Abstract
Introduction
Maternal T-cells reactive towards paternally inherited fetal minor histocompatibility antigens are expanded during pregnancy. Placental trophoblast cells express at least four fetal antigens, including human minor histocompatibility antigen 1 (HA-1). We investigated oxygen as a potential regulator of HA-1 and whether HA-1 expression is altered in preeclamptic placentas.
Methods
Expression and regulation of HA-1 mRNA and protein were examined by qRT-PCR and immunohistochemistry, using first, second, and third trimester placentas, first trimester placental explant cultures, and term purified cytotrophoblast cells. Low oxygen conditions were achieved by varying ambient oxygen, and were mimicked using cobalt chloride. HA-1 mRNA and protein expression levels were evaluated in preeclamptic and control placentas.
Results
HA-1 protein expression was higher in the syncytiotrophoblast of first trimester as compared to second trimester and term placentas (P<0.01). HA-1 mRNA was increased in cobalt chloride-treated placental explants and purified cytotrophoblast cells (P=0.04 and P<0.01, respectively) and in purified cytotrophoblast cells cultured under 2% as compared to 8% and 21% oxygen (P<0.01). HA-1 mRNA expression in preeclamptic vs. control placentas was increased 3.3-fold (P=0.015). HA-1 protein expression was increased in syncytial nuclear aggregates and the syncytiotrophoblast of preeclamptic vs. control placentas (P= 0.02 and 0.03, respectively).
Discussion
Placenta HA-1 expression is regulated by oxygen and is increased in the syncytial nuclear aggregates and syncytiotrophoblast of preeclamptic as compared to control placentas. Increased HA-1 expression, combined with increased preeclamptic syncytiotrophoblast deportation, provides a novel potential mechanism for exposure of the maternal immune system to increased fetal antigenic load during preeclampsia.
1. Introduction
Pregnancy is a unique biological process wherein the female is confronted with an antigenically foreign fetus that is permitted and encouraged to flourish, despite a surrounding immunological environment that can recognize it as foreign. Thus, the success of pregnancy depends on maternal immunological acceptance of the fetus. Disruption of the biological processes governing maternal-fetal interactions can lead to the development of serious pregnancy complications. Preeclampsia is a common and dangerous complication that is specific to pregnancy and affects 5–8% of pregnancies worldwide [1]. It is a syndrome characterized by new onset maternal hypertension after 20 weeks gestation and proteinuria, and if untreated, can lead to maternal seizures, multi-system organ failure and even death of both mother and baby. The only successful treatment of preeclampsia is delivery of the baby and the placenta; making preeclampsia one of the leading causes of iatrogenic prematurity [2]. Numerous studies have indicated a central, causative role for the placenta in the manifestation of preeclampsia; for example, preeclampsia can develop in molar pregnancies, in which no fetus is present [3], and further, delivery of the placenta, not the baby, relieves the maternal symptoms [4]. Although the pathophysiology of preeclampsia is not well understood, alterations in vascular reactivity leading to both increased placental oxidative stress and enhanced maternal immune activation are key components of this syndrome [5].
The placenta plays an important role in modulating the response of the maternal immune system to the baby during pregnancy. While the placenta tightly restricts its expression of the highly polymorphic human leukocyte antigens [6] [7], which govern adaptive immune recognition, it does express polymorphic fetal proteins encoded outside the HLA complex, known as minor histocompatibility antigens (mHAg) [8], which can also be recognized by maternal immune cells. Minor antigens are important in the success or failure of hematopoietic stem cell and solid organ transplants [9–11]. Exposure of an untolerized individual to these antigens, either as the result of pregnancy or allogeneic transplantation, can lead to the expansion of minor antigen-specific T cells [12, 13]. Further, the presence of minor antigen-specific T cells in allogeneic transplants has been linked to increased rates of graft rejection, graft-versus-host disease, and graft-versus-leukemia effects, indicating that, at least in the context of transplantation, these T cells are functional and pathophysiologically relevant [9, 11, 14, 15]. Recent work by our lab and others has demonstrated that the placenta is a source of fetal minor antigens during pregnancy, and thus contributes to maternal immunological recognition of the fetus [8]. T cells with specificity for paternally-inherited fetal antigens expand during pregnancy and can persist for up to 22 years following delivery of the baby [13, 16, 17]. HA-1 (also called HMHA1 for human minor histocompatibility antigen 1) functions as a Rho GTPase activating protein [23], is expressed in the human placenta [8] and is important for graft outcomes in HLA-matched hematopoietic stem cell transplantations. A study of 148 HLA-matched bone marrow transplant pairs noted that a mismatch of HA-1 alone was significantly correlated with increased graft-versus-host disease [11]. HA-1 is antigenic due to a single nucleotide polymorphism in which the arginine in the peptide sequence KECVLRDDLLEA is changed to a histidine, giving rise to the immunogenic peptide KECVLHDDLLEA. This single nucleotide polymorphism results in increased binding affinity of the peptide to the appropriate major histocompatibility complex (MHC) for immune recognition, in this case HLA-A*0201, A*0206, B*60 or B*40012 [24–28]. HA-1-specific CD8+ T cells have been identified in human pregnancies in which an HLA-A2-positive mother expresses the non-immunogenic form of the peptide and the fetus expresses the immunogenic form [13]. Given the expansion of specific T cells during pregnancy and the importance of HA-1 in transplantation immunology, we investigated the expression and regulation of HA-1 in the human placenta during pregnancy, when the placenta and fetus together act as an allograft that can be recognized by the maternal immune system.
2. Materials and Methods
2.1 Sample Collection
Samples were collected in accordance with the Helsinki Declaration using protocols approved by the Institutional Review Boards of the University of Kansas Medical Center and Saint Luke’s Hospital. First trimester placentas were obtained from women undergoing elective pregnancy terminations at the Center for Women’s Health in Overland Park, Kansas. Normal term placentas, defined as placentas from pregnancies at 37 gestational weeks or later with no noted complications other than fetal malpresentation (including intrauterine growth restriction and diabetes), were obtained following non-laboring Caesarian deliveries at the University of Kansas Medical Center and Saint Luke’s Hospital. Fixed and frozen tissues from preeclamptic placentas, together with gestational age- and mode of delivery-matched control placentas were obtained from the Research Centre for Women’s and Infants’ Health Biobank at Mount Sinai Hospital, Toronto, Canada. Control placentas were selected primarily based on the mode of delivery and gestational age, and excluded pregnancies complicated by gestational hypertension or intrauterine growth restriction. We compared the clinical parameters outlined in Table 1 and the only significant difference between the control and preeclampsia groups was that infants in the preeclampsia group weighed significantly less than those in the control group (p<0.01). This is not unexpected given the high concordance of preeclampsia and intrauterine growth restriction [29].
Table 1.
Clinical Characteristics of Preeclamptic and Matched Control Placentas
| Sample | Clinical Information |
GA (weeks/ days) |
Ethnicity | Age | Parity (Gravidity/ Parity) |
Antenatal glucocorticoids? (Y/N) |
Maternal cigarette use? (Y/N) |
BMI | Mode of Delivery |
Fetal Birth Weight |
Fetal Sex (M/F) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 - PE | PE | 34/6 | East Indian | 37 | 3/1 | N | N | 19.83 | Vaginal | 2140g | F |
| 1 - C | PTL | 34/5 | Caucasian | 31 | 1/0 | N | N | 25.91 | Vaginal | 2710g | M |
|
| |||||||||||
| 2 - PE | PE | 37/2 | Caucasian | 37 | 3/0 | N | N | 19.47 | Vaginal | 2710g | M |
| 2 - C | Labor | 37/0 | Asian | 30 | 1/0 | N | N | 21.51 | Vaginal | 2870g | M |
|
| |||||||||||
| 3 - PE | PE/HELLP | 36/5 | Caucasian | 31 | 1/0 | N/A | N | 24.34 | C-section | 2640g | M |
| 3 - C | Placenta praevia | 36/3 | Black | 35 | 2/1 | N | N | 26.78 | C-section | 2665g | M |
|
| |||||||||||
| 4 - PE | PE/HELLP/IUGR | 32/4 | Caucasian | 39 | 2/0 | Y | N | 33.96 | C-section | 1220g | F |
| 4 - C | PTL | 32/4 | Caucasian | 33 | 1/0 | Y | N | 21.1 | C-section | 2080g | M |
|
| |||||||||||
| 5 - PE | PE | 36/2 | Asian | 38 | 1/0 | N | N | 21.64 | Vaginal | 2265g | M |
| 5 - C | PTL | 35/6 | Asian | 32 | 1/0 | Y | N | 19.88 | Vaginal | 2920g | F |
|
| |||||||||||
| 6 - PE | PE/IUGR | 34/1 | Black | 24 | 1/0 | N | N | 23.62 | C-section + labor | 1550g | F |
| 6 - C | PTL | 34/1 | Caucasian | 27 | 4/1 | Y | N | 26.18 | C-section + labor | 2360g | M |
|
| |||||||||||
| 7 - PE | PE | 36/0 | Caucasian | 37 | 1/0 | N | N | 26.4 | C-section + labor | 2280g | M |
| 7 - C | PTL | 35/4 | Other | 36 | 1/0 | N | N | 21.19 | C-section + labor | 3030g | F |
|
| |||||||||||
| 8 - PE | PE/IUGR | 31/5 | Black | 38 | 4/1 | Y | N | 26.22 | Vaginal | 890g | F |
| 8 - C | PTL | 32/1 | Caucasian | 29 | 2/1 | Y | N | 17.99 | Vaginal | 1810g | M |
|
| |||||||||||
| 9 - PE | PE/IUGR | 34/4 | Native Canadian | 37 | 4/0 | Y | N | 25.15 | C-section + labor | 1673g | F |
| 9 - C | PTL | 34/6 | Caucasian | 36 | 2/1 | N | N | 35.16 | C-section + labor | 2180g | M |
GA = Gestational Age, PE = Preeclampsia, C = Control, PTL = Pre-term Labor, HELLP = Hemolysis, Elevated Liver enzymes, Low Platelets, IUGR = Intrauterine Growth Restriction, N/A = Not Available, BMI = Body Mas Index, M = Male, F = Female
2.2 Placental explant preparation
First trimester (n=6) villi were dissected and explants of approximately 500mg were cultured in 12-well plates fitted with Netwell inserts (Corning, Tewksbury, MA). Explants were cultured in DMEM-F12 media (Corning Cellgro, Herndon, VA) containing 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 5ng/ml epidermal growth factor (PeproTech, Rocky Hill, NJ), 5μg/ml insulin (Sigma-Aldrich, St. Louis, MO), 10μg/ml transferrin (Sigma-Aldrich), 100μg/ml L-glutamine (Corning Cellgro), 20nM sodium selenite (Sigma-Aldrich), 400 IU hCG (Sigma-Aldrich), 100μg/ml streptomycin (Corning Cellgro) and 100 IU penicillin (Corning Cellgro).
2.3 Cytotrophoblast isolation
Cytotrophoblast cells were isolated as previously described [30, 31]. Briefly, term placentas were collected following normal, non-laboring, Caesarian deliveries and approximately 40g of villous material was dissected. The tissue was enzymatically digested using Trypsin (Gibco, Grand Island, NY) and DNase (Sigma-Aldrich), washed, and layered over a Percoll gradient (Sigma-Aldrich). Cells were further washed and subjected to negative immuno-magnetic selection using an anti-class I (W6/32) MHC antibody. Only cell preparations resulting in greater than 90% purity as assessed by cytokeratin-7 positivity by flow cytometry were used. Spontaneous differentiation of cytotrophoblast cells cultured for 24 hours at 2%, 8% and ambient oxygen concentrations is minimal, as assessed by quantification of human chorionic gonadotropin [31].
2.4 Cobalt chloride treatment
First trimester placental explants (n=6) and purified term cytotrophoblast cells (n=5) were plated overnight at 37°C with 5% carbon dioxide, 95% normal air. The following day, cells were treated with either medium alone or media containing 100μM cobalt chloride. After 24 hours, cytotrophoblast cells were lysed in TRI Reagent (Sigma-Aldrich) and processed for RNA isolation as described below; placental explants were frozen in liquid nitrogen prior to mRNA analysis.
2.5 Oxygen cultures
After overnight plating, cytotrophoblast cells (n=6) were washed with pre-equilibrated medium and were then cultured under 2% or 8% oxygen or ambient air (21% oxygen) for 24 hours using an InVivo 300 (Ruskinn/Baker, Sanford, ME) or an H35 (Hypoxygen, Frederick, MD) hypoxia chamber, or in a standard incubator for the ambient air condition. After 24 hours, cells were collected in TRI Reagent and processed as described below.
2.6 RNA Isolation and RT-PCR
RNA was extracted from purified cytotrophoblast cells, first trimester placental explants and frozen placental tissues using TRI Reagent according to the manufacturer’s directions. The RNA concentration was quantified using spectrophotometry and 0.5μg were reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) and random primers (Invitrogen, Carlsbad, CA) in a reaction volume of 20 μl. 2 μl of the reverse transcription (RT) reaction was subjected to polymerase chain reaction (PCR) using Taq DNA polymerase (Fermentas, Glen Burnie, MD). RT-PCR for HA-1 was carried out using a TaqMan Gene Expression Assay for HA-1 (Hs00299628_m1, Applied Biosystems, Foster City, CA) or beta-actin (Hs99999903). Average fold change for each sample was calculated using ddCt values and normalized to the beta-actin control.
2.7 Immunohistochemistry
Paraformaldehyde-fixed (4%), paraffin embedded placentas cut to a thickness of 5μm were collected from preeclamptic (n=8) and gestational age- and mode of delivery-matched control women (n=8) at the Research Center for Women’s and Infants’ Health Biobank at Mount Sinai Hospital, Toronto, Canada. Slides were deparaffinized in Histo-clear (Fisher Scientific, Pittsburgh, PA), rehydrated and stained as previously described [32]. Tissues were subjected to heat antigen retrieval using Reveal buffer (BioCare Medical, Walnut Creek, CA), and nonspecific antibody binding was blocked in 10% goat serum (Sigma-Aldrich). A rabbit polyclonal anti human HA-1 antibody (Sigma-Aldrich, 12 μg/ml) or rabbit IgG (Vector Labs, Burlingame, CA, 12ug/ml) was added to tissue sections overnight at 4°C. Following addition of biotinylated goat anti-rabbit secondary antibodies (Vector Labs, 10 μg/ml) and depletion of endogenous peroxidases, binding of primary antibody was detected using streptavidin-peroxidase and aminoethyl carbazole (Invitrogen, Carlsbad, CA).
2.8 H-score Quantification of HA-1 Expression
Expression of HA-1 in first trimester, second trimester, term uncomplicated and preeclamptic placental tissues was calculated using the H-score method [33]. In the preeclamptic and matched control placentas, expression of HA-1 in the syncytiotrophoblast, syncytial nuclear aggregates and Hofbauer cells was quantified. Identification of syncytiotrophoblast and Hofbauer cells was based on location and morphology. Syncytial nuclear aggregates (SNAs) were defined as 10 or more syncytial nuclei clustered together that either protruded from a single villus or were not in contact with any other villi, as previously described [34]. Briefly, 10–20 400X images were taken of each placenta using a Nikon 80i microscope. The expression of HA-1 in each placental villus, cell or SNA was scored by a blinded reviewer as 3 (strong immunoreactivity), 2 (moderate), 1 (weak), or 0 (no immunoreactivity). At least 200 villi were quantified for each placenta. The H-score was quantified using the following formula: H-score = 3*(%strong) + 2*(%moderate) + 1*(%weak).
2.9 Statistical Analyses
All statistical analyses were conducted using the SigmaStat software (Systat, Chicago, IL). The differences between HA-1 mRNA expression in cobalt chloride-treated and untreated first trimester placental explants and purified term cytotrophoblast cells were calculated using two-tailed, paired T-tests. The differences in HA-1 mRNA expression across gestation and in purified term cytotrophoblast cells cultured at 2%, 8% and 21% oxygen were calculated using one-way ANOVAs on Ranks with Student-Newman-Keuls post-tests. The differences between HA-1 mRNA expression and H-scores in preeclamptic and normal placental tissues were calculated using two-tailed, paired T-tests. All results with a P-value of ≤ 0.05 were considered significantly different.
3. Results
3.1 HA-1 is expressed in placental tissues across gestation
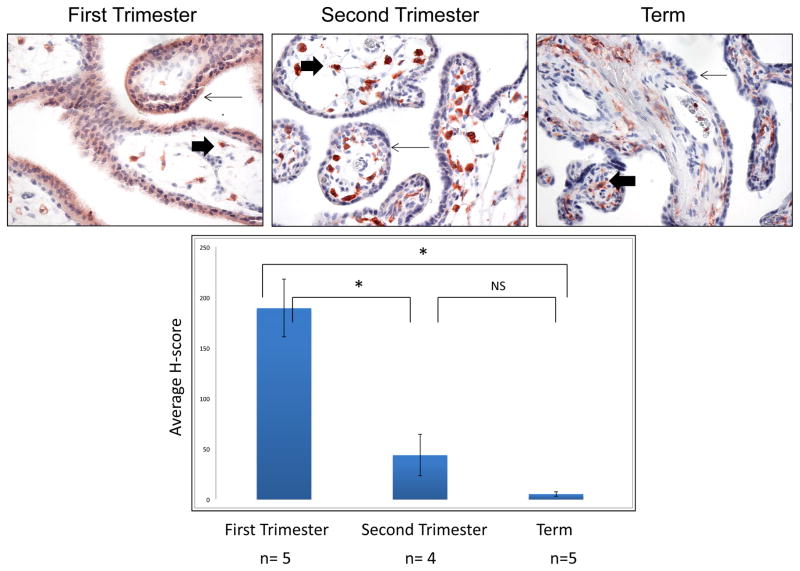
Previous work by our lab has shown that HA-1 and other mHAgs are expressed in placental lysate, purified cytotrophoblast cells and fetal cord blood [8]. Nonquantitative analysis suggested that HA-1 is strongly expressed in the syncytiotrophoblast of first trimester, but is reduced in second and third trimester placentas. To further explore the possibility of differential regulation of HA-1 in the placenta across gestation, we used semiquantitative analysis to examine expression of HA-1 protein in the syncytiotrophoblast of first (n=5), second (n=4) and third (n=5) trimester placentas (Figure 1). The overall cellular distribution of HA-1 agreed with our previous findings: Hofbauer cells from all stages of gestation exhibited strong staining for HA-1, while immunoreactivity in trophoblast populations was most evident in the first trimester. Furthermore, H-score analysis revealed that HA-1 protein was more than 4- and nearly 40-fold higher in the syncytiotrophoblast of the first trimester placentas as compared to the second (P=0.001) and third (P<0.001) trimester placentas, respectively. In contrast, second trimester syncytiotrophoblast HA-1 expression was minimal, and remained so at term (P>0.05). Although there was a small subset of second trimester and term placentas with clear expression of HA-1 in the syncytiotrophoblast, most second trimester and term placentas showed little or no HA-1 expression in the syncytiotrophoblast, suggesting that in many cases HA-1 expression in the syncytiotrophoblast may be confined to the first trimester.
Figure 1. Expression of HA-1 in the human placenta across gestation.
HA-1 expression in the syncytiotrophoblast (small arrows) and Hoffbauer cells (large arrows) of first trimester (n=5), second trimester (n=4) and term placental sections (n=5) was examined by immunohistochemistry and quantified using the H-score method. Expression of HA-1 in the syncytiotrophoblast was significantly higher in first trimester placentas as compared to second trimester (P<0.01) and third trimester placentas (P<0.01). There was no difference in HA-1 protein expression between the second and third trimester placentas (P=0.277). * = P<0.01, ns=no significance.
3.2 Cobalt chloride treatment increases the expression of HA-1 mRNA in first trimester placental explants and purified term cytotrophoblast cells
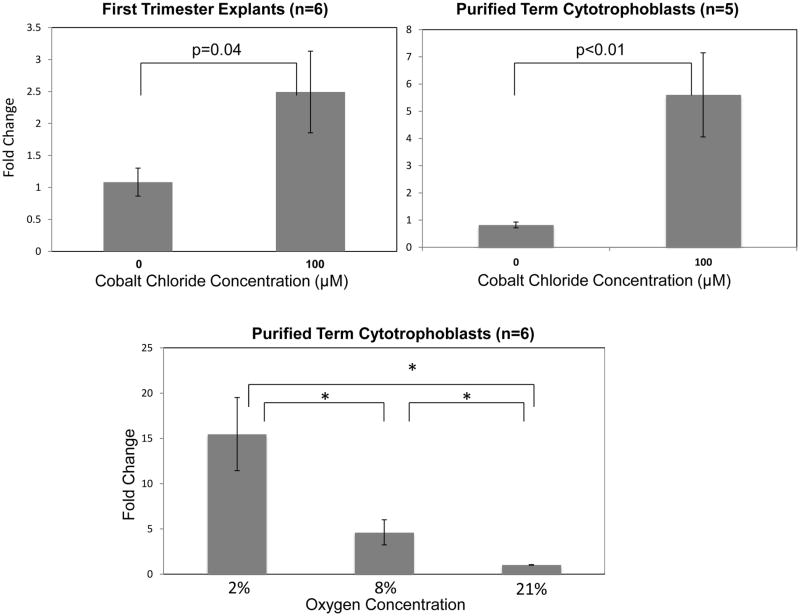
Oxygen tension in the placenta increases dramatically from the first to the second trimester of pregnancy as a result of trophoblast remodeling of the maternal spiral arteries, suggesting that oxygen could be, at least in part, responsible for the observed alterations in HA-1 expression across gestation [35]. Therefore, to examine potential regulation of HA-1 expression by oxygen, we treated first trimester placental explants and purified term cytotrophoblast cells with 100μM of the hypoxia mimetic, cobalt chloride (CoCl2). Cells or explants were treated in the presence or absence of 100μM CoCl2 for twenty-four hours, and HA-1 mRNA was quantified by real time RT-PCR (Figure 2). There was a significant increase in HA-1 mRNA expression in the first trimester placental explants (n=6, p=0.04) and in the purified term cytotrophoblast cells (n=5, p<0.01) treated with cobalt chloride as compared to the untreated controls.
Figure 2. Cobalt chloride and low oxygen increase HA-1 mRNA expression in culture.
First trimester explants (n=6) and purified term trophoblast cells (n=5) were cultured in the presence of cobalt chloride, a hypoxia mimetic. Purified term trophoblast cells from normal, C-section deliveries (n=6) were cultured in 2%, 8% or 21% oxygen for twenty-four hours. HA-1 mRNA expression, determined using qRT-PCR, was normalized to beta-actin and fold change relative to the untreated controls or 21% oxygen condition, as appropriate, was calculated using the ddCt method. HA-1 mRNA expression was elevated in the first trimester placental explants and purified term cytotrophoblast cells treated with 100μM cobalt chloride as compared to the untreated controls (P=0.04 and P<0.01, respectively). HA-1 mRNA expression was significantly higher in the 8% and 2% oxygen conditions as compared to 21% oxygen (P<0.01). HA-1 mRNA expression was also significantly higher in the 2% oxygen condition as compared to the 8% oxygen condition (P<0.01). *=P<0.01.
3.3 Low oxygen increases the expression of HA-1 mRNA in purified term cytotrophoblast cells
To directly examine a potential role of oxygen in regulating HA-1 mRNA expression, purified term cytotrophoblast cells (n=6) were cultured at 2% (hypoxia for the term placenta), 8% (normoxia for the term placenta) and 21% (ambient) oxygen for twenty-four hours. The expression of HA-1 mRNA was then determined using qRT-PCR. HA-1 mRNA expression was significantly different between all three groups (p<0.01), with the highest expression seen in the cells cultured at 2% oxygen (Figure 2).
3.4 HA-1 is increased in the syncytiotrophoblast and syncytial nuclear aggregates of preeclamptic placentas
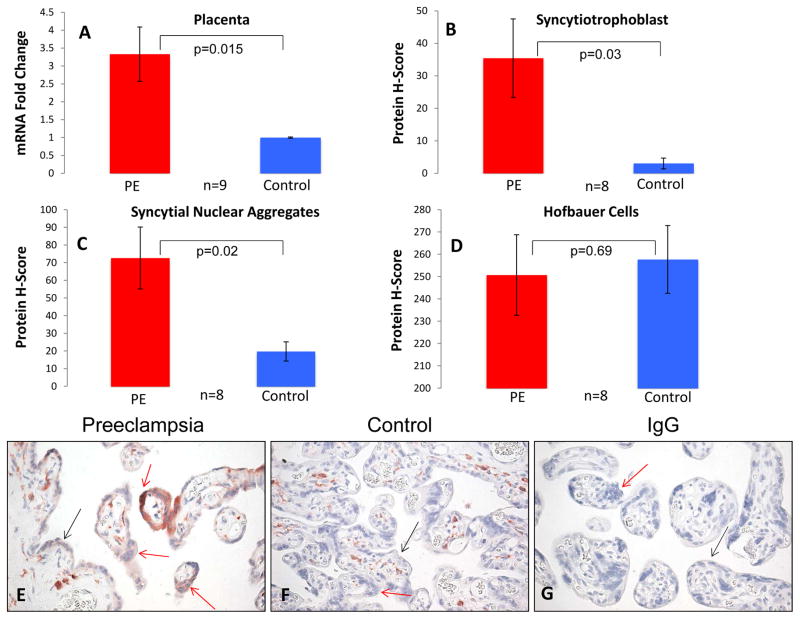
Previously published observations of increased expression of HIF-1α in preeclamptic placentas [36], the disrupted placental blood flow characteristic of this disease [37, 38], and the observed upregulation of HA-1 mRNA by low oxygen, led us to postulate that HA-1 expression may be dysregulated in placentas from preeclamptic patients. Preeclamptic and control placentas were matched for gestational age and mode of delivery (Table 1). Using qRT-PCR, we found that HA-1 mRNA expression (n=9 matched pairs) was significantly increased in placentas from preeclamptic pregnancies as compared to control placentas (Figure 3A, p=0.015). We also used immunohistochemistry to determine the expression of HA-1 protein in the Hofbauer cells, syncytial nuclear aggregates (SNAs) and syncytiotrophoblast layers of preeclamptic and control placentas (n=8 matched pairs; pair 4 was excluded due to poor tissue fixation). Expression of HA-1 in the SNAs and syncytiotrophoblast of preeclamptic placentas was highly variable and showed an uneven distribution within tissue sections, possibly as a result of local differences in oxygen tension and/or HIF-1α expression. Nonetheless, HA-1 protein expression was significantly higher in the SNAs and syncytiotrophoblast of preeclamptic placentas when compared to the matched control placentas (Figure 3B, C, E, F, p=0.02 and 0.03, respectively). It is worth noting that the majority (approximately 80%) of the SNAs observed are likely sectioning artifacts; in the absence of analysis of serial sections, we cannot discriminate between true syncytial knots, villous tips, and bridges between adjacent villi [33]. In addition, we quantified the number of SNAs/villi and found that there was no difference between the preeclamptic and control placentas (0.39 vs. 0.28 SNAs/villi, p=0.15). Despite the observed differences of trophoblast HA-1 expression the between control and preeclamptic placentas, HA-1 expression in the Hofbauer cells (placental macrophages) was similarly high in both groups, (p=0.69), suggesting that regulation of HA-1 expression differs among different cell types.
Figure 3. HA-1 expression is increased in preeclamptic placentas as compared to controls.
Whole placental lysate and placental sections were obtained from preeclamptic and gestational-age and mode of delivery matched controls. (A) HA-1 mRNA expression was determined using qRT-PCR normalized to beta-actin. HA-1 mRNA expression (fold change) was significantly higher in the preeclamptic placentas as compared to the control placentas (n=9, P=0.015). (B–D) HA-1 protein expression in the syncytiotrophoblast and syncytial nuclear aggregates was significantly elevated in the preeclamptic placentas as compared to the controls (n=8, p=0.03 and p=0.02, respectively), whereas there was no difference in HA-1 expression in the Hofbauer cells of preeclamptic placentas as compared to controls (n=8, p=0.69). (E–G) HA-1 protein expression in the syncytiotrophoblast layer (black arrows), syncytial nuclear aggregates (red arrows) and Hofbauer cells of preeclamptic and control placentas was determined by a blinded reviewer using the H-score method. PE = Preeclampsia.
IV. Discussion and Conclusions
Herein we report the first work to investigate mechanisms that regulate the expression of the minor histocompatibility antigen HA-1. We found that expression of HA-1 is significantly higher in the syncytiotrophoblast of first as compared to second and third trimester placentas. We also found that treatment with cobalt chloride, culture in hypoxic conditions, and preeclampsia all increase trophoblast expression of HA-1. One of the major changes that occurs during the transition from the first to the second trimester of pregnancy is a dramatic increase in placental oxygen delivery as a result of maternal spiral artery remodeling by invasive trophoblast cells and the loss of cytotrophoblast cell aggregates which block arterial blood flow to the placenta during early pregnancy [35, 39]. This process may help explain why HA-1 expression in the syncytiotrophoblast decreases as normal gestation progresses but appears to be maintained in preeclampsia, where the remodeling of the spiral arteries is defective.
The increased expression of HA-1 in purified cytotrophoblast cells following treatment with CoCl2 and culture in a low oxygen environment suggests that HA-1 expression may be regulated, at least in part, by oxygen in vivo. This regulation likely occurs via the oxygen-sensitive transcription factor, hypoxia inducible factor (HIF) 1α. Under normoxic conditions, HIF-1α is ubiquitinated and degraded by prolyl hydroxylase, thus preventing its action, while under hypoxic or stress conditions, or in vitro in the presence of cobalt ions, the prolyl hydroxylase is impaired and HIF-1α function is maintained [40–42]. In support of the idea that HIF-1α regulates HA-1 expression in trophoblast cells, there are at least 3 sequences (5′-GCGTG-3′) in the upstream promoter region of the HA-1 gene that could serve as HIF-1α binding sites (800, 219 and 100 base pairs upstream of the HA-1 transcription start site). HIF-1α expression is also increased in preeclamptic as compared to normal placentas [36]. HIF-1α expression in the preeclamptic placenta may be due to changes in oxygen tension, but HIF-1α can also be upregulated by other processes that occur in preeclampsia, including oxidative stress [43] and heightened proinflammatory cytokines [44]. One limitation of this study as a model of pre-eclampsia is the culture of purified cytotrophoblast cells at static oxygen conditions, rather than cycling conditions of hypoxia and normoxia, which would more closely mimic pre-eclampsia in vitro [45]. We chose to examine HA-1 expression at static oxygen conditions in order to better clarify the precise role of oxygen in directly regulating HA-1 expression and remain interested in future studies examining the role of varying oxygen conditions in regulating HA-1 expression.
HA-1-specific CD8+ T cells have been found in the peripheral blood following human pregnancy [13], indicating that exposure of the maternal immune system to fetal HA-1 occurs during normal pregnancies. Several factors may alter maternal exposure to HA-1, and possibly other minor histocompatibility antigens, in preeclampsia. First, the alteration in placental blood flow at the maternal-fetal interface in preeclamptic women leads to areas of ischemia/reperfusion [46], the production of reactive oxygen species (ROS) [45, 47–49] and an increased deportation of syncytiotrophoblast fragments [50–54]. The increased release of syncytiotrophoblast microparticles, including the subset of syncytial nuclear aggregates known as syncytial knots, into the peripheral blood, together with increased HA-1 expression could mean that the maternal immune system is exposed to higher concentrations of HA-1 in preeclampsia than in normal pregnancies.
Second, the production of acute phase reactants and pro-inflammatory cytokines, specifically TNF-α and IL-6 [55], is characteristic of preeclampsia. This increase in pro-inflammatory cytokines together with increased expression of fetal antigen could combine to elicit a heightened antigen-specific maternal immune response in pregnancies complicated by preeclampsia. Although heightened fetal antigen-specific immune responses in preeclampsia have not been definitively shown to date, some authors have suggested the possibility that disruptions in maternal-fetal tolerance [4, 56, 57], a shift from Th2-mediated immunity to Th1-mediated immunity [58, 59], and a reduction in quantity or function or regulatory T cells [60] may play important roles in the pathophysiology of preeclampsia.
In conclusion, we have shown that low oxygen levels as well as preeclampsia increase HA-1 expression by trophoblast cells of the human placenta. The increased expression of HA-1 in the syncytiotrophoblast and syncytial nuclear aggregates of preeclamptic placentas combined with increased syncytiotrophoblast deportation and elevated levels of proinflammatory cytokines may have significant effects on maternal immune activation during preeclamptic as compared to normal pregnancies. The effects of this enhanced immune activation on current and subsequent pregnancies is unknown and may provide important insights into the manifestation of preeclampsia, other pregnancy complications and the success or failure of HA-1-mismatched transplantations. Further, given the function HA-1 as a Rho GTPase activating protein, and the potential for HA-1 expression to influence actin cytoskeletal remodeling [23], the regulation of HA-1 expression is likely to be important for understanding not only the maternal immune response to the fetus during uncomplicated and preeclamptic pregnancies, but other biological processes as well.
Highlights.
Higher expression of HA-1 in 1st as compared to 2nd and 3rd trimester syncytiotrophoblast.
Expression of HA-1 mRNA is increased in trophoblast cells treated with cobalt chloride.
HA-1 mRNA expression is increased in term trophoblast cells cultured in low oxygen (2%).
Increased expression of HA-1 mRNA and protein in preeclamptic as compared to control placentas.
Acknowledgments
The authors thank Adam Krieg for use of the oxygen chambers and for assistance with this project. The authors would also like to thank the staff and patients at the University of Kansas Medical Center, Kansas City, KS, Saint Luke’s Hospital, Kansas City, MO, the Center for Women’s Health, Overland Park, KS and Mount Sinai Hospital, Toronto, CA. This work was supported by NIH grants R01-HD045611 and P20-GM103418.
Footnotes
Conflict of Interest Statement
This statement is to confirm that there are no conflicts of interest associated with this manuscript and that we have no financial relationships that could be construed as a conflict of interest.
In addition, we confirm that all of the listed authors have read and approved this manuscript and that there are no other individuals who should be listed as authors and are not included.
Further, we confirm that there are no intellectual property concerns about this work and that all of the studies involving human subjects have been approved by the appropriate ethical entities.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
VI. References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Sem Perinatol. 2009;33(3):130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Disease models & mechanisms. 2012;5(1):9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soper JT, Mutch DG, Schink JC. Diagnosis and treatment of gestational trophoblastic disease: ACOG Practice Bulletin No. 53. Gynecol Oncol. 2004;93(3):575–85. doi: 10.1016/j.ygyno.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response--a review. Placenta. 2003;24 (Suppl A):S21–7. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 5.Tannetta D, Sargent I. Placental disease and the maternal syndrome of preeclampsia: missing links? Curr Hypertens Rep. 2013;15(6):590–9. doi: 10.1007/s11906-013-0395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hladunewich MA, Derby GC, Lafayette RA, Blouch KL, Druzin ML, Myers BD. Effect of L-arginine therapy on the glomerular injury of preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;107(4):886–95. doi: 10.1097/01.AOG.0000207637.01824.fe. [DOI] [PubMed] [Google Scholar]
- 7.Hunt JS, Andrews GK, Wood GW. Normal trophoblasts resist induction of class I HLA. J Immunol. 1987;138(8):2481–7. [PubMed] [Google Scholar]
- 8.Holland OJ, Linscheid C, Hodes HC, Nauser TL, Gilliam M, Stone P, Chamley LW, Petroff MG. Minor histocompatibility antigens are expressed in syncytiotrophoblast and trophoblast debris: implications for maternal alloreactivity to the fetus. Am J Pathol. 2012;180(1):256–66. doi: 10.1016/j.ajpath.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dierselhuis M, Goulmy E. The relevance of minor histocompatibility antigens in solid organ transplantation. Curr Opin Organ Transplant. 2009;14(4):419–25. doi: 10.1097/MOT.0b013e32832d399c. [DOI] [PubMed] [Google Scholar]
- 10.Goulmy E. Minor histocompatibility antigens: from transplantation problems to therapy of cancer. Hum Immunol. 2006;67(6):433–8. doi: 10.1016/j.humimm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Goulmy E, Schipper R, Pool J, Blokland E, Falkenburg JH, Vossen J, Gratwohl A, Vogelsang GB, van Houwelingen HC, van Rood JJ. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 1996;334(5):281–5. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan NS, Higgins RM, Lam FT, Kashi H, Jobson S, Ramaiyan K, Rahman M, Morris A. HA-1 mismatch has significant effect in chronic allograft nephropathy in clinical renal transplantation. Transplant Proc. 2007;39(5):1439–45. doi: 10.1016/j.transproceed.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 13.Verdijk RM, Kloosterman A, Pool J, van de Keur M, Naipal AM, van Halteren AG, Brand A, Mutis T, Goulmy E. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103(5):1961–4. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 14.Goulmy E, Gratama JW, Blokland E, Zwaan FE, van Rood JJ. A minor transplantation antigen detected by MHC-restricted cytotoxic T lymphocytes during graft-versus-host disease. Nature. 1983;302:159–61. doi: 10.1038/302159a0. [DOI] [PubMed] [Google Scholar]
- 15.Goulmy E, Termijtelen A, Bradley BA, van Rood JJ. Alloimmunity to human H-Y. Lancet. 1976;2(7996):1206. doi: 10.1016/s0140-6736(76)91727-x. [DOI] [PubMed] [Google Scholar]
- 16.Piper KP, McLarnon A, Arrazi J, Horlock C, Ainsworth J, Kilby MD, Martin WL, Moss PA. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod. 2007;76(1):96–101. doi: 10.1095/biolreprod.106.055426. [DOI] [PubMed] [Google Scholar]
- 17.Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PA. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. Journal of immunology. 2012;189(2):1072–80. doi: 10.4049/jimmunol.1200544. [DOI] [PubMed] [Google Scholar]
- 18.Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102(1):106–9. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80(5):1036–45. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James E, Chai JG, Dewchand H, Macchiarulo E, Dazzi F, Simpson E. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood. 2003;102(1):388–93. doi: 10.1182/blood-2002-10-3170. [DOI] [PubMed] [Google Scholar]
- 21.Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182(12):8080–93. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- 22.Perchellet AL, Jasti S, Petroff MG. Maternal CD4(+) and CD8(+) T cell tolerance towards a fetal minor histocompatibility antigen in T cell receptor transgenic mice. Biol Reprod. 2013;89(4):102. doi: 10.1095/biolreprod.113.110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Kreuk BJ, Schaefer A, Anthony EC, Tol S, Fernandez-Borja M, Geerts D, Pool J, Hambach L, Goulmy E, Hordijk PL. The human minor Histocompatibility Antigen1 is a RhoGAP. PLoS One. 2013;8(9):e73962. doi: 10.1371/journal.pone.0073962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholls S, Piper KP, Mohammed F, Dafforn TR, Tenzer S, Salim M, Mahendra P, Craddock C, van Endert P, Schild H, Cobbold M, Engelhard VH, Moss PA, Willcox BE. Secondary anchor polymorphism in the HA-1 minor histocompatibility antigen critically affects MHC stability and TCR recognition. Proc Natl Acad Sci U S A. 2009;106(10):3889–94. doi: 10.1073/pnas.0900411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mommaas B, Kamp J, Drijfhout JW, Beekman N, Ossendorp F, Van Veelen P, Den Haan J, Goulmy E, Mutis T. Identification of a novel HLA-B60-restricted T cell epitope of the minor histocompatibility antigen HA-1 locus. J Immunol. 2002;169(6):3131–6. doi: 10.4049/jimmunol.169.6.3131. [DOI] [PubMed] [Google Scholar]
- 26.Bleakley M, Riddell SR. Exploiting T cells specific for human minor histocompatibility antigens for therapy of leukemia. Immunol Cell Biol. 2011;89(3):396–407. doi: 10.1038/icb.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.den Haan JM, Meadows LM, Wang W, Pool J, Blokland E, Bishop TL, Reinhardus C, Shabanowitz J, Offringa R, Hunt DF, Engelhard VH, Goulmy E. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279(5353):1054–7. doi: 10.1126/science.279.5353.1054. [DOI] [PubMed] [Google Scholar]
- 28.Torikai H, Akatsuka Y, Miyauchi H, Terakura S, Onizuka M, Tsujimura K, Miyamura K, Morishima Y, Kodera Y, Kuzushima K, Takahashi T. The HLA-A*0201-restricted minor histocompatibility antigen HA-1H peptide can also be presented by another HLA-A2 subtype, A*0206. Bone Marrow Transplant. 2007;40(2):165–74. doi: 10.1038/sj.bmt.1705689. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 30.Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS. Isolation and culture of term human trophoblast cells. Methods Mol Med. 2006;121:203–17. doi: 10.1385/1-59259-983-4:201. [DOI] [PubMed] [Google Scholar]
- 31.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68(5):1496–504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 32.Holets LM, Hunt JS, Petroff MG. Trophoblast CD274 (B7-H1) is differentially expressed across gestation: Influence of oxygen concentration. Biol Reprod. 2006;74(2):352–8. doi: 10.1095/biolreprod.105.046581. [DOI] [PubMed] [Google Scholar]
- 33.Goulding H, Pinder S, Cannon P, Pearson D, Nicholson R, Snead D, Bell J, Elston CW, Robertson JF, Blamey RW, et al. A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalin-fixed tissue samples. Human pathology. 1995;26(3):291–4. doi: 10.1016/0046-8177(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 34.Coleman SJ, Gerza L, Jones CJ, Sibley CP, Aplin JD, Heazell AE. Syncytial nuclear aggregates in normal placenta show increased nuclear condensation, but apoptosis and cytoskeletal redistribution are uncommon. Placenta. 2013;34(5):449–55. doi: 10.1016/j.placenta.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. The American journal of pathology. 2000;157(6):2111–22. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caniggia I, Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies--a review. Placenta. 2002;23 (Suppl A):S47–57. doi: 10.1053/plac.2002.0815. [DOI] [PubMed] [Google Scholar]
- 37.North RA, Ferrier C, Long D, Townend K, Kincaid-Smith P. Uterine artery Doppler flow velocity waveforms in the second trimester for the prediction of preeclampsia and fetal growth retardation. Obstet Gynecol. 1994;83(3):378–86. [PubMed] [Google Scholar]
- 38.Steel SA, Pearce JM, McParland P, Chamberlain GV. Early Doppler ultrasound screening in prediction of hypertensive disorders of pregnancy. Lancet. 1990;335(8705):1548–51. doi: 10.1016/0140-6736(90)91376-l. [DOI] [PubMed] [Google Scholar]
- 39.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. American journal of obstetrics and gynecology. 1999;181(3):718–24. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 40.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 41.Lee X, Keith JC, Jr, Stumm N, Moutsatsos I, McCoy JM, Crum CP, Genest D, Chin D, Ehrenfels C, Pijnenborg R, van Assche FA, Mi S. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta. 2001;22(10):808–12. doi: 10.1053/plac.2001.0722. [DOI] [PubMed] [Google Scholar]
- 42.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–82. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 43.Wang T, Leng YF, Zhang Y, Xue X, Kang YQ. Oxidative stress and hypoxia-induced factor 1alpha expression in gastric ischemia. World J Gastroenterol. 2011;17(14):1915–22. doi: 10.3748/wjg.v17.i14.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thornton RD, Lane P, Borghaei RC, Pease EA, Caro J, Mochan E. Interleukin 1 induces hypoxia-inducible factor 1 in human gingival and synovial fibroblasts. Biochem J. 2000;350(Pt 1):307–12. [PMC free article] [PubMed] [Google Scholar]
- 45.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215(1):27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. The American journal of pathology. 2001;159(3):1031–43. doi: 10.1016/S0002-9440(10)61778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–82. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12(6):747–55. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90(12):1274–81. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 50.Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H, Kaufmann P. Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta. 2003;24(2–3):181–90. doi: 10.1053/plac.2002.0903. [DOI] [PubMed] [Google Scholar]
- 51.Huppertz B, Kaufmann P, Kingdom J. Trophoblast turnover in health and disease. Fetal Maternal Med Rev. 2002;13:103–18. [Google Scholar]
- 52.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105(6):632–40. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 53.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von Dadelszen P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27(1):56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Reddy A, Zhong XY, Rusterholz C, Hahn S, Holzgreve W, Redman CW, Sargent IL. The effect of labour and placental separation on the shedding of syncytiotrophoblast microparticles, cell-free DNA and mRNA in normal pregnancy and pre-eclampsia. Placenta. 2008;29(11):942–9. doi: 10.1016/j.placenta.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 55.Kronborg CS, Gjedsted J, Vittinghus E, Hansen TK, Allen J, Knudsen UB. Longitudinal measurement of cytokines in pre-eclamptic and normotensive pregnancies. Acta Obstet Gynecol Scand. 2011;90(7):791–6. doi: 10.1111/j.1600-0412.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Varea A, Pellicer B, Perales-Marin A, Pellicer A. Relationship between Maternal Immunological Response during Pregnancy and Onset of Preeclampsia. Journal of immunology research. 2014;2014:210241. doi: 10.1155/2014/210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Redman CW, Sargent IL. Preeclampsia and the systemic inflammatory response. Semin Nephrol. 2004;24(6):565–70. doi: 10.1016/s0270-9295(04)00127-5. [DOI] [PubMed] [Google Scholar]
- 58.Szarka A, Rigo J, Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC immunology. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am J Reprod Immunol. 2002;47(2):91–7. doi: 10.1034/j.1600-0897.2002.1o020.x. [DOI] [PubMed] [Google Scholar]
- 60.Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, Nanan R. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol. 2012;181(6):2149–60. doi: 10.1016/j.ajpath.2012.08.032. [DOI] [PubMed] [Google Scholar]