-3776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikloska Z, Bosnjak L, Cunningham AL. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J Virol 75:5958–5964. doi: 10.1128/JVI.75.13.5958-5964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sodeik B, Ebersold MW, Helenius A. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol 136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. 2006. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 44.Budt M, Hristozova T, Hille G, Berger K, Brune W. 2011. Construction of a lytically replicating Kaposi's sarcoma-associated herpesvirus. J Virol 85:10415–10420. doi: 10.1128/JVI.05071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLauchlan J, Rixon FJ. 1992. Characterization of enveloped tegument structures (L particles) produced by alphaherpesviruses: integrity of the tegument does not depend on the presence of capsid or envelope. J Gen Virol 73:269–276. doi: 10.1099/0022-1317-73-2-269. [DOI] [PubMed] [Google Scholar]
- 46.Szilagyi JF, Cunningham C. 1991. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J Gen Virol 72:661–668. doi: 10.1099/0022-1317-72-3-661. [DOI] [PubMed] [Google Scholar]
- 47.Dargan DJ, Patel AH, Subak-Sharpe JH. 1995. PREPs: herpes simplex virus type 1-specific particles produced by infected cells when viral DNA replication is blocked. J Virol 69:4924–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibiricu I, Maurer UE, Grunewald K. 2013. Characterization of herpes simplex virus type 1 L-particle assembly and egress in hippocampal neurons by electron cryo-tomography. Cell Microbiol 15:285–291. doi: 10.1111/cmi.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldwich A, Prechtel AT, Muhl-Zurbes P, Pangratz NM, Stossel H, Romani N, Steinkasserer A, Kummer M. 2011. Herpes simplex virus type I (HSV-1) replicates in mature dendritic cells but can only be transferred in a cell-cell contact-dependent manner. J Leukoc Biol 89:973–979. doi: 10.1189/jlb.0310180. [DOI] [PubMed] [Google Scholar]
- 50.Raftery MJ, Moncke-Buchner E, Matsumura H, Giese T, Winkelmann A, Reuter M, Terauchi R, Schonrich G, Kruger DH. 2009. Unravelling the interaction of human cytomegalovirus with dendritic cells by using SuperSAGE. J Gen Virol 90:2221–2233. doi: 10.1099/vir.0.010538-0. [DOI] [PubMed] [Google Scholar]
- 51.Dargan DJ, Subak-Sharpe JH. 1997. The effect of herpes simplex virus type 1 L-particles on virus entry, replication, and the infectivity of naked herpesvirus DNA. Virology 239:378–388. doi: 10.1006/viro.1997.8893. [DOI] [PubMed] [Google Scholar]
- 52.Silverman JM, Reiner NE. 2011. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol 13:1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 53.Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Strobel T, Breakefield XO, Saydam O. 2013. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther 21:101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 56.Hosseini HM, Fooladi AA, Nourani MR, Ghanezadeh F. 2013. The role of exosomes in infectious diseases. Inflamm Allergy Drug Targets 12:29–37. doi: 10.2174/1871528111312010005. [DOI] [PubMed] [Google Scholar]
- 57.Wurdinger T, Gatson NN, Balaj L, Kaur B, Breakefield XO, Pegtel DM. 2012. Extracellular vesicles and their convergence with viral pathways. Adv Virol 2012:767694. doi: 10.1155/2012/767694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM. 2010. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. 2010. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A 107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smiley JR. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J Virol 78:1063–1068. doi: 10.1128/JVI.78.3.1063-1068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weir JP. 2001. Regulation of herpes simplex virus gene expression. Gene 271:117–130. doi: 10.1016/S0378-1119(01)00512-1. [DOI] [PubMed] [Google Scholar]