Abstract
Patient: Male, 49
Final Diagnosis: Type 2 diabetes
Symptoms: —
Medication: —
Clinical Procedure: —
Specialty: Endocrinology and Metabolic
Objective:
Unusual clinical course
Background:
Type 2 diabetes is a well described extra-hepatic manifestation of hepatitis C infection (HCV). Eradication of HCV has led to improvements in insulin resistance but to date has not been shown to induce remission of diabetes.
Case Report:
We report a case of a 49-year-old man with HCV and a 2-year history of T2DM on oral agents. He was initially treated with peg-interferon/ribavirin (peg-IFN/rib) but did not achieve a HCV treatment response. Four years later he was retreated with peg-IFN/rib plus an HCV protease inhibitor (boceprevir). His HbA1c at the start of treatment was 7.9%. Antiviral response to HCV-therapy correlated with a significant improvement in glucose control without a change in diabetes therapy or improvement in adherence. He achieved a sustained virological response and within a year of completing antiviral therapy he no longer required medical therapy for diabetes. Two years after the completion of HCV treatment, the patient has maintained an HbA1c of 5.8% without any diabetes medications.
Conclusions:
This case provides evidence of the important relationship between HCV and diabetes and highlights the potential reversibility of glucose abnormalities with successful eradication of HCV. Increased awareness of this association may improve detection of undiagnosed HCV infection, identify patients with reversible causes of diabetes, guide therapeutic decisions for HCV treatment, and improve outcomes in patients with both diseases.
MeSH Keywords: alpha-Fetoproteins; Antiviral Agents; Carcinoma, Hepatocellular; Diabetes Mellitus, Type 2; Hepatitis C; Insulin Resistance
Background
The World Health Organization estimates that approximately 185 million people are infected with HCV worldwide [1]. Diabetes is also a global health care challenge. Approximately 347 million individuals worldwide have diabetes, with type 2 diabetes accounting for 90% of all cases [2]. An association between these two chronic diseases was first recognized in 1994 and since then several epidemiological studies have demonstrated an increased prevalence of type 2 diabetes among HCV-infected patients compared with HBV infection, chronic liver disease from other causes, and healthy controls [3–8]. The mechanism by which HCV impairs glucose metabolism is not clear. Although alterations in insulin-signalling pathways have been implicated, the process is likely multifactorial, including host factors and HCV genotype [6].
The presence of insulin resistance and type 2 diabetes in HCV has been associated with poor response to interferonribavirin HCV antiviral therapy, acceleration of liver fibrosis, and increased risk of hepatocellular carcinoma (HCC) [9–12]. Several observational studies have demonstrated that eradication of HCV is associated with improved insulin sensitivity and may reduce the long-term risk of diabetes in this population [13–15]. Case reports have described improvements in glycemic control with treatment of HCV with both IFN/Ribavirin and IFN/Ribavirin/telaprevir; however, the improvements were limited to the treatment phase [16,17]. In both these cases, there was evidence of recurrence of diabetes once HCV anti-viral therapy ended. This is the first reported case to demonstrate complete remission of diabetes with viral clearance beyond the treatment phase.
Case Report
A 49-year-old man with no history of diabetes was referred to Endocrinology with polyuria, polydipsia, fasting blood sugar of 18 mmol/L, and a hemoglobin A1c (HbA1c) of 10% (Table 1). His past medical history included hemophilia, blood transfusion acquired HCV, non-alcoholic steatohepatitis, and early-stage cirrhosis. He had previously received pegylated-interferon/ribavirin (peg-IFN/rib) treatment for HCV and did not achieve a sustained virological response (SVR). The patient was diagnosed with type 2 diabetes and was started on Metformin 500 mg twice daily and Gliclazide 80 mg twice daily. Within a month, his HbA1c was 7.7% and fasting blood sugars ranged between 5–7 mmol/L. He continued to adhere to the prescribed treatment of oral agents over the next 2 years and maintained an HbA1c between 4.6 and 8.6%
Table 1.
Hematologic and biochemical laboratory measures pre and post HCV-antiviral therapy (IFN/Rib/boceprevir) for HCV genotype 1 b.
| Investigations | June 2009 | Pre-treatment with IFN/Rib (Oct 2011) | Pre-treatment with Bocepravir (Nov 2011) | 12-months after start of treatment (Oct 2012) | 24-months after start of treatment (Oct 2013) |
|---|---|---|---|---|---|
| HbA1c (%) | 10.0 | 8.6 | 7.9 | 4.6 | 5.8a |
| Fasting glucose (mmol/L) | 18.4 | – | 9.2 | – | – |
| Random glucose (mmol/L) | – | 10.3 | 10.7 | 6.4 | 4.5 |
| Hemoglobin (g/L) | 138 | 133 | 115 | 108 | 138 |
| AST (U/L) | 175 | 168 | 129 | 42 | 24 |
| ALT (U/L) | 165 | 233 | 175 | 58 | 41 |
| GGT (U/L) | 297 | 336 | 304 | 156 | 39 |
| HCV RNA (IU/ml) | 4.68×106 b | – | 1.43×106 | Not detected | Not detected |
| Total cholesterol (mmol/L) | 5.1 c | 4.0 d | – | 4.2 | 3.36 a |
| HDL-c (mmol/L) | 0.9 c | 0.9 d | – | 1.24 | 1.50 a |
| LDL-c (mmol/L) | 3.2 c | 2.2 d | – | 2.5 | 1.56 a |
| Triglycerides (mmol/L) | 2.0 c | 1.8 d | – | 1.01 | 0.66 a |
| TSH (mU/L) | 1.90 | 1.40 | 0.89 | 2.43 | – |
| Free T4 (pmol/L) | 12.4 | – | – | – | – |
| Creatinine (umol/L) | 74 | 77 | 81 | 79 | 88 |
| Albumin (g/L) | 42 | 39 | 38 | 43 | 46 |
| AFP (ug/L) | 75.4 | 160.9 e | N/A | 2.8 f | 2.2 |
| Liver Biopsy (May 2009) | Stage III fibrosis; grade II portal and lobular activity; severe steatosis | ||||
| Fibroscan (June 2013) | Cirrhosis (left/caudate lobe hypertrophy; nodular hepatic contour). Fibrosis score of F4. | ||||
a – December 2013; b – January 2009; c – November 2009; d – January 2011; e – July 2012; f – June 2012. HbA1c – hemoglobin A1c; AST-; ALT-; GGT-; HCV – hepatitis C virus; HDL-c – high density lipoprotein cholesterol; LDL-c – low density lipoprotein cholesterol; TSH – thyroid stimulating hormone; Free T4 – free thyroxine; AFP – alpha fetoprotein.
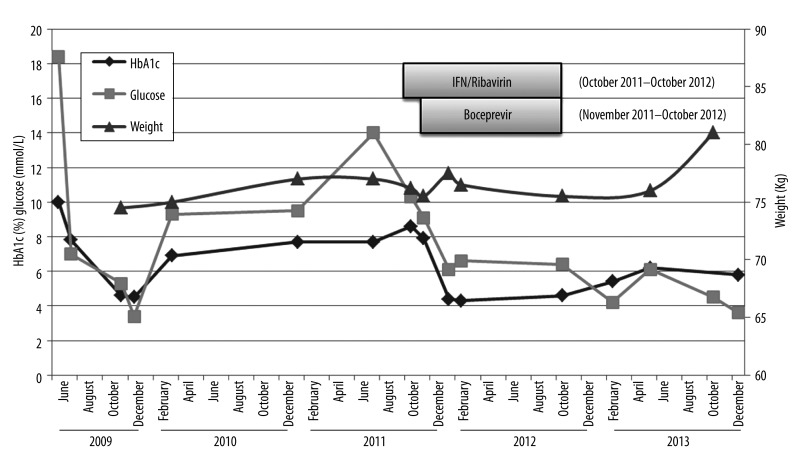
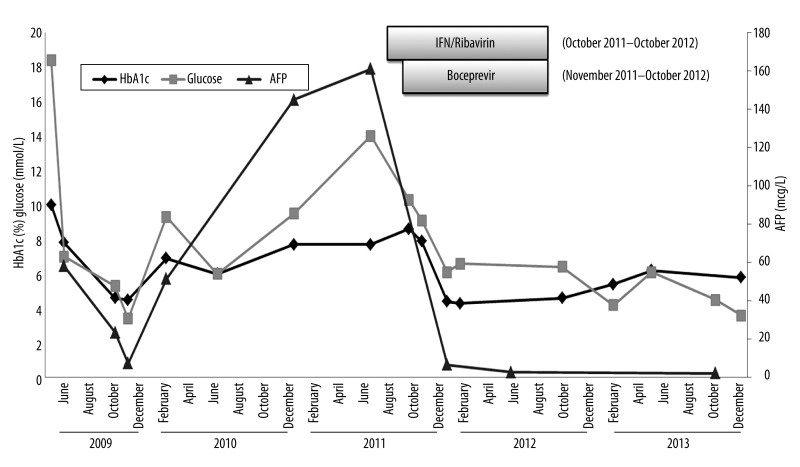
In 2011, he was retreated with a 48-week course of peg-IFN/ rib plus a HCV protease inhibitor (boceprevir). His HbA1c at the start of treatment was 7.9%, with no change in diabetes medications. Antiviral response to HCV-therapy correlated with a significant improvement in glucose control based on random glucose levels and HbA1c measurements (Figure 1). He achieved a sustained virological response (free of viremia 6 months following completion of HCV treatment) and within a year of completing antiviral therapy no longer required diabetes medications. More than 2 years after the completion of HCV antiviral treatment, the patient remains aviremic and has maintained an HbA1c of 5.8% without diabetes medication. His weight remained relatively stable during this period with no significant lifestyle changes (Figure 1). In addition to the improvements in glucose metabolism described, we also observed decreasing levels of alpha-fetoprotein (AFP) during treatment, which paralleled the improvements in HbA1c (Figure 2).
Figure 1.
Changes in glucose metabolism with Interferon/Ribavirin/boceprevir treatment for HCV genotype 1b. The above depicts the changes in hemoglobin A1c (blue line) and glucose levels (red line) that were observed in a 49-year-old man with hepatitis C genotype 1b before, during, and after hepatitis C antiviral treatment (Interferon/Ribavirin/boceprevir). Abbreviations: HbA1c-hemoglobin A1c.
Figure 2.
Changes in AFP and glucose levels with Interferon/Ribavirin/boceprevir treatment for HCV genotype 1b. The above depicts the reduction in alpha-fetoprotein levels (red line) that paralleled improvements in glucose metabolism [hemoglobin A1c (green line), glucose levels (blue line)] that were observed in a 49-year-old man with HCV genotype 1b after receiving HCV-antiviral treatment (Interferon/Ribavirin/boceprevir). Abbreviations: HbA1c hemoglobin A1c; AFP alpha-fetoprotein.
Discussion
Despite the extensive literature describing an association, type 2 diabetes is often under-recognized as an extrahepatic manifestation of HCV. Epidemiological studies estimate the prevalence type 2 diabetes among HCV-infected patients to vary between 14.5% and 33% [18]. Insulin resistance has been implicated as the precursor to development of HCV-medicated type 2 diabetes and may be present in up to 70% of HCV-infected patients [19]. This case report illustrates the relationship between HCV and type 2 diabetes, highlights the potential reversibility of glucose abnormalities with successful HCV eradication, and draws attention to the need for further research into the association between impaired insulin signalling and alpha-fetoprotein levels (AFP).
Mechanisms for impaired insulin sensitivity in HCV infection
Despite extensive research in this area, the exact mechanism by which HCV impairs insulin sensitivity and leads to increased burden of diabetes is not well understood and is likely multifactorial. HCV infection is specific to the liver. However, insulin sensitivity appears to be impaired both in the liver and the periphery [20,21]. HCV impairs insulin signaling through direct and indirect mechanisms [22]. HCV directly impacts insulin signaling by interacting with specific proteins such as serine/threonine kinases that subsequently lead to inhibition or increased degradation of insulin signaling molecules [6,23]. HCV may also indirectly cause insulin resistance in the periphery by inducing the production of pro-inflammatory cytokines that impair insulin signaling pathways in uninfected tissues [20]. Changes in metabolic parameters described with chronic HCV infection differ by genotype, suggesting that the mechanisms by which these signaling molecules are impacted are genotype-dependent. Viral steatosis is more common among HCV-genotype 3-infected patients, and, in contrast, there is a higher prevalence of insulin resistance among HCV-genotype 1 and 4 infection [6,24].
HCV treatment, insulin resistance, and glycemic control
Several observational studies have demonstrated that successful treatment of HCV with IFN/Ribavirin leads to improved insulin sensitivity and may reduce the long-term risk of type 2 diabetes [10,13–15]. Tahrani et al. reported a case of improved glycemic control and hypoglycemic episodes requiring cessation of diabetes therapies during treatment with IFNα-Ribavirin (Table 2) [16]. In contrast to our patient, the improvements in glycemic control were not maintained. After 24 weeks of treatment, HCV polymerase chain reaction remained positive and HCV treatment was stopped. Six months post-treatment, the glycemic control had deteriorated based on an HbA1c of 8.2%. The difference between the above-cited case and our patient may be due to the differences in antiviral treatment response and persistent viremia in the patient described above.
Table 2.
Case reports of HCV-infected individuals experiencing improvements in glycemic control with antiviral treatment of HCV.
| References | Sex | Age | BMI | HCV and therapy | Treatment outcome | Diabetes treatment and HbA1c (pre-antiviral therapy) | Diabetes outcomes |
|---|---|---|---|---|---|---|---|
| Tahrani et al. (2006) [15] | M | 40 | 30.8 kg/m2 | 24 weeks IFN-α/Rib | No SVR | Lispro (Humalog Mix) and Metformin HbA1c 7.7% | 6 months post-treatment: A1c 8.2% on diet alone. |
| TallÓn de Lara et al. (2014) [16] | F | 56 | 44.0 kg/m2 | IFN-α2a/Rib (48 weeks) Telaprevir (12-weeks) |
SVR | Sitagliptin 50 mg od Metformin 500 mg od HbA1c not provided |
1 month after telaprevir stopped patient resumed diabetes medications |
IFN – interferon; Rib – ribavarin; HbA1c – hemoglobin A1c.; SVR – sustained virological response.
In recent years, the treatment of HCV has evolved rapidly with the development of direct-acting antiviral (DAA) therapies (protease inhibitors, NS5a inhibitors, and nucleotide and non-nucleotide polymerase inhibitors). The effect of DAA HCV treatments on insulin resistance and long-term risk of type 2 diabetes has yet to be clearly established. A randomized controlled trial of HCV mono-infected study participants receiving 14 days of monotherapy with the protease inhibitor Danoprevir found that serum HCV RNA and HOMA-IR correlated significantly (Spearman rho=0.379, p<0.0001) [25]. At the end of 14 days of Danoprevir monotherapy, the mean decrease in HCV RNA was 2.2±1.3 log10 IU/ml (p<0.0001) in patients who received the active drug (n=40), which correlated with a decrease in mean HOMA-IR score by 1.6±1.1 (p<0.0001). In contrast, HCVRNA and HOMA-IR remained unchanged in placebo recipients.
A recently published case report further suggests that telaprevir, a NS3/4A protease inhibitor, may improve glucose metabolism (Table 2) [17]. In this case, a patient developed recurrent episodes of hypoglycemia shortly after initiating triple therapy (IFN/Ribavirin and telaprevir), which required discontinuation of all diabetes medications. Similar to the previously described case, but in contrast with our patient, improvements in glycemic control were not maintained when antiviral therapy ended, despite achieving an SVR. While the differences in outcomes may be due to host factors, the authors of this case report suggest that the protease inhibitor may have a direct antidiabetic effect. Interestingly, our patient continued to show improvement in glucose metabolism post-treatment, with diabetes therapy being reduced and eventually discontinued over 12 months. This suggests a mechanism of impairment that extends beyond the direct viral effects of HCV or HCV therapy and improvements in glucose metabolism, which may reflect decreased inflammation and liver fibrosis with improved liver function. Further studies are needed to establish the mechanisms by which HCV induces insulin resistance and the effects that different HCV therapies have in improving glycemic outcomes.
HCV, insulin resistance, and AFP
In addition to the improvements in glucose metabolism described, we observed decreasing levels of alpha-fetoprotein (AFP) during treatment, which paralleled the improvements in HbA1c (Figure 2). AFP is an oncofetal protein associated with hepatic malignancies and liver regeneration [26,27]. HCV core protein, inflammation, necrosis, and hepatocellular injury have all been suggested as causes for elevated AFP levels in chronic HCV infection [26–29]. Although type 2 diabetes and insulin resistance have been identified as risk factors for the progression of liver fibrosis and development of hepatocellular carcinoma, the mechanism by which this occurs is not clear [9–12]. The relationship between AFP and insulin resistance was recently examined in a retrospective analysis of 300 HCV-infected patients [30]. This study demonstrated that whole-body insulin resistance and hepatic fibrosis correlated directly with elevated levels of AFP. In addition, this group conducted a pilot study examining the effects of a lifestyle modification program on insulin resistance and AFP levels. Lifestyle modification over a 3-month period correlated with improved insulin resistance and a reduction in AFP levels. The parallel improvements in AFP and glycemic control demonstrated in our patient are likely in part a reflection of resolving inflammation and hepatocellular injury. However, these findings draw attention to the need for further prospective studies to understand the relationship among insulin resistance, AFP, and hepatocarcinogenesis.
Conclusions
This case highlights the interaction between HCV and type 2 diabetes, as well as the potential reversibility of impaired glucose metabolism with viral eradication. It further suggests the need for close monitoring of glucose levels and the potential need for dose adjustments of diabetes therapies during treatment of HCV to prevent hypoglycemia. Studies are needed to delineate the impact of different HCV antiviral therapies on insulin signalling pathways and the potential for improving glucose metabolism. This knowledge will help inform patient care, guide therapeutic selection, and improve liver and metabolic outcomes for HCV-infected patients with type 2 diabetes.
Furthermore, this case highlights the association between insulin resistance and AFP levels. Although the improvements in both parameters with HCV antiviral therapy are in part a reflection of decreased inflammation and liver injury, additional evaluation for causative relationship(s) among HCV, insulin resistance, AFP, and HCC are necessary.
Footnotes
Conflicts of interest
None declared.
Source of Suport: Mary-Anne Doyle was supported by a Gilead/CTN Postdoctoral Fellowship Award
References:
- 1.World Health Organization(WHO). Guidelines for the screening, care and treatment of persons with hepatitis C infection. 2014. http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/ [PubMed]
- 2.World Health Organization (WHO).\ Diabetes Fact Sheet (No.312). 2015. http://www.who.int/mediacentre/factsheets/fs312/en/
- 3.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–39. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 4.el-Zayadi AR, Selim OE, Hamdy H, et al. Association of chronic hepatitis C infection and diabetes mellitus. Trop Gastroenterol. 1998;19:141–44. [PubMed] [Google Scholar]
- 5.Huang JF, Dai CY, Hwang SJ, et al. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am J Gastroenterol. 2007;102:1237–43. doi: 10.1111/j.1572-0241.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–44. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasso A, Malfatti F, De Leo P, et al. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirin. J Hepatol. 2009;51:984–90. doi: 10.1016/j.jhep.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SH, Brancati FL, Sulkowski MS, et al. Prevalence of Type 2 diabetes mellitus among persons with hepatitix C virus infection in the United States. Ann Intern Med. 2000;133:592–99. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hung CH, Wang JH, Hu TH, et al. Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J Gastroenterol. 2010;16:2265–71. doi: 10.3748/wjg.v16.i18.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurito MP, Parise ER. Association between insulin resistance and sustained virologic response in hepatitis C treatment, genotypes 1 versus 2 and 3: systematic literature review and meta-analysis. Braz J Infect Dis. 2013;17:555–63. doi: 10.1016/j.bjid.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taura N, Ichikawa T, Hamasaki K, et al. Association between liver fibrosis and insulin sensitivity in chronic hepatitis C patients. Am J Gastroenterol. 2006;101:2752–59. doi: 10.1111/j.1572-0241.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 13.Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–44. doi: 10.1002/hep.22703. [DOI] [PubMed] [Google Scholar]
- 14.Simo R, Lecube A, Genesca J, et al. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462–66. doi: 10.2337/dc06-0456. [DOI] [PubMed] [Google Scholar]
- 15.Thompson AJ, Patel K, Chuang WL, et al. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut. 2012;61:128–34. doi: 10.1136/gut.2010.236158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahrani A, Bowler L, Singh P, Coates P. Resolution of diabetes in type 2 diabetic patient treated with IFN-alpha and ribavirin for hepatitis C. Eur J Gastroenterol Hepatol. 2006;18:291–93. doi: 10.1097/00042737-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Tallon de Lara P, Himschoot T, Frossard JL, Negro F. Does telaprevir possess a direct antidiabetic effect? Liver Int. 2014;34:967–69. doi: 10.1111/liv.12440. [DOI] [PubMed] [Google Scholar]
- 18.Huang JF, Yu ML, Dai CY, Chuang WL. Glucose abnormalities in hepatitis C virus infection. Kaohsiung J Med Sci. 2013;29:61–68. doi: 10.1016/j.kjms.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison SA. Insulin resistance among patients with chronic hepatitis C: etiology and impact on treatment. Clin Gastroenterol Hepatol. 2008;6:864–76. doi: 10.1016/j.cgh.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Milner KL, van der Poorten D, Trenell M, et al. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology. 2010;138:932–41.e1-3. doi: 10.1053/j.gastro.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 21.Vanni E, Abate ML, Gentilcore E, et al. Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C. Hepatology. 2009;50:697–706. doi: 10.1002/hep.23031. [DOI] [PubMed] [Google Scholar]
- 22.Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012;56(Suppl.1):S56–65. doi: 10.1016/S0168-8278(12)60007-5. [DOI] [PubMed] [Google Scholar]
- 23.Walsh MJ, Jonsson JR, Richardson MM, et al. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529–35. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheridan DA, Neely RD, Bassendine MF. Hepatitis C virus and lipids in the era of direct acting antivirals (DAAs) Clin Res Hepatol Gastroenterol. 2013;37:10–16. doi: 10.1016/j.clinre.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Moucari R, Forestier N, Larrey D, et al. Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut. 2010;59:1694–98. doi: 10.1136/gut.2010.219089. [DOI] [PubMed] [Google Scholar]
- 26.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74–83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- 27.Liaw YF, Tai DI, Chen TJ, et al. Alpha-fetoprotein changes in the course of chronic hepatitis: relation to bridging hepatic necrosis and hepatocellular carcinoma. Liver. 1986;6:133–37. doi: 10.1111/j.1600-0676.1986.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen TM, Huang PT, Tsai MH, et al. Predictors of alpha-fetoprotein elevation in patients with chronic hepatitis C, but not hepatocellular carcinoma, and its normalization after pegylated interferon alfa 2a-ribavirin combination therapy. J Gastroenterol Hepatol. 2007;22:669–75. doi: 10.1111/j.1440-1746.2007.04898.x. [DOI] [PubMed] [Google Scholar]
- 29.Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434–41. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi Y, Mizuta T, Eguchi Y, et al. Whole-body insulin resistance is associated with elevated serum alpha-fetoprotein levels in patients with chronic hepatitis C. Intern Med. 2013;52:2393–400. doi: 10.2169/internalmedicine.52.0992. [DOI] [PubMed] [Google Scholar]