Abstract
Objective
To study the effects of an acute lifestyle change in human semen oxidative stress (OS) by applying seminal parameters and OS markers and to study the feasibility of mid-infrared spectroscopy with Fourier transform infrared spectroscopy (FT-IR) as a complementary tool to evaluate the effects of OS on human sperm samples.
Material and methods
Sperm samples were collected from healthy male students (n=8) who voluntarily submitted themselves to acute lifestyle changes during academic festivities. The samples were obtained before and after the academic festivities and were compared by basic semen analyses and OS markers, namely with thiobarbituric acid reactive species (TBARS) and total thiol (SH) groups by spectrophotometric assays and carbonyl (CO) groups by slot blot. The samples were also submitted for spectroscopic analysis to evaluate the feasibility of FT-IR coupled with multivariate analysis to calibrate OS biomarkers. Statistical analysis was performed applying paired Wilcoxon tests.
Results
Acute lifestyle alterations during academic week festivities were associated with a significant decrease in the percentage of normal spermatozoa in the ejaculate (p=0.011) and a decrease in sperm concentration and in semen volume. Regarding OS, acute lifestyle changes promoted a significant increment of TBARS (p=0.018) and an increasing trend in the SH group. With FT-IR and multivariate analysis, it was possible to develop calibration models to the following protein OS biomarkers: SH groups and CO.
Conclusions
Acute lifestyle changes during academic festivities have negative effects on sperm quality, in both conventional seminal parameters and OS markers. The evaluation of OS biomarkers and FT-IR could improve andrology diagnosis and therapeutic follow-up.
Keywords: Human sperm, infrared spectroscopy, lifestyle, reactive oxygen species
Introduction
In the last decades, developed countries have experienced a decline in fertility rates to below replacement level, which is primarily caused by environmental and socio-cultural changes.[1,2] Approximately 15% of couples in the fertile age are affected by fertility problems, and 50% of the problems are related to males.[2–4] In a recent study, human sperm quality was shown to decrease by an acute lifestyle alteration (mainly alcohol and tobacco consumption), clearly showing that external factors may influence sperm quality, even when present for a short period.[5] Lifestyle factors, such as tobacco and alcohol, increase reactive oxygen species (ROS) production, leading to sperm oxidative stress (OS).[3,6,7] These ROS have been implicated in 30–80% of male infertility cases.[8] ROS promote the damage of spermatozoa membrane and deoxyribonucleic acid (DNA), affecting its ability to fertilize the oocyte. Male germ cells are highly prone to OS damage due to its membrane constitution, low cytoplasm content, which does not allow the storage of endogenous antioxidants and finally, alterations in transcription and translation related to the incapacity of making de novo synthesis being incapable of producing antioxidants.
These cellular events are counteracted by the high amount of antioxidants present in seminal plasma. However, semen from infertile and subfertile men was reported to have lower antioxidant compounds than from fertile men.[9]
Reactive oxygen species are continually generated in biological systems and play an important role in physiological pathways (spermatozoon maturation, capacitating, hyperactivation, acrosome reaction, and spermatozoa/oocyte fusion).[9–12] Nevertheless, if ROS are produced at levels above a certain level, due to environmental factors, primary pathologies of the male reproductive system, and systemic pathologies, all types of biomolecules will be injured.[13]
This study aimed to assess the effects of an acute lifestyle change using a validated model to evaluate OS in semen[5] and to apply Fourier transform infrared spectroscopy (FT-IR) as a novel methodological tool to measure OS damage in human sperm samples by detecting changes in the metabolic profile. This approach has the potential to select patients who would benefit most from antioxidant therapy allowing the follow-up of these therapies.
Material and methods
Sample collection and basic semen analyses
Samples were collected from[5] eight volunteers who filled a questionnaire (Appendix 1, 2) in each time point (TP) of the study [one week before (TP1) and one week after (TP2) the academic festivities] about their relevant past clinical information, periods of sexual abstinence, and alcohol and tobacco consumption. In Portugal, there is a strong tradition of the acute abuse of alcohol, tobacco, and drugs during academic festivities. In TP1, alcohol consumption was analyzed per week during the month prior to the academic festivities. The samples obtained in this TP will be used as control samples. TP2 reflected the alcohol consumed during the week of academic festivities. Semen samples obtained in TP2 will be used to evaluate the impact of lifestyle on epididymal sperm. Semen sample collection and semen basic analyses were performed according to the World Health Organization (WHO) guidelines, excluding sexual abstinence, after a signed informed consent and local ethical committee approval were obtained. Macroscopic (volume, appearance, viscosity, and liquefaction) and microscopic (concentration, motility, and morphology) analyses were performed.[5,14]
Seminal plasma was separated from spermatozoa by a 5-min centrifugation at 600 g at 4°C. Semen samples were stored at −80°C (pellet, supernatant, and total), within 1 h and 30 min following collection. The aliquots used for semen sample analyses were neither centrifuged nor frozen (Figure 1). Ethical approval was obtained from the ethics committee of Hospital Infante D. Pedro, Aveiro.
Figure 1.
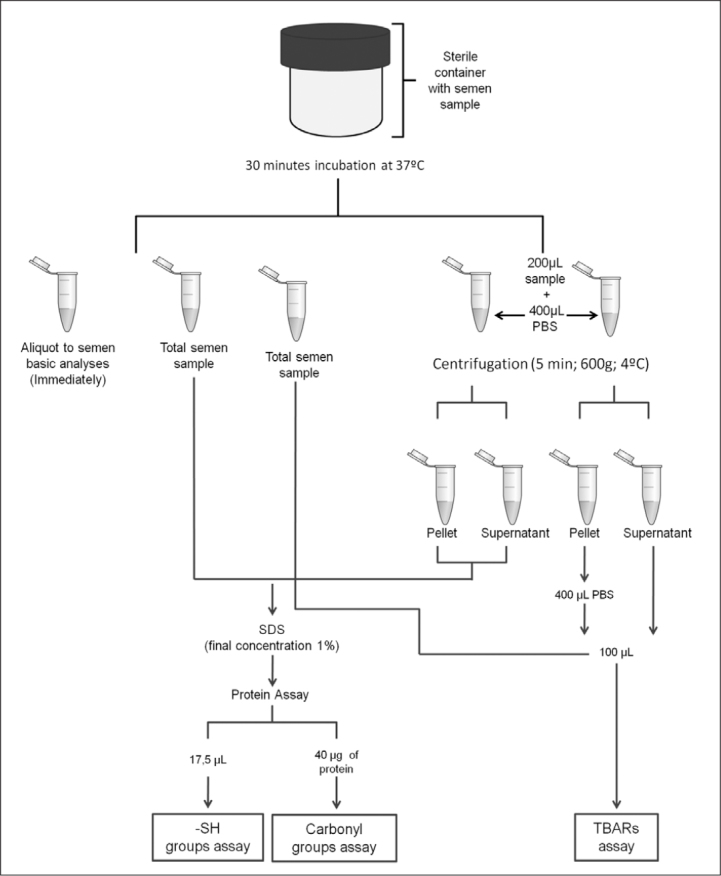
Illustrative scheme of sample handling and processing
Protein assay
Total protein measurements were conducted using Pierce bicinchoninic acid protein assay kit (Fisher Scientific, Lisbon, Portugal), following the manufacturer’s instructions.
Lipid peroxidation
Lipid peroxidation was conducted using a method based on that mentioned by Ohkawa et al.[15] Briefly, it consisted of the addition of 100 μL of sample to 200 μL of 10% trichloroacetic acid (TCA), followed by centrifugation for 20 min at 15000 g. The supernatant (200 μL) was added to 200 μl of 1% thiobarbituric acid (TBA). The samples were placed in a thermoblock for 10 min at 100°C. Next, the samples were cooled for 20 min. Absorbance was measured at 535 nm.[16]
Sulfhydryl thiol group determination
For sulfhydryl thiol (SH) group determination, the method developed by Miao-Lin Hu[17] was followed. In a tube, 17.5 μL of sample was added to 52.5 μL of Tris-ethylenediamine tetraacetic acid, 3.5 μL of 5.5′-dithiobis (2-nitrobenzoic acid) (DTNB), and 276.5 μL of methanol. The mixture was then incubated for 20 min at room temperature, followed by centrifugation at 3000 g for 10 min. Absorbance was measured at 412 nm. Three blanks were made: one without DTNB, another without the sample, and another without both.
Carbonyl group determination
For carbonyl (CO) group determination, the slot blot method was used followed by immunodetection.[18] A volume containing 40 μg of protein was derivatized with 20 mM of 2.4-dinitrophenylhydrazine dissolved in 10% trifluoroacetic acid and neutralized with 1.5 volumes of 2 M of Tris with 18% of mercaptoethanol. To perform the slot blot, the sample was diluted to 0.002 μg/μL. The samples (200 μl) were applied in the slot blot with a nitrocellulose membrane. The nitrocellulose membrane was blocked for 1 h in 5% milk in Tris-buffered saline with 1× Tween 20 (T-TBS). Next, 1:5000 rabbit anti-2.4-dinitrophenol (Merck–Millipore, Lisbon, Portugal) was added, incubated overnight, and washed twice in 1× T-TBS. The membrane was then incubated with a secondary antibody, anti-rabbit (Li-cor Bioscience, Germany), for 1 h, and was washed twice with 1× T-TBS and then twice with 1× TBS. The detection was performed in Odyssey Infrared Imaging System.
Mid infra-red spectra acquisition
Mid-infrared spectroscopy was performed for the analysis of sperm pellet samples. FT-IR spectra were recorded from 4000 cm−1 to 900 cm−1 at 8 cm−1 resolution with 128 co-added scans using a Golden Gate single reflectance attenuated total reflection in a Perkin–Elmer instrument. Three independent aliquots of each sample were analyzed. The spectra were obtained after drying kinetics. Sample aliquot (8 μl) was placed in the Golden Gate crystal and was allowed to dry, while the spectra were constantly acquired (total of 16 spectra). This concentration increases the method sensitivity by the elimination of water contribution. For multivariate calibration, the data set used included the last three spectra acquired because they were completely dry and did not compromise the reproducibility.
Multivariate calibration of infrared spectra
Spectra in the range of 4000–900 cm−1 were transferred through Joint Committee on Atomic and Molecular Physical data-Data Exchange format into the data analysis software.[19]
Several spectral pre-treatment procedures were used (standard normal deviates, SNV, and 1st and 2nd Savitzky–Golay derivatives) and spectral regions (4000–2400 cm−1, 1800–900 cm−1, and 1750–1700 cm−1) were tested to select the enhanced conditions of analysis and to find the one that gave the lower prediction error. The dimensionality (number of latent variables, LV) of the calibration model was assessed by internal cross-validation at each iteration level by examining the root mean square error of cross-validation (RMSECV) the replicates were kept together in this process.
After setting the number of LV and spectral region, multivariate calibration (partial least squares regression, PLS1) was applied to the data set (pellet semen samples, Figure 1). The spectra data set calibrated against the raw values (before normalization to protein content) obtained for OS biomarkers analysis (SH group, CO group, and lipid peroxidation) were used to calculate the values shown in Table 1.
Table 1.
Results of basic semen analyses and oxidative stress assays on TP1 and TP2 determined on the semen samples of all volunteers (C1 to C8)
| TP1 | TP2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||||
| Parameter | Volunteers | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 |
| Basic semen analyses | Volume (mL) | 8.5 | 3 | 2.5 | 2.5 | 1.5 | 1.5 | 2.5 | 2.5 | 3.5 | 2 | 2.5 | 4 | 2 | 2 | 2 | 2 |
| Concentration (106 spz/mL) | 73 | 60 | 253 | 69 | 65 | 33 | 110 | 101 | 53 | 13 | 101 | 61 | 439 | 69 | 111 | 38 | |
| Normal forms (%) | 10 | 9 | 11 | 11 | 8 | 9 | 6 | 7 | 5 | 6 | 4 | 4 | 6 | 7 | 5 | 4 | |
| Motility (%) | 74 | 58 | 67 | 65 | 54 | 59 | 63 | 71 | 70 | 51 | 53 | 71 | 63 | 46 | 74 | 67 | |
|
| |||||||||||||||||
| Lifestyle | Alcohol consumption (g/day) | 2.69 | 4.28 | 16 | 15.8 | 0 | 0 | 0 | 16 | 114 | 47.5 | 507 | 206 | 111 | 80 | 48 | 207 |
| Nicotine (mg/day) | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 2.4 | 24 | 0.34 | 0 | 0 | 0 | |
| Sexual abstinence (days) | 7 | 3 | 3 | 7 | 3 | 1 | 60 | 1 | 1 | 1 | 4 | 5 | 3 | 1 | 60 | 0 | |
|
| |||||||||||||||||
| Oxidative stress markers | TBARS (nmol/108spz) | ||||||||||||||||
| Supernatant | 0.7 | 0.3 | 0.3 | 1.7 | 0.4 | 0.9 | 0.2 | 1.3 | 0.8 | 1.8 | 2 | 1.3 | 4.8 | 1.4 | |||
| SH (μmol/μg) | |||||||||||||||||
| Total | 1.8 | 0.8 | 1.5 | 1.0 | 2.4 | 1.5 | 3.5 | 2.7 | 1.5 | 3.1 | 1.6 | 2.4 | 3.3 | 1.0 | |||
| CO (arbitrary units/μg protein) | |||||||||||||||||
| Total | 1.0 | 0.8 | 0.4 | 1.0 | 0.8 | 0.8 | 0.5 | 1.2 | 1.7 | 1.3 | 0.7 | 1.9 | 0.6 | 0.5 | |||
TP: time period; TBARS: thiobarbituric acid reactive substances; SH: thiol group; CO: carbonyl group
Statistical analysis
A longitudinal study approach was used to estimate changes in sperm quality at two distinct time periods. Each individual was followed-up at TP1 and TP2. To assess the influence of acute exposure, paired Wilcoxon tests were applied. The use of parametric or non-parametric paired t-tests was judged by Kolmogorov–Smirnov test for normality, QQ-plot graphs, and box plot. All computations were conducted in Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) 17.0.
Results
Aiming to study the changes in seminal quality after an acute lifestyle alteration basic semen analyses and OS assays were performed before (TP1) and after the academic festivities (TP2). The sample was composed of 8 male students (C1-C8) who participate in the academic festivities in Aveiro (Table 1).
Basic semen and lifestyle analyses
The volunteers had a mean age of 22.1±1.5 years (mean±SD). Sperm samples were normal according to the guidelines of the WHO except for the concentration of volunteer C2 in TP2. Regarding morphology, the results indicated a significant decrease in the percentage of normal spermatozoa in TP2 (p=0.011). Volume, concentration, and total motile spermatozoa did not reveal statistically significant differences (p=0.606, p=0.484, and p=0.575, respectively), but the results showed a tendency toward lower levels after the academic festivities (TP2).
The extent of alcohol consumption and cigarette smoking during the festivities were assessed. Considering the values obtained in the Wilcoxon test, there was a significant increase in alcohol consumption (p=0.012); however, nicotine consumption (p=0.102) and sexual abstinence (p=0.136) were not statistically significant.
Oxidative markers
Thiobarbituric acid reactive species
The thiobarbituric acid reactive species (TBARS) assay results were normalized to the spermatozoa. When comparing the volunteers from TP1 to TP2, there was a significant increase in supernatant (p=0.018) TBARS (Figure 2), indicating an increase of lipid peroxidation. Oxidative markers were not determined in volunteer C6 because the sample was too viscous and could not be normally processed.
Figure 2.
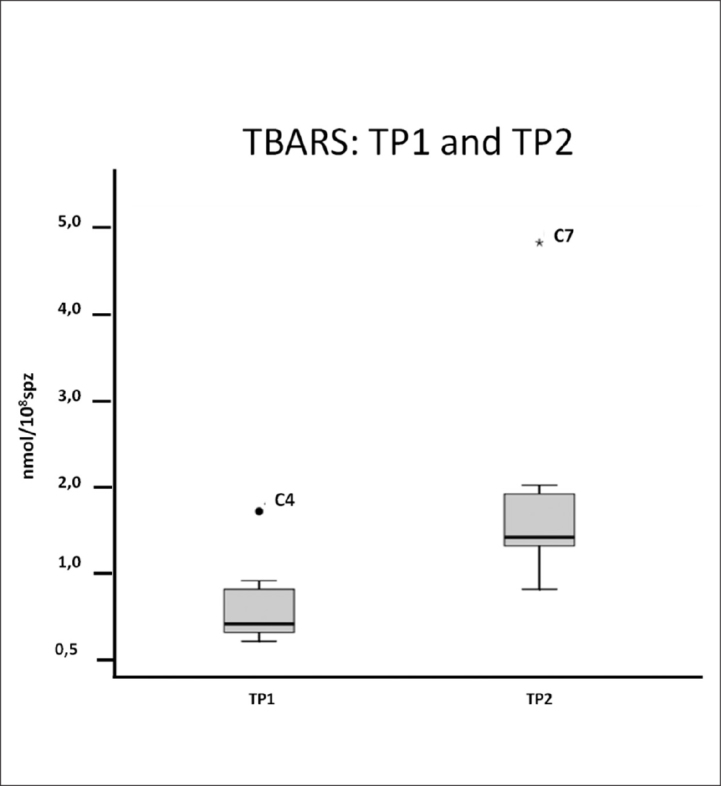
Box plot of the TBARS assay in the supernatant of the sample. An increase in median in TP2 was observed. Volunteer C4 is considered an outlier in TP1 and C7 is in TP2
Total SH group determination
To address the total SH group content, values were normalized to the amount of protein. Regarding the Wilcoxon analysis, no significant differences (p=0.345) were observed between the volunteers from TP1 to TP2, but there was an increasing trend when considering the results of the total sample (Figure 3).
Figure 3.
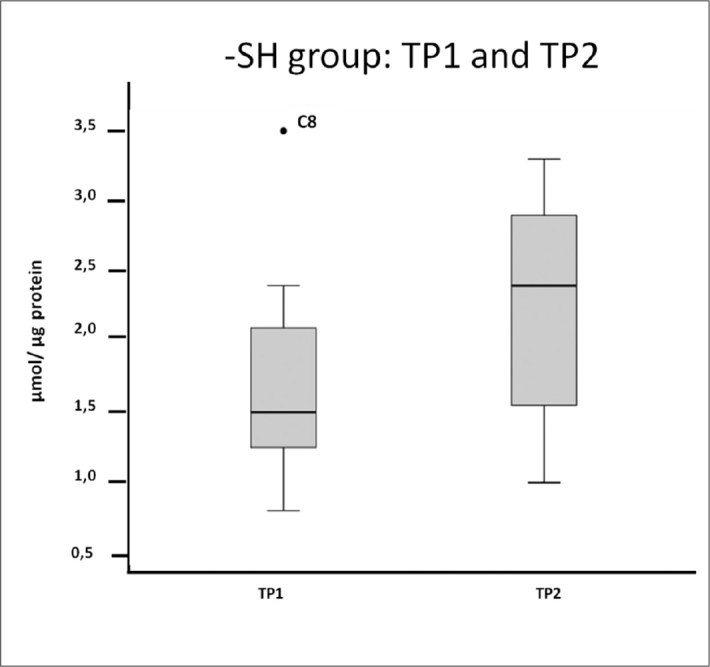
Box plot of total SH groups in the total sample. An increase in the median in TP2 was observed. Volunteer C8 is considered an outlier in TP1
CO groups
Protein susceptibility to oxidation was also evaluated in TP2 and was compared with that in TP1 by the CO group assay with the slot blot technique. The results showed no significantly statistical alterations between TP1 and TP2 (Figure 4, 5).
Figure 4.

Carbonyl group determined by slot blot in TP1 and TP2. Box plot of the densitometry of the bands obtained in the slot blot (n=3)
Figure 5.
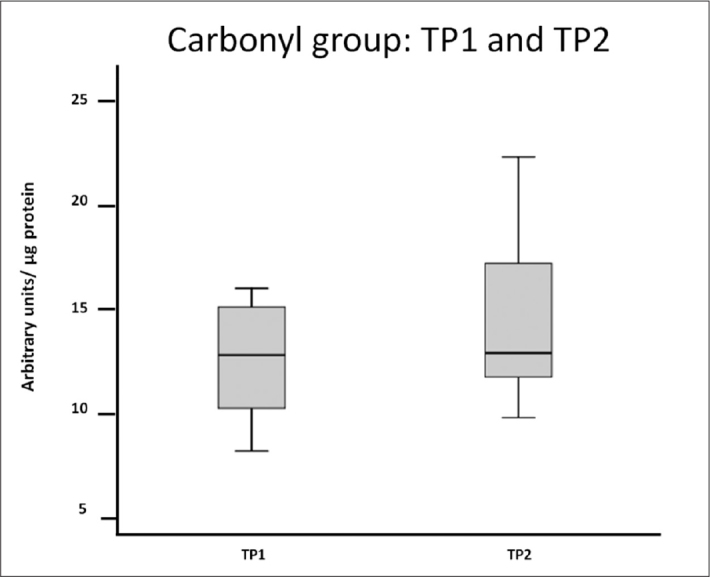
Carbonyl group determined by slot blot in TP1 and TP2. Samples were normalized to μg of protein
Fourier transform: Infrared spectroscopy calibration
Figure 6 shows the typical sperm pellet FT-IR. The spectral region 1800–900 cm−1 has a larger amount of information. The bands at 1634 cm−1 and 1538 cm−1 were respectively assigned to the amide I (C=O symmetric stretching) and amide II (strong N-H in plane bending) absorption of cell proteins. The band around 1450 cm−1 and 1394 cm−1 arises from the CH3 bending modes of proteins with the contribution of the vibrations of fatty acids. The bands at 1238 cm−1 and around 1060 cm−1 were assigned to the phosphate of nucleic acids (phosphate ester C-O-P). The band at around 976 cm−1 was assigned to C-O and C-C of nucleic acids.
Figure 6.
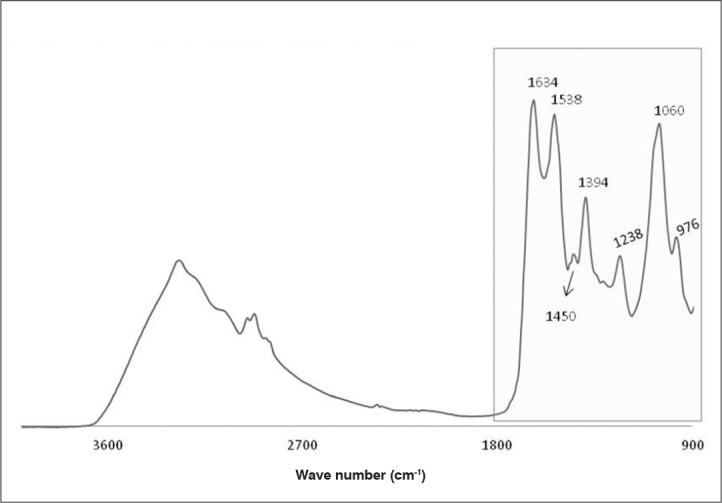
Typical FT-IR of pellet semen sample after dry drop
Multivariate calibration was tested for OS biomarkers (SH and CO groups and lipid peroxidation). After test data pre-treatments and spectral regions, the calibration was possible for the SH and CO groups.
The model for the SH group content estimation was built using 1800–900 cm−1 spectral region, whereas 1750–1700 cm−1 was used for the CO group. SNV was the pre-treatment applied to both calibrations with reasonable calibration/prediction errors (Figure 6). In b vectors, it is possible to identify the main FT-IR regions related to the quantification of the SH (Figure 7) and CO groups (Figure 8).
Figure 7.
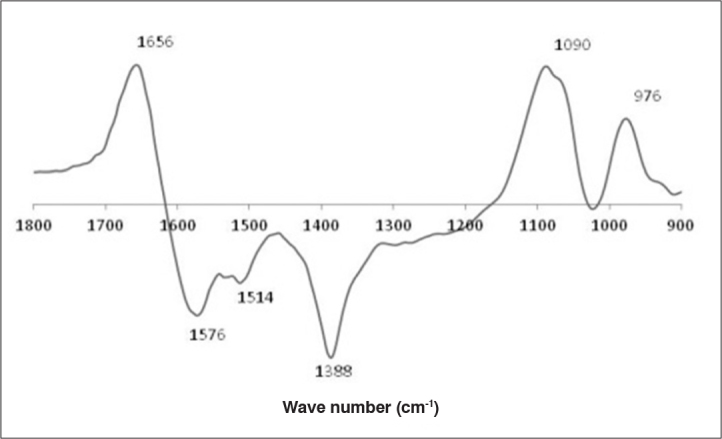
b vector plot for the calibration of protein oxidative stress thiol groups (SH)
Figure 8.
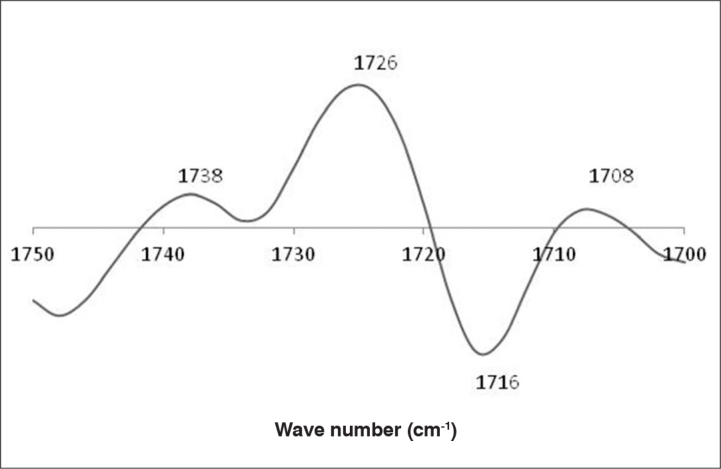
b vector plot for the calibration of protein oxidative stress carbonyl groups (CO)
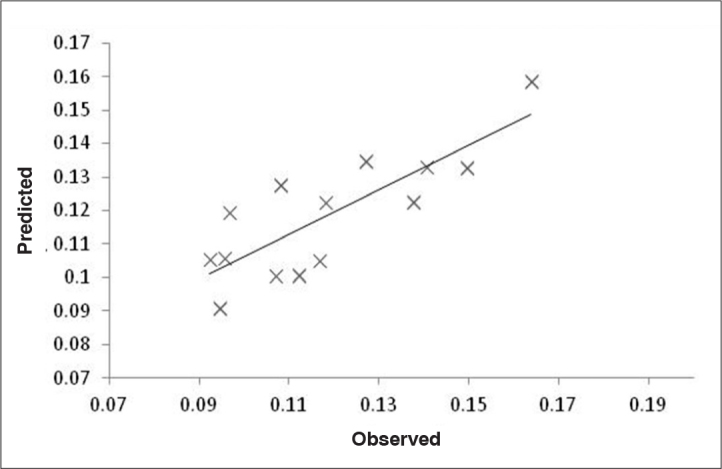
The calibration of SH groups was positively correlated to spectroscopic signals at 1656, 1090, and 976 cm−1 and negatively correlated to spectroscopic signals at 1576, 1514, and 1388 cm−1. The signals at 1656 and 1576 cm−1 were related to protein conformation and are therefore indirectly correlated to the presence of SH groups in semen proteins (Figure 7). The calibration of CO groups was positively correlated to spectroscopic signals at 1738, 1726 and 1708 cm−1 and negatively correlated to 1716 cm−1. All these signals were correlated to the CO groups in lipids (1738 and 1726 cm−1) and proteins (1716 and 1708 cm−1) (Figure 7). The relationship between the observed and predicted values shows that the calibration model has a reasonable predictive ability for the considered range of the SH (Figure 9) and CO (Figure 10) groups.
Figure 9.
Regression line built using observed and predicted values for protein oxidative stress SH. SH calibration needed 1 latent variable to capture the main sources of variations. Those calibrations are associated with reasonable calibration/prediction errors. For SH groups, a predictive power with a correlation of 0.72, a relative RMSECV of 12.5%, and a root mean square error of prediction (RMSEP) of 13.4% were obtained
Figure 10.
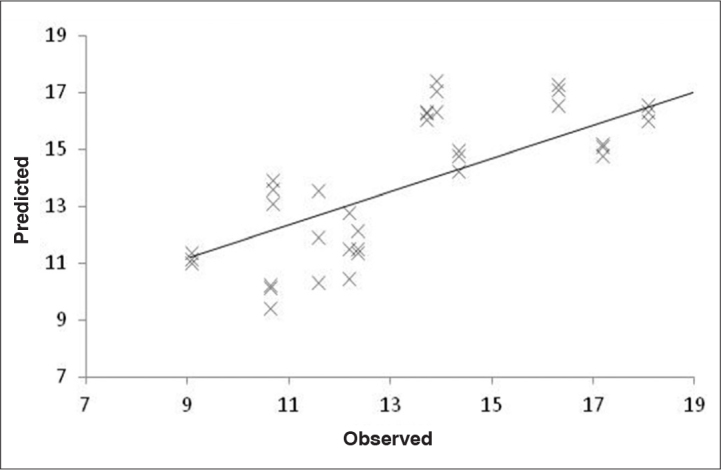
Regression line built using observed and predicted values for protein oxidative stress CO. CO calibration needed 2 latent variables to capture the main sources of variations. Those calibrations are associated with reasonable calibration/prediction errors. For CO groups, a predictive power with a correlation of 0.70, a relative RMSECV of 17.2%, and RMSEP of 19.1% were obtained
Discussion
To determine if an acute lifestyle change alters the basic seminal parameters and oxidative seminal state, human semen samples were collected on the week before (TP1) and one week after (TP2) Aveiro academic festivities, which have a strong tradition of abusive nicotine and alcohol consumption.[5] The volunteers answered a lifestyle questionnaire in each sampling TP (1 and 2) regarding, in the prior month and preceding week.[5]
Concerning the answers of the 8 volunteers to the questionnaire (Table 1), a significant increase in alcohol consumption during the academic festivities was verified. Concerning nicotine consumption, there is an increase in 3 (C3, C4, and C5) of the 8 volunteers. The results for sexual abstinence in the second collection revealed a tendency to decrease. There are other factors that may interfere in semen quality that are not possible to correctly quantify, such as alterations in circadian cycle, dietary habits, etc.
Basic semen analyses were performed for the same of the 8 volunteers. The seminal parameters evaluated revealed a significant decrease in the percentage of the normal forms (p=0.011). The motility in both collections was similar in median. The calculus of motility was based on the guidelines of WHO 2010, which consider progressive motile, non-progressive motile, and immotile spermatozoa. This may influence the motility results because the progressive motility increase could not be synonymous of rapid progressive motility. Regarding concentration, the values decreased on average in TP2. In volume, there were no significant differences despite the decreasing trend observed in TP2. The results presented are in accordance with those of a previous study where the same study model was used.[5] The innovation of the present study is the evaluation of oxidative state alterations after the academic festivities as a result of a massive acute lifestyle alteration. The statistical analysis to the TBARS supernatant content confirmed that there were significant differences when normalized to the number of spermatozoa. Lipid peroxidation reflects the sum effect of several factors including radical production, high polyunsaturated fatty acid content in the membrane, temperature in the extracellular medium, and antioxidant defense mechanism. TBARS increase in TP2 suggests an increase in ROS production or a decrease in antioxidants due to a marked increase in alcohol consumption, for example. It has been described by Kumar et al.[3] that the consumption of alcohol and tobacco, for example, increases the amount of leukocytes, which are a major producer of ROS in sperm, and decreases antioxidant defense in the supernatant. The increase verified in TBARS concentration could be related to the decrease in normal forms observed in basic semen analyses because lipid peroxidation compromises spermatozoa membrane integrity.
To analyze total semen protein oxidation, the SH and CO groups were evaluated. The free SH groups from TP1 to TP2 have a tendency to increase, suggesting lower protein susceptibility to oxidation.[20] This fact could be justified by an increase in the amount of glutathione, a peptide with antioxidant functions through its cysteine residue.[21,22] This increase could reveal that the semen sample is under oxidant conditions caused by acute lifestyle changes and that the organism is producing more antioxidants as a defense mechanism. However, the CO groups revealed no statistical differences between TP1 and TP2 (Figure 4, 5). Probably an increase in the sample number would be necessary to draw any conclusions.
FT-IR in tandem with PLS1 regression was used to quantify OS biomarkers in human sperm pellet samples, indicating its potential to be used as an alternative method to quantify these parameters. It is important to emphasize that besides the apparent good predictive power (considering the nature of the samples and size of the data set), prevision with an external data set was not possible due to the dimension of the data set (n=7).
The impossibility of building a calibration model for lipid OS could be related to the fact that lipid peroxidation includes complex chain reactions. It is possible that biochemical determination and spectrum acquisition may not have coincided in the reaction step. Additionally, this OS biomarker is quantified by an indirect methodology of the analysis of lipid peroxidation (TBARS analysis) that may not be correlated to functional groups behind spectroscopic signals.
Reactive oxygen species can damage spermatozoa biomolecules, contributing to the increase in abnormal forms, and induce sperm apoptosis, explaining the reduction in sperm concentration from TP1 to TP2. This process can be justified by the disruption of mitochondrial membrane integrity, thus releasing cytochrome c, which leads to apoptosis signaling cascade events and activation of caspase 3 and 9. A previous study based on samples from young donors in academic festivities studied cleaved poly (ADP-ribose) polymerase (PARP) showed that there was a statistically significant increase of 53% in cleaved PARP levels in the motile fraction sperm ejaculated from volunteers from TP1 to TP2. [5] OS increase in TP2 shown here may explain PARP increase in the “Para o Frasco 2010.”[5] Spectroscopic results suggest that it is possible to apply protein oxidative calibration models for real-time analysis in a clinical context and use them as a potential diagnostic tool to more clearly understand male infertility. Furthermore, when compared to the classical approaches of analysis, FT-IR is environmentally sustainable and also has economic advantages. It also requires a less amount of sample and few processing steps and is more precise and robust.
In conclusion, in this pilot study, we have shown that acute lifestyle changes (particularly increase in alcohol consumption) may influence sperm oxidative parameters. This occurs with TBARS, which significantly increased from TP1 to TP2 correlating positively with alcohol consumption. The other evaluated parameters (level of the CO and SH groups) did not change significantly, possibly due to the small number of samples. Nevertheless, we demonstrated that the application of the techniques used to evaluate oxidative damage is possible in human sperm. Further, FT-IR has the potential to be used as an alternative method to quantify human sperm oxidative sperm parameters.
Appendix 1
Study on the influence of student’s abusive behavior on the sperm during the academic week festivities
Note: This questionnaire is confidential and exclusively designed to be used in this research project and subsequent studies. Personal information related to the e-mail address and telephone contact will only be used to send the respective spermogram results and to give information related to the next phases of the study
| Volunteer no.: |
|---|
| E-mail address |
| Telephone number (optional) |
| Date of collection |
| Hour of collection |
| Sample reception hour |
| Spermogram examination hour |
| Do you want to receive the spermograms results? (Yes/No) |
| General information |
|---|
| Age: |
| No. of abstinence days: |
| Current or past relevant diseases (this includes past oncological treatments and current consumption of medicines and dietary supplements): |
| No. of children: |
| Usual consumption |
|---|
| Tobacco (average no. of cigarettes per day in the last 30 days) |
| Alcohol (daily average of the last month) describe the type of drinks and their respective amounts |
| Drugs (daily average of the last month) describe the type and quantities |
| Drugs (history of past consumption) |
Appendix 2
Study on the influence of student’s abusive behaviors on the sperm during the academic week festivities
Note: This questionnaire is confidential and exclusively designed to be used in this research project and subsequent studies. Personal information related to the e-mail address and telephone contact will only be used to send the respective spermogram results and to give information related to the next phases of the study
| Volunteer no.: |
|---|
| E-mail address |
| Telephone number (optional) |
| Date of collection |
| Hour of collection |
| Sample reception hour |
| Spermogram examination hour |
| Do you want to receive the spermogram results? (Yes/No) |
| General information |
|---|
| Age: |
| No. of abstinence days: |
| Current or past relevant diseases (this includes past oncologic treatments and current consumption of medicine and dietary supplements): |
| No. of children: |
| Consumption during the academic week |
|---|
| Tobacco (average no. of cigarettes per day) |
| Alcohol: describe the type of drinks and their respective amounts (during the academic week) |
| Drugs: describe the type and quantities (during the academic week) |
Footnotes
Ethics Committee Approval: Ethics committee approval was obtained.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - J.V.S., V.S., M.F.; Design - J.V.S., V.S., M.F.; Supervision - A.N., R.F., M.F.; Funding - M.F.; Materials - A.N., R.F., M.F.; Data Collection and/or Processing - C.L., A.N., I.C., R.S.; Analysis and/or Interpretation - D.F.C., J.V.S., A.N.; Literature Review - D.F.C., C.L., J.V.S., A.N.; Writer - D.F.C., C.L., J.V.S., A.N.; Critical Review - A.N., R.F., J.V.S., M.F.
Conflict of Interest: The authors declared no conflict of interest.
Financial Disclosure: This work was funded by Centre for Fertility Studies (FertiCentro). The work is also supported by “FCT-Fundação para a Ciência e Tecnologia (PTDC/DTP-PIC/0460/2012) and cofinanced by FEDER through “Eixo I do Programa Operacional Fatores de Competitividade (POFC) do QREN” (COMPETE: FCOMP-01-0124-FEDER-028692).
References
- 1.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–12. doi: 10.1093/humrep/dem046. http://dx.doi.org/10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 2.Cui W. Mother or nothing: the agony of infertility. Bull World Health Organ. 2010;88:881–2. doi: 10.2471/BLT.10.011210. http://dx.doi.org/10.2471/BLT.10.011210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, Kumari A, Murarka S. Lifestyle factors in deteriorating male reproductive health. Indian J Exp Biol. 2009;47:615–24. [PubMed] [Google Scholar]
- 4.Lombardo F, Sansone A, Romanelli F, Paoli D, Gandini L, Lenzi A. The role of antioxidant therapy in the treatment of male infertility: an overview. Asian J Androl. 2011;13:690–7. doi: 10.1038/aja.2010.183. http://dx.doi.org/10.1038/aja.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira M, Silva JV, Silva V, Barros A, Cruz e Silva OAB, Fardilha M. Lifestyle influences human sperm functional quality. Asian Pac J Reprod. 2012;1:224–30. http://dx.doi.org/10.1016/S2305-0500(13)60081-0. [Google Scholar]
- 6.Moustafa MH, Sharma RK, Thornton J, Mascha E, Abdel-Hafez MA, Thomas AJ, Agarwal A. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod. 2004;19:129–38. doi: 10.1093/humrep/deh024. http://dx.doi.org/10.1093/humrep/deh024. [DOI] [PubMed] [Google Scholar]
- 7.Toro J, Rodrigo R. Oxidative Stress: Basic Overview. In: Rodrigo R, editor. Oxidative Stress and Antioxidants-Their Role in Human Disease. New York: Nova Biomedical Books; 2009. pp. 1–24. [Google Scholar]
- 8.Swan SH. Semen quality in fertile US men in relation to geographical area and pesticide exposure. Int J Androl. 2006;29:62–8. doi: 10.1111/j.1365-2605.2005.00620.x. http://dx.doi.org/10.1111/j.1365-2605.2005.00620.x. [DOI] [PubMed] [Google Scholar]
- 9.Shamsi MB, Venkatesh S, Tanwar M, Talwar P, Sharma RK, Dhawan A, et al. DNA integrity and semen quality in men with low seminal antioxidant levels. Mutat res. 2009;665:29–36. doi: 10.1016/j.mrfmmm.2009.02.017. http://dx.doi.org/10.1016/j.mrfmmm.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Aitken RJ, Baker MA, De Iuliis GN, Nixon B. New insights into sperm physiology and pathology. Handb Exp Pharmacol. 2010:99–115. doi: 10.1007/978-3-642-02062-9_7. http://dx.doi.org/10.1007/978-3-642-02062-9_7. [DOI] [PubMed] [Google Scholar]
- 11.Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl. 2011;13:43–52. doi: 10.1038/aja.2010.76. http://dx.doi.org/10.1038/aja.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010;48:425–35. [PubMed] [Google Scholar]
- 13.Agarwal A, Sekhon LH. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil (Camb) 2010;13:217–25. doi: 10.3109/14647273.2010.532279. http://dx.doi.org/10.3109/14647273.2010.532279. [DOI] [PubMed] [Google Scholar]
- 14.WHO. WHO laboratory manual for the Examination and processing of human semen. 5th edition. World Health Organization; 2010. [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. http://dx.doi.org/10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Magalhães J, Ascensão A, Soares JM, Ferreira R, Neuparth MJ, Oliveira J, et al. Acute and chronic exposition of mice to severe hypoxia: the role of acclimatization against skeletal muscle oxidative stress. Int J Sports Med. 2005;26:102–9. doi: 10.1055/s-2004-817858. http://dx.doi.org/10.1055/s-2004-817858. [DOI] [PubMed] [Google Scholar]
- 17.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–5. doi: 10.1016/s0076-6879(94)33044-1. http://dx.doi.org/10.1016/S0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 18.Ascensao A, Magalhaes J, Soares J, Ferreira R, Neuparth M, Marques F, et al. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol. 2005;100:451–60. doi: 10.1016/j.ijcard.2004.11.004. http://dx.doi.org/10.1016/j.ijcard.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Barros A. PhD Thesis. France: Institut National Agronomique Paris-Grignon; 1999. Contribution à la sélection et la comparaison de variables caractéristiques. [Google Scholar]
- 20.Shiva M, Gautam AK, Verma Y, Shivgotra V, Doshi H, Kumar S. Association between sperm quality, oxidative stress, and seminal antioxidant activity. Clin Biochem. 2010;44:319–24. doi: 10.1016/j.clinbiochem.2010.11.009. http://dx.doi.org/10.1016/j.clinbiochem.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Bansal AK, Bilaspuri GS. Oxidative stress alters membrane sulfhydryl status, lipid and phospholipid contents of crossbred cattle bull spermatozoa. Anim Reprod Sci. 2008;104:398–404. doi: 10.1016/j.anireprosci.2007.06.017. http://dx.doi.org/10.1016/j.anireprosci.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Bansal AK, Kaur ARJ. Cooperative functions of manganese and thiol redox system against oxidative stress in human spermatozoa. J Hum Reprod Sci. 2009;2:76–80. doi: 10.4103/0974-1208.57227. http://dx.doi.org/10.4103/0974-1208.57227. [DOI] [PMC free article] [PubMed] [Google Scholar]


