Abstract
Background: the term frailty refers to a condition of increased vulnerability to stressors among older people, leading to a decline in homeostatic reserve. Frailty often leads to falls, hospitalisation and mortality, hence its importance for the delivery of health care to older adults. The pathophysiological mechanisms behind frailty are not well understood, but the decreased steroid-hormone production and elevated chronic systemic inflammation of older people appear to be major contributors.
Method: we used a sample of 3,160 individuals aged 50 or older from the English Longitudinal Study of Ageing and assessed their frailty status according to a Frailty Index. We selected 620 single nucleotide polymorphisms in genes involved in the steroid hormone or inflammatory pathways. We performed linear association analysis. The outcome variable was the square root transformation of the Frailty Index, with age and sex entered as covariates.
Results: the strongest signal was detected in the pro-inflammatory Interleukin-18 gene (rs360722, P = 0.0021, β = −0.015). Further significant signals were observed in the Interleukin-12 (rs4679868, P = 0.0062, β = −0.008 and rs9852519, P = 0.0077, β = −0.008), low density lipoprotein receptor-related protein 1 (rs1799986, P = 0.0065, β = 0.011) and Selectin-P (rs6131, P = 0.0097, β = −0.01) genes. None of these associations remain significant after Bonferroni correction.
Conclusions: we show potential associations between genetic variants of four genes and the frailty index. These genes are involved in the cholesterol transport and inflammatory pathway and, as such, our results provide further support for the involvement of the immunological processes in frailty of the elderly.
Keywords: genetics, SNP, Frailty Index, inflammation, older people
Introduction
While it is accepted that the core feature of frailty is increased vulnerability to stressors due to impairments in multiple, inter-related systems that lead to decline in homeostatic reserve and resilience [1], the pathophysiological pathways behind this condition are not well understood.
Performance-based measures of frailty, such as the frailty phenotype [2] have found associations between decreased sex hormone levels such as testosterone, dehydroepiandrosterone (DHEA) and its sulphate derivative (DHEAS) and frailty [3, 4]. The levels of these hormones gradually decline with age, around 1–3%/year [5, 6]. The role that these hormones play in body mass [7, 8], muscle strength [9, 10] and bone mineral density [11, 12] gives biological support to the relation between these sex hormone levels and the performance-based measures of frailty.
Moreover, these hormones have an effect on inflammatory cytokines. Testosterone exerts a suppressive effect on pro-inflammatory cytokines, such as Interleukin-1β (IL-1β) and tumor necrosis factor (TNF) and increases anti-inflammatory cytokine Interleukin-10 (IL-10) [13], whereas DHEA seems to have an effect on Interleukin-6 (IL-6) levels [14].
In line with these results, age-related increases in cytokines and their association with performance measured frailty have been described in the literature [15, 16].
These results indicate a possible frailty mechanism, where the decreasing sex hormone and increasing inflammatory cytokine production during ageing yields a chronic low-level inflammatory state, which is responsible for the frailty symptoms.
Another robust and flexible measure of frailty is based on the concept of deficit accumulation quantified as a Frailty Index (FI) [17]. Deficits can be symptoms, or functional impairments that accumulate with age, such as problems carrying out daily activities, or chronic illnesses. Although this measure has received less attention in association studies, the FI was shown to be associated with lower levels of total and free testosterone and DHEAS in men [18] and increased levels of biomarkers of inflammation: basal IL-6, basal TNF and C-reactive protein [19].
The aim of this study was to conduct a candidate gene association study using the FI as a phenotypic measure in 3,160 community-dwelling individuals over the age of 50 in the English Longitudinal Study of Ageing (ELSA) panel study.
We selected genes involved in the steroid hormone biosynthesis and inflammation pathways, as evidence in the literature indicates their possible involvement in frailty pathophysiology (for example DHEAS [18], IL-6, TNF and C-reactive protein [19]). We hypothesised that single nucleotide polymorphisms (SNPs) within these genes, especially those ones which have been shown to affect the expression of their genes such as rs1800795 for IL6 [20] and rs1800629 for TNF [21] will show significant association to FI.
Methods
Participants
The analyses are performed on a sample of 3,160 participants drawn from Wave 2 (2004) of the ELSA. Detailed description of ELSA can be found elsewhere ([22] www.elsa-project.ac.uk/, 14 September 2015, date last accessed). Briefly ELSA is a prospective panel study representative of community-dwelling men and women over 50 living in England. The participants have answered a computer assisted personal interview biannually collecting a wide range of information on social, psychological, functional and health domains. All participants provided signed consent and ethical approval was granted by the London Multi-Centre Research Ethics Committee.
Genetic data
Genotype data of 620 SNPs for 3,160 participants were obtained from the publicly available ELSA DNA Repository (EDNAR).
Genotyping was performed by Illumina (San Diego, CA, USA) as part of a 1,536 Goldengate custom SNP panel using high-throughput BeadArrayTM technology.
Phenotypic measures
An ELSA Frailty Index (FI) was created following guidance in the literature [17] using Wave 2 data. Briefly, the FI counts health-related problems (deficits) in a range of domains (activities of daily living, cognitive function, falls and fractures, joint replacement, vision, hearing, chronic diseases, cardiovascular diseases, depression). Only individuals with non-missing values for at least 30 of the 62 frailty components were included. A full list of the components is available in Appendix 1 (Supplementary data in Age and Ageing online). The FI had a negatively skewed distribution so we performed square root transformation.
Statistical analysis
We used the Plink software for genetic association analyses [23]. Associations between genotypes and square root transformed FI were tested with linear regression using sex and age as covariates. Stata12 software (Stata Corporation, http://www.stata.com/, 14 September 2015, date last accessed) was used for the demographic and phenotypic analysis. Genetic power calculation was performed by Quanto software (http://biostats.usc.edu/software, 14 September 2015, date last accessed).
Results
Demographic and phenotypic results
Table 1 shows the demographic and phenotypic results. There were more females than males in the sample with no significant differences between the mean ages. The mean FI score was significantly higher in women than in men (two sample t-test, P < 0.0001).
Table 1.
Demographic and phenotypic results
| Total sample | Males | Females | |
|---|---|---|---|
| Number of participants | 3,160 | 1,466 | 1,694 |
| Mean age years (SE) in Wave 2 | 68.3 (0.10) | 68.27 (0.14) | 68.32 (0.14) |
| Mean FI scores (SE) in Wave 2 | 0.153 (0.0018) | 0.140 (0.0025) | 0.164 (0.0025) |
SE, standard error; FI, Frailty Index.
The distribution of the 62 item FI was skewed towards lower scores; therefore we performed square root transformation to normalise the distribution before the genetic association analysis (Figure 1). The lowest untransformed score was 0, the highest 0.634. As expected, age was positively associated with the FI which was lowest in the youngest group (age 60–64, mean FI = 0.133, SE = 0.003) and increased in the consecutive age groups (age 65–69, mean FI = 0.144, SE = 0.003, age 70–74, mean FI = 0.166, SE = 0.004, age 75–79, mean FI = 0.185, SE = 0.004).
Figure 1.
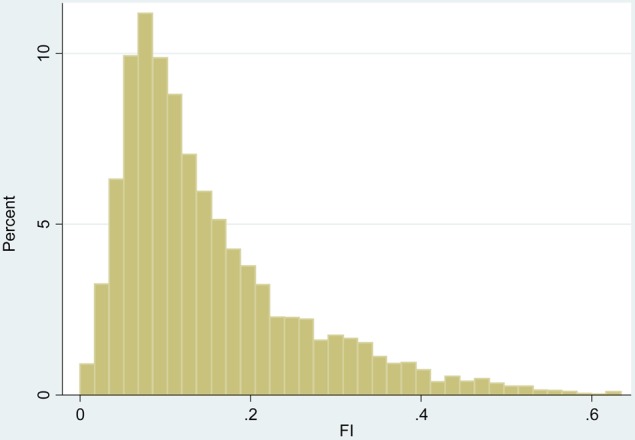
Frailty Index distribution in Wave 2 before square root transformation. FI, Frailty Index.
Genetic association analysis results
Of the received 620 SNPs, 15 were out of Hardy–Weinberg equilibrium (P < 0.01), and a further 15 had a minor allele frequency (MAF) below 5%, so these were excluded from the genetic association analysis, resulting in 590 SNPs used. The full list of 590 SNPs can be found in Appendix 2 (Supplementary data, available in Age and Ageing online).
The most significant results of the genetic association analysis can be seen in Table 2. One SNP reached significance level below P = 0.005, rs360722 (IL18), which decreased the FI (β = −0.015, uncorrected P-value =0.0021). Four SNPs were below the P = 0.01 threshold, rs4679868 (IL12A) (β = −0.008, uncorrected P-value = 0.0062), rs9852519 (IL12A) (β = −0.008, uncorrected P-value = 0.0077) and rs6131 (SELP) (β = −0.01, uncorrected P-value =0.0097), all decreasing the FI, whilst rs1799986 (LRP1) (β = 0.011, uncorrected P-value =0.0065) increased the FI. A further 12 SNPs were below the P = 0.05 significance level (see Supplementary data, Appendix 2, available in Age and Ageing online).
Table 2.
Most significant results of genetic association analysis
| SNP | Minor allele | MAF | β | P-value | Gene |
|---|---|---|---|---|---|
| rs360722 | A | 0.10 | −0.015 | 0.0021 | IL-18 |
| rs4679868 | A | 0.38 | −0.008 | 0.0062 | IL-12A |
| rs1799986 | A | 0.15 | 0.011 | 0.0065 | LRP |
| rs9852519 | A | 0.37 | −0.008 | 0.0077 | IL-12A |
| rs6131 | A | 0.16 | −0.01 | 0.0097 | SELP |
SNP, small nucleotide polymorphism; MAF, minor allele frequency.
Several of these SNPs were in high linkage disequilibrium (rs4679868 and rs9852519, r2 = 0.92, rs2886666 and rs747825, r2 = 0.97 (all IL12A), rs6131 and rs3917729, r2 = 0.65, both SELP); therefore the number of independent signals is fewer.
None of these results survives the Bonferroni correction (P < 8.474E-05).
Discussion
Our candidate gene association study is the first to investigate the association between the Frailty Index and genes involved in steroid hormone metabolism and inflammatory pathways.
Of the five top hits, inflammatory pathways are represented by four SNPs that are located in three genes, IL18, IL12 and SELP. IL-18 is a powerful pro-inflammatory cytokine which plays a pivotal role in systemic and local inflammation, whereas IL12 is a major cytokine produced by antigen presenting cells and represents the link between the cellular and humoral branches of an effective host immune defence [24]. The third gene SELP encodes a cell adhesion molecule with a role in inflammation and haemostasis. During the former, it takes part in the tethering, rolling and weak adhesion of leukocytes, whereas in haemostasis it stabilises platelet aggregates and determines the size of aggregates [25].
IL-18 levels increase with age, similarly to IL-6 and CRP levels [26]. To our knowledge, IL-18 levels have not been investigated in relation to frailty, but higher IL-18 levels have been significantly associated with poorer physical performance (including balance, walk test and chair rise test) and with difficulties performing six activities of daily living (including bathing, dressing, eating, grooming, toileting and continence) [27]. Physical activity was also reported to be significantly and negatively associated with serum IL-18 levels in Japanese men [28]. The rs360722 A allele appeared to be protective against rheumatoid arthritis in another Japanese study [29]. This allele appeared to be protective in our study too, as it was associated with a decrease in the FI.
There is little information in the literature about the function of the other SNPs that we identified as potentially important. Rs4679868 A and rs9852519T alleles (both in IL-12A) have been associated with increased risk of primary biliary cirrhosis [30] whereas the A allele of rs6131 (SELP) has been found to lower platelet activation upon release of the cross-clamp in patients undergoing coronary artery bypass graft [31].
Naturally, these gene-products are all part of the very complex immune system and they are in interaction with several other molecules. For example, both IL18 and IL12 stimulate the interferon-γ production (IFN-γ) of Th1 cells, with a synergistic effect between them [24]. Moreover, rs360722 A allele (IL18) itself has been associated with a dose-dependent decrease in the secretion of the pro-inflammatory cytokine tumor necrosis factor (TNF) following smallpox vaccination [32].
It appears that SELP also has an effect on TNF. Adhesion of monocytes to SELP increased the secretion of TNF by the leukocytes when they were stimulated with platelet-activating factor in vitro [33].
Despite this, none of the eight SNPs in the IFNG and TNF gene regions showed an association with FI in our study. Other important inflammatory pathway genes, such as CRP or IL6 (and its receptor IL6R), were also not associated with the FI, although CRP and IL6 levels have been associated with this phenotype [19]. One possible explanation for this is that IL-18 and IL-12 exert their effect on frailty independently from each other, not via increasing the IFN-γ or TNF levels, perhaps via pathways not related to IL6 or CRP. The inflammatory pathways consist of many members with complex interactions between them and it is not possible to evaluate these interactions fully within a candidate gene study of a limited number of genes.
It is important to note though, that as our results did not remain significant after the Bonferroni-correction; it is possible that they are false positive associations, although their agreement is compatible with some previous findings in the literature.
We also found a relationship below the P = 0.01 level for rs1799986, a synonymous variant located within the third exon of low density lipoprotein receptor-related protein 1 (LRP1) gene on chromosome 12. LRP1 is a large multiligand receptor involved in several cellular processes, including intracellular signalling, lipid homeostasis including cholesterol transport, and clearance of apoptotic cells [34] and thought to have a role in the pathology of Alzheimer disease (AD) [34, 35]. Analysis of post-mortem brain tissue showed that LRP1 levels normally decrease with ageing and were significantly lower in the brains of AD patients compared to controls [35]. There is contradictory evidence in the literature on the functionality of the rs1799986 polymorphism in AD [36, 37, 38]. Our results showed that the A allele appeared to increase the FI which is in agreement with a Spanish study, in which it was associated with increased risk of premature cardiovascular disease [39]. On the other hand, as rs1799986 is a synonymous mutation, it is possible that another SNP nearby is responsible for the effect. And this result did not remain statistically significant after correction for multiple testing.
This study has a number of limitations. First, only a proportion of the ELSA sample was genotyped, thus it may not be as representative as the full sample of the older adults in England. Secondly, the FI is based on self-reported questions which may introduce bias into the measure. The distribution of FI is skewed towards the lower end of the scale; therefore those with severe frailty are underrepresented in this sample of community-residing older people. This distribution however is in line with other reports from the literature. Thirdly, the genes and genotypic variants were selected from a publicly available dataset with limited genotyping, rather than specifically selected for the purpose of this study. Finally, we did not have the opportunity to measure serum IL-18 levels to corroborate our findings as data on this were not available in the dataset.
The strengths of our study include the derivation of the sample from a representative population study of older adults in England using a panel survey with standardised interviewer delivered validated protocols. Another important strength is the achieved sample size. The effective sample size required to test whether a gene main effect is associated with a continuous trait for 99% power, α = 0.05 (two-sided test), assuming outcomes that are modelled using linear regression (mean = 0.153, SD = 0.10, genetic effect = 0.01, MAF = 0.05) is 1,828, which is given in our study. As with all genetic association studies it would be advantageous to replicate our findings.
In summary, our candidate gene association study is the first one to investigate the genetic components of the Frailty Index and indicates the role of inflammation and cholesterol transport. Although the results are in line with some previous findings in the literature, none of the associations remains statistically significant after correction for multiple testing. This report generates hypotheses and hopefully will encourage further research into the molecular determinants of frailty in older adults.
Key points.
Limited evidence exists of plausible biological markers of frailty.
To address this we conducted a candidate gene association study using the Frailty Index in the ELSA.
The most significant signal was within IL-18 gene with lesser associations in the IL-12, LRP1 and SELP genes.
Our findings support that inflammatory pathways are implicated in frailty.
Conflicts of interest
None declared.
Funding
This work was supported by the Medical Research Council (G1001375), UK. The funder had no further role in the conduct of the research.
Supplementary data
Supplementary data mentioned in the text is available to subscribers in Age and Ageing online.
Acknowledgement
The authors are very grateful to Dr Meena Kumari and Mr Jorgen Engmann for providing the phenotypic and genotypic data and to all the participants in ELSA.
References
The very long list of references supporting this research paper has meant that only the most important are listed here and represented by bold type throughout the text. The full list of references is available in Appendix 3 (Supplementary data in Age and Ageing online).
- 1.Bergman H, Ferrucci L, Guralnik J et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62A: 731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56. [DOI] [PubMed] [Google Scholar]
- 3.Carcaillon L, Blanco C, Alonso-Bouzón C, Alfaro-Acha A, Garcia-García FJ, Rodriguez-Mañas L. Sex differences in the association between serum levels of testosterone and frailty in an elderly population: the Toledo Study for Healthy Aging. PLoS One 2012; 7: e32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 1991; 73: 1016–25. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas-Shankar U, Roberts SA, Connolly MJ et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2010; 95: 639–50. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Swerdloff RS, Iranmanesh A et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85: 2839–53. [DOI] [PubMed] [Google Scholar]
- 11.Khosla S, Melton LJ III, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 1998; 83: 2266–74. [DOI] [PubMed] [Google Scholar]
- 13.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 2004; 89: 3313–8. [DOI] [PubMed] [Google Scholar]
- 14.Straub RH, Konecna L, Hrach S et al. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. J Clin Endocrinol Metab 1998; 83: 2012–7. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard RE, O'Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med 2009; 13: 3103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tajar A, O'Connell MD, Mitnitski AB et al. Frailty in relation to variations in hormone levels of the hypothalamic-pituitary-testicular axis in older men: results from the European male aging study. J Am Geriatr Soc 2011; 59: 814–21. [DOI] [PubMed] [Google Scholar]
- 19.Collerton J, Martin-Ruiz C, Davies K et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev 2012; 133: 456–66. [DOI] [PubMed] [Google Scholar]
- 20.Fishman D, Faulds G, Jeffery R et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 1998; 102: 1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA 1997; 94: 3195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purcell S, Neale B, Todd-Brown K et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biet F, Locht C, Kremer L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med (Berl) 2002; 80: 147–62. [DOI] [PubMed] [Google Scholar]
- 25.Chen M, Geng JG. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch Immunol Ther Exp (Warsz) 2006; 54: 75–84. Review. [DOI] [PubMed] [Google Scholar]
- 26.Ferrucci L, Corsi A, Lauretani F et al. The origins of age-related proinflammatory state. Blood 2005; 105: 2294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frayling TM, Rafiq S, Murray A et al. An interleukin-18 polymorphism is associated with reduced serum concentrations and better physical functioning in older people. J Gerontol A Biol Sci Med Sci 2007; 62: 73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oda K, Miyatake N, Sakano N et al. Serum interleukin-18 levels are associated with physical activity in Japanese men. PLoS One 2013; 8: e81497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiura T, Kawaguchi Y, Ikari K et al. Interleukin-18 promoter polymorphisms in Japanese patients with rheumatoid arthritis: protective effect of the T allele and T/T genotype at rs360722. Mod Rheumatol 2011; 21: 359–64. [DOI] [PubMed] [Google Scholar]
- 30.Hirschfield GM, Liu X, Xu C et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med 2009; 360: 2544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathew JP, Podgoreanu MV, Grocott HP et al. Genetic variants in P-selectin and C-reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol 2007; 49: 1934–42. [DOI] [PubMed] [Google Scholar]
- 32.Ovsyannikova IG, Haralambieva IH, Kennedy RB et al. Impact of cytokine and cytokine receptor gene polymorphisms on cellular immunity after smallpox vaccination. Gene 2012; 510: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest 1995; 95: 2297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev 2008; 88: 887–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang DE, Pietrzik CU, Baum L et al. Modulation of amyloid beta-protein clearance and Alzheimer's disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest 2000; 106: 1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollenbach E, Ackermann S, Hyman BT, Rebeck GW. Confirmation of an association between a polymorphism in exon 3 of the low-density lipoprotein receptor-related protein gene and Alzheimer's disease. Neurology 1998; 50: 1905–7. [DOI] [PubMed] [Google Scholar]
- 39.Aledo R, Alonso R, Mata P, Llorente-Cortés V, Padró T, Badimon L. LRP1 gene polymorphisms are associated with premature risk of cardiovascular disease in patients with familial hypercholesterolemia. Rev Esp Cardiol (Engl Ed) 2012; 65: 807–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


